Abstract
Objective:
In the U.S., uterine cancer incidence is rising, with racial and ethnic minorities experiencing the largest increases. We performed age-period-cohort analyses using novel methods to examine the contribution of age at diagnosis (age), year of diagnosis (period), and birth cohort (cohort), to trends in uterine cancer incidence.
Methods:
We used uterine cancer incidence data from the Surveillance, Epidemiology, and End Result (SEER) 12 database (1992-2019), and performed hysterectomy-correction. We generated hexamaps to visualize age, period, and cohort effects, and used mutual information to estimate the percent contribution of age, period, and cohort effects, individually and combined, on uterine cancer incidence, overall and by race and ethnicity and histology.
Results:
Hexamaps showed an increase in uterine cancer in later time periods, and a cohort effect around 1933 showing a lower incidence compared with earlier and later cohorts. Age, period, and cohort effects combined contributed 86.6% (95% CI: 86.4%, 86.9%) to the incidence. Age effects had the greatest contribution (65.1%, 95% CI: 64.3%, 65.9), followed by cohort (20.7%, 95% CI: 20.1%, 21.3%) and period (14.2%, 95% CI: 13.7%, 14.8%) effects. Hexamaps showed higher incidence in recent years for non-Hispanic Blacks and non-endometrioid tumors.
Conclusions:
Age effects had the largest contribution to uterine cancer incidence, followed by cohort and period effects overall and across racial and ethnic groups and histologies.
Impact:
These findings can inform uterine cancer modeling studies on the effects of interventions that target risk factors which may vary across age, period, or cohort.
Keywords: Uterine cancer, endometrial cancer, age, cohort, period, hysterectomy
Introduction
Uterine cancer is the fourth most common cancer among females and the sixth most common cause of cancer-associated death in women the United States, with an estimated 66,200 new cases and 13,030 deaths in 2023.1 The incidence of uterine cancer is increasing and expected to surpass colorectal cancer as the third leading cause of cancer in females, and fourth leading cause of cancer death by 2040.2-4 Specifically, since 2001, uterine cancer has increased across racial and ethnic groups from 40.8 per 100,000 in 2001 to 42.9 per 100,000 in 2016 (Annual Percent Change (APC) = 0.5, p<0.001), with the largest increases observed in non-Hispanic Black, Hispanic, and Asian women.5
There are three main histologic types of uterine cancer: endometrioid, non-endometrioid, and sarcoma. Endometrioid cancers make up approximately 75% of all uterine cancers and have the best prognosis. Non-endometrioid cancers, which include serous and clear cell carcinomas, account for approximately 15-20% of all uterine cancers, are more aggressive, and are associated with worse outcomes. Lastly, uterine sarcomas, which arise in the myometrium, are the least common group and the least well-studied.2,3 Non-Hispanic Black women have a two- to four-fold increased risk of developing non-endometrioid uterine cancers compared with non-Hispanic White women, which may contribute to the higher mortality observed in these women.5
Age-period-cohort (APC) analysis plays an important role in understanding temporal elements in epidemiology by classifying three types of time-varying phenomena: age effects, period effects, and cohort effects, birth cohort effects specifically in this analysis. Age effects are variations associated with the biological changes and social experiences of aging; period effects are variations across time that affect all age groups equally; and cohort effects are variations due to common exposures or experiences of a group of people (birth cohort) as they move through time.6,7 APC analysis can help elucidate factors that may be contributing to observed uterine cancer incidence trends. For example, changes in the prevalence of established risk factors for uterine cancer, including obesity and diabetes, which increase risk, and parity and hormonal contraceptive use, which decreases risk8, may vary across age, time period, and birth cohort. Further, this information can be used to predict future uterine cancer incidence and prioritize interventions and populations for reducing uterine cancer risk.
There is limited research on age-period-cohort analyses of uterine cancer incidence in the U.S. Further, given that age at diagnosis, year of diagnosis, and patient birth year are linearly dependent, traditional regression models cannot effectively distinguish their relative contributions to changes in incidence without additional assumptions or constraints.9 By utilizing a novel data visualization method in conjunction with a quantitative information theory-based analytic approach, we are better able to capture and describe these competing temporal effects which can inform model designs and targeted prevention efforts. This analysis aims to estimate and compare the effects of age, period, and cohort on uterine cancer incidence in the U.S.
Materials and Methods
We extracted uterine cancer incidence data from the Surveillance, Epidemiology, and End Result (SEER) 12 registry database (1992-2019)10 for patients with known age between 0 and 84 years. We included patients whose cancers had a primary site ICD-10 diagnosis code in C54.0-C55.9. We included only cancers that had diagnostic confirmation by either microscopic confirmation or positive histology. We categorized uterine cancers into the following three histologic groups with respective ICD-0-3 histology codes: endometrioid: 8140, 8380-8383, 8480, 8570; non-endometrioid: 8000, 8020, 8050, 8255, 8260, 8310, 8323, 8441, 8460-8461, 8481,8560, 8950-8951,8980-8982; and sarcoma: 8800-8805, 8810-8811, 8813-8815, 8890-8891, 8894-8896, 8900-8902, 8910, 8912, 8930-8931, 8933, 8935, 8990-8991. We extracted the following variables from SEER: age at diagnosis, year of diagnosis, and race and ethnicity defined as Non-Hispanic White, Non-Hispanic Black, Non-Hispanic American Indian/Alaska Native, Non-Hispanic Asian-American/Pacific Islander, and Hispanic (all races). Although hysterectomy effectively removes a woman from the at-risk population, and hysterectomy prevalence in the U.S. can reach over 40%11, information about prior hysterectomy is not available in SEER. In separate analyses, we estimated hysterectomy-adjusted incidence rates of uterine cancer based on age- and period-specific hysterectomy prevalence data from several nationally-representative databases, including The National Hospital Discharge Survey and databases from The Healthcare Cost and Utilization Project (i.e., The Naitonal Inpatient Sample, State Inpatient Databases, and The State Ambulatory Surgery and Services Databases).12
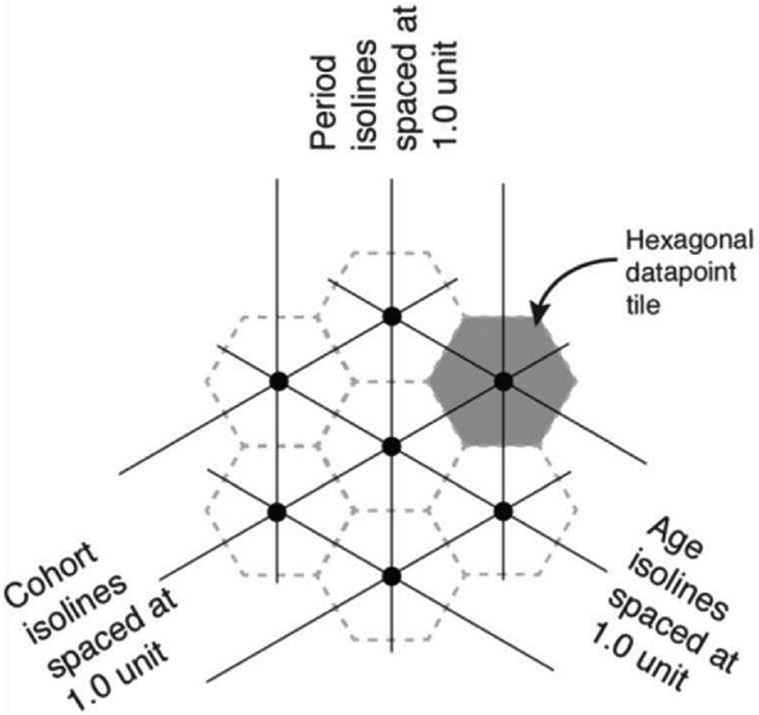
We generated hexamaps to visualize the simultaneous effects of age, period, and cohort on uterine cancer incidence. Hexamaps are similar to traditional heatmaps, but use hexagonal instead of rectilinear tiled maps, which create equally spaced isobars for all three of the age, period, and cohort axes, and enable accurate trend recognition. Color change perpendicular to an isobar indicates greater effect strength.13 (Figure 1) For age, period, and cohort effects, we used age at uterine cancer diagnosis, year of uterine cancer diagnosis, and birth year, which was determined by subtracting age from year, respectively.
Figure 1.
Illustration of a hexagonal heatmap (hexamap) depicting the age, period, and cohort isolines
Quantifying the comparative effect strengths of our collinear predictors (age, period, and cohort) can be achieved by recognizing non-linear data features. Mutual information is a quantity that measures non-linear associations between variables.14 Functionally, mutual information serves as a generalization of the correlation coefficient, where the reduction in variance of one variable (e.g., incidence rate), due to a single observation of another variable (e.g., age at diagnosis), is used to quantify the association. Thus, we used a composite information theory-based metric to parse out the relative importance of age, period and cohort in our observational data. The dataset is first discretized into equal width bins, with the number of bins equal to the number of calendar years present in the data. This is done to avoid bias due to differences in data ranges between the age at diagnosis, year at diagnosis, and birth year variables.15 Our composite measure is broken down into four percentage-based components. The first is the Shannon’s Entropy of the incidence with variables conditioned on the age, period, and cohort variables subtracted from the unconditional Shannon’s Entropy of incidence, divided by the Shannon’s Entropy of incidence and finally rescaled to a percentage. This serves as a measure of the total amount of information contained in the predictor variables (age, period, and cohort) as a percentage of the total amount of information needed to specify the incidence rate. This is sensitive to the variance in the underlying incidence data. The second and third components are measures of the contribution of age and period predictors. Formally, this is defined as the minimum of the mutual information between incidence and predictors, and the conditional mutual information between incidence and predictors when conditioned on cohort. This value is then converted into a percentage of the total information contained in the predictors. As age and period separately have non-zero mutual information with cohort in observational data, conditioning is necessary in case the single predictor effects are mediated via the cohort predictor. Practically this can be interpreted as the contribution of the predictor alone to the incidence rate. The fourth component is similar to the second and third however the minimum is taken out of the mutual information between incidence and cohort, the mutual information conditioned on age, and the mutual information conditioned on period. This is done for the same reason, to extract the contribution of cohort alone on the incidence.16 We then developed 95% confidence intervals around the mutual information contribution estimates using a method developed by Stefani and colleagues.17
We estimated the percent contribution of age, period, and cohort effects to the uterine cancer incidence rate, individually and combined, overall with and without hysterectomy-correction. Then, using only hysterectomy-corrected uterine cancer incidence rates, we generated new hexamaps and estimated the percent contribution of age, period, and cohort stratified by race and ethnicity, and by histology. We also plotted hysterectomy-corrected and - uncorrected incidence trends by age, period, and cohort separately (Supplemental Figures 1a-1c). We analyzed all data using R Version 4.3.1.
Data availability
The data analyzed in this study are available from the SEER database, which is available from the National Cancer Institute (https://seer.cancer.gov/data-software/).
Results
We identified 132,754 uterine cancer cases diagnosed between 1992 and 2019. The number of cases increased across time periods with 45.4% of cases coming from the most recent 10 years of data (2010-2019). While the greatest proportion of cases were non-Hispanic White, the proportion of non-Hispanic White cases decreased over time from 78.8% in 1992-2000 to 60.3% in 2010-2019. Correspondingly, the proportion of cases in all other racial and ethnic groups increased over time. While the proportion of non-endometrioid cases increased over time from 14.6% in 1992-2000 to 21.1% in 2010-2019, the proportion of endometrioid cases slightly decreased over time from 78.4% in 1992-2000 to 72.8% in 2010-2019. The proportion of local stage cancers decreased over time (73.6% in 1992-2000 and 68.4% in 2010-2019) with corresponding increases in regional and distant stage disease (14.4% and 8.2% in 1992-2000 and 19.8% and 9.2% in 2010-2019, respectively). (Table 1)
Table 1.
Baseline characteristics of uterine cancer cases in SEER 12 (1992-2019)
| Baseline Characteristics | Year at Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall 1992-2019 |
1992-2000 | 2001-2009 | 2010-2019 | |||||
| N | % | N | % | N | % | N | % | |
| Age at diagnosis (years) | 132,754 | 100.0 | 33,219 | 25.0 | 39,216 | 29.5 | 60,319 | 45.4 |
| 0-10 | 4 | 0.0 | 0 | 0.0 | 2 | 0.0 | 2 | 0.0 |
| 11-20 | 30 | 0.0 | 9 | 0.0 | 9 | 0.0 | 12 | 0.0 |
| 21-30 | 969 | 0.8 | 213 | 0.7 | 266 | 0.7 | 490 | 0.9 |
| 31-40 | 5196 | 4.3 | 1,274 | 4.2 | 1,593 | 4.4 | 2,329 | 4.2 |
| 41-50 | 15657 | 12.8 | 4,147 | 13.6 | 5,163 | 14.4 | 6,347 | 11.4 |
| 51-60 | 37929 | 31.0 | 7,909 | 25.9 | 12,213 | 34.0 | 17,807 | 32.0 |
| 61-70 | 40887 | 33.5 | 9,303 | 30.5 | 10,848 | 30.2 | 20,736 | 37.2 |
| 71-80 | 26168 | 21.4 | 8,442 | 27.6 | 7,122 | 19.8 | 10,604 | 19.0 |
| 81-84 | 5914 | 4.8 | 1,922 | 6.3 | 2,000 | 5.6 | 1,992 | 3.6 |
| Race and Ethnicity | ||||||||
| NH White | 89,459 | 67.4 | 25,919 | 78.0 | 27,163 | 69.3 | 36,377 | 60.3 |
| NH Black | 9,840 | 7.4 | 1,756 | 5.3 | 2,726 | 7.0 | 5,358 | 8.9 |
| NH AI/AN | 1,118 | 0.8 | 181 | 0.5 | 342 | 0.9 | 595 | 1.0 |
| NH API | 15,050 | 11.3 | 2,624 | 7.9 | 4,329 | 11.0 | 8,097 | 13.4 |
| Hispanic | 16,610 | 12.5 | 2,649 | 8.0 | 4,488 | 11.4 | 9,473 | 15.7 |
| Histology | ||||||||
| Non-Endometriod | 24,727 | 18.6 | 4,855 | 14.6 | 7,157 | 18.3 | 12,715 | 21.1 |
| Endometriod | 99,195 | 74.7 | 26,043 | 78.4 | 29,243 | 74.6 | 43,909 | 72.8 |
| Sarcoma | 6,055 | 4.6 | 1,540 | 4.6 | 1,912 | 4.9 | 2,603 | 4.3 |
| Other | 2,777 | 2.1 | 781 | 2.4 | 904 | 2.3 | 1,092 | 1.8 |
| Region | ||||||||
| Northeast | 16,745 | 12.6 | 4,584 | 13.8 | 5,228 | 13.3 | 6,933 | 11.5 |
| Midwest | 14,014 | 10.6 | 3,843 | 11.6 | 4,276 | 10.9 | 5,895 | 9.8 |
| South | 9,370 | 7.1 | 1,985 | 6.0 | 2,548 | 6.5 | 4,837 | 8.0 |
| West | 92,625 | 69.8 | 22,807 | 68.7 | 27,164 | 69.3 | 42,654 | 70.7 |
| Summary Stage | ||||||||
| Local | 92,948 | 70.0 | 24,447 | 73.6 | 27,243 | 69.5 | 41,258 | 68.4 |
| Regional | 23,822 | 17.9 | 4,790 | 14.4 | 7,076 | 18.0 | 11,956 | 19.8 |
| Distant | 12,098 | 9.1 | 2,730 | 8.2 | 3,829 | 9.8 | 5,539 | 9.2 |
| Unstaged/Unknown | 3,886 | 2.9 | 1,252 | 3.8 | 1,068 | 2.7 | 1,566 | 2.6 |
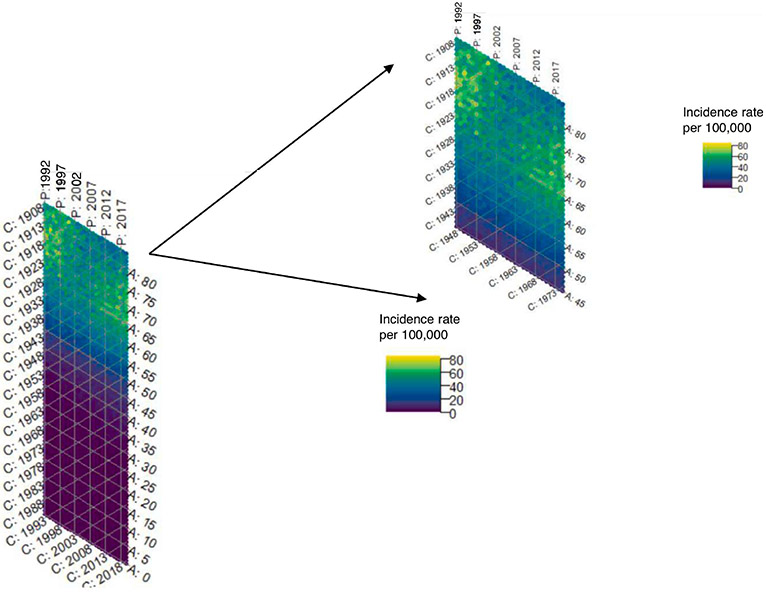
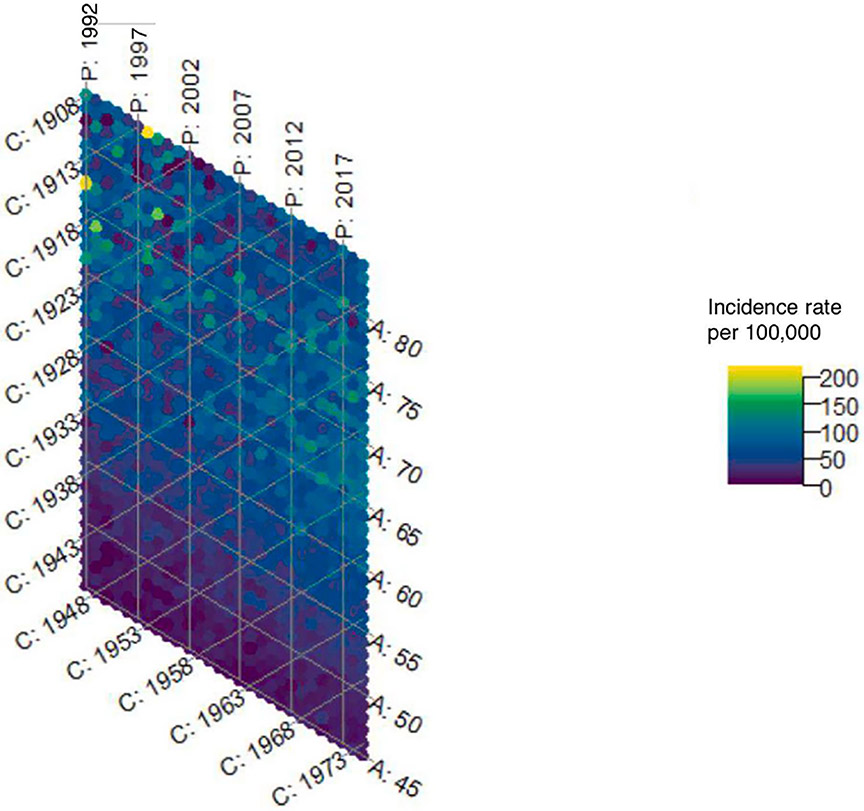
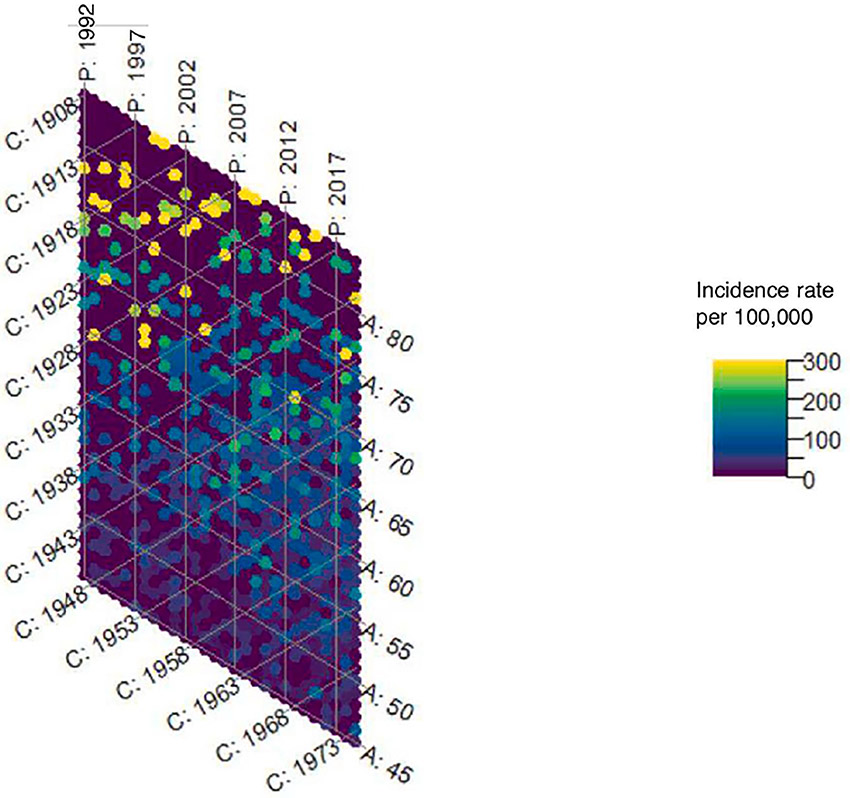
The hexamaps for uterine cancer incidence overall show a strong age effect which is expected, given that the majority of uterine cancers are diagnosed in postmenopausal women. In order to better visualize the color gradients in women most at risk of uterine cancer, we truncated the hexamaps at age 45. (Figures 2a and 2b) Consistent with prior literature on the importance of using hysterectomy-corrected data to accurately estimate the uterine cancer incidence in women who are at risk of the disease3,18-21, we observed substantial differences in incidence between hysterectomy-corrected and –uncorrected data, that varied across age, time period, and birth cohort. (Supplemental Figures 1a-1c) However, the total contribution of age, period, and cohort effects to uterine cancer incidence was similar whether using hysterectomy uncorrected (86.2%, 95% CI 85.9%-86.4%) or corrected data (86.6%, 95% CI 86.4%-86.9%). (Figure 2c) Using the hysterectomy-corrected data, the hexamap shows a slight cohort effect, with women born around 1933 having a lower rate of uterine cancer, and a period effect with higher rates in earlier and later time periods. (Figure 2b) Age effects had the greatest contribution to incidence (65.1%, 95% CI: 64.3%, 65.9%), followed by cohort effects (20.7%, 95% CI: 20.1%, 21.3%) then period effects (14.2%, 95% CI: 13.7%, 14.8%). (Figure 2c)
Figure 2a.
Hexamap for the uterine cancer incidence rate (per 100,000) overall without hysterectomy correction (SEER 12)
* Abbreviations: C = Cohort; P = Period; A = age
Figure 2b.
Hexamap for the uterine cancer incidence rate (per 100,000) overall with hysterectomy correction (SEER 12)
* Abbreviations: C = Cohort; P = Period; A = age
Figure 2c.
Percent contribution to the incidence rate for age, period, and cohort effects individually and combined stratified by hysterectomy correction
*No Hysterectomy Correction:
Cohort: 22.9 (95% CI: 22.4, 23.5)
Period: 12.7 (95% CI: 12.3, 13.2)
Age: 64.3 (95% CI: 63.6, 65.1)
With Hysterectomy Correction:
Cohort: 20.7 (95% CI: 20.1, 21.3)
Period: 14.2 (95% CI: 13.7, 14.8)
Age: 65.1 (95% CI: 64.3, 65.9)
The hexamaps also showed differences in age, period, and cohort effect contributions to uterine cancer incidence by race and ethnicity. (Figures 3a-3e) The hexamap for non-Hispanic White patients shows a higher incidence in earlier time periods, followed by a decrease, and then a slight increase in later time periods. There was a cohort effect between birth years 1933 and 1938 showing a lower incidence rate. The hexamap for non-Hispanic Black patients shows an increase in incidence in later time periods, particularly for women aged 60-75 years. The hexamaps for Non-Hispanic AI/ANs and APIs show an increase in incidence in later time periods and younger birth cohorts. The hexamap for Hispanic patients shows a slight increase in incidence in later time periods, particularly for women aged 60-70 years. The contribution of age, period, and cohort effects combined had the largest contribution to incidence for non-Hispanic White patients (86.0%, 95% CI: 85.7, 86.4) and the smallest contribution for non-Hispanic AI/ANs (78.1%, 95% CI: 72.2%, 83.4%). While age effects still had the largest contribution to uterine cancer incidence for all racial and ethnic groups, it had the greatest contribution for non-Hispanic White and Hispanic patients (62.5%, 95% CI: 61.4%, 63.7% and 62.2%, 95% CI: 60.0%, 64.5%, respectively), and the lowest contribution for non-Hispanic AI/ANs (43.2%, 95% CI: 32.4%, 56.1%). Cohort effects had the next largest contribution to uterine cancer incidence for all racial and ethnic groups, but was highest for non-Hispanic AI/ANs (38.2%, 95% CI: 27.7%, 50.6%) and lowest for non-Hispanic Whites (21.8%, 95% CI: 20.9%, 22.7%). (Figure 3f)
Figure 3a.
Hexamap for the uterine cancer incidence rate (per 100,000) in Non-Hispanic White women (SEER 12)
* Abbreviations: NH = Non-Hispanic; C = Cohort; P = Period; A = age
Figure 3e.
Hexamap for the uterine cancer incidence rate (per 100,000) in Hispanic women (SEER 12)
*Abbreviations: C = Cohort; P = Period; A = Age
Figure 3f.
Percent contribution to the uterine cancer incidence rate for age, period, and cohort effects individually and combined stratified by race and ethnicity
*NH White:
Cohort: 21.8 (95% CI: 20.9, 22.7)
Period: 15.7 (95% CI: 14.8, 16.5)
Age: 62.5 (95% CI: 61.4, 63.7)
NH Black:
Cohort: 28.5 (95% CI: 25.9, 31.1)
Period: 14.1 (95% CI: 11.8, 16.4)
Age: 57.5 (95% CI: 54.3, 60.7)
NH API:
Cohort: 30.4 (95% CI: 28.7, 32.3)
Period: 12.4 (95% CI: 10.9, 13.9)
Age: 57.2 (95% CI: 55.2, 59.3)
NH AI/AN:
Cohort: 38.2 (95% CI: 27.7, 50.6)
Period: 18.6 (95% CI: 9.6, 29.2)
Age: 43.2 (95% CI: 32.4, 56.1)
Hispanic:
Cohort: 27.6 (95% CI: 25.8, 29.4)
Period: 10.2 (95% CI: 8.7, 11.7)
Age: 62.2 (95% CI: 60.0, 64.5)
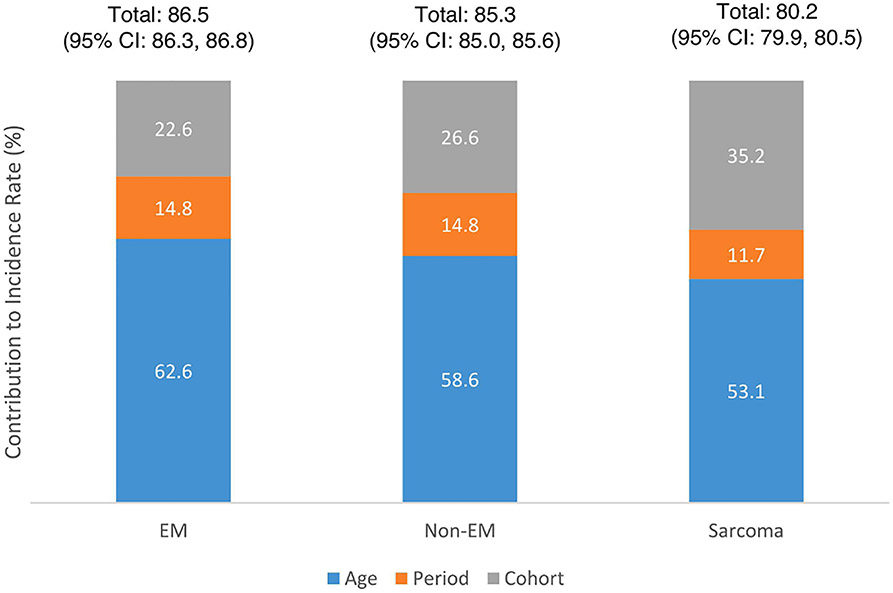
The hexamaps by uterine cancer histology show differences in incidence trends across ages, periods, and cohorts. (Figures 4a-4c) The hexamap for endometrioid cancers shows a period effect with an overall decreasing incidence over time, but a slight cohort effect around the 1938 birth year shows a lower incidence compared to the older and younger birth cohorts. The hexamap for non-endometroid cancers shows a period effect with a higher incidence in later time periods. Period and cohort effects were more difficult to distinguish in the hexamap for sarcomas given the smaller sample size and lower incidence rate. While age effects contributed the most to incidence across histologies, it had the greatest contribution for endometrioid cancers (62.6%, 95% CI: 61.8%, 63.4%) and the smallest contribution for sarcomas (53.1%, 95% CI: 52.3%, 53.9%). Cohort effects played the next largest role in incidence across histologies, contributing 35.2% (95% CI: 34.5%, 36.0%) for sarcomas, 26.6% (95% CI: 25.9%, 27.3%) for non-endometrioid cancers, and 22.6% (95% CI: 22.0%, 23.2%) for endometrioid cancers. While period effects were similar for endometrioid and non-endometrioid cancers, contributing 14.8% to the incidence rate, they had a smaller contribution for sarcomas (11.7%, 95% CI: 11.1%, 12.3%). (Figure 4d) Percent contribution estimates for all racial and ethnic and histologic subtype groups are reported in Supplemental Table 1.
Figure 4a.
Hexamap for the incidence rate (per 100,000) of Endometrioid uterine cancers (SEER 12)
*Abbreviations: Em = Endometriod; C = Cohort; P = Period; A = age
Figure 4c.
Hexamap for the incidence rate (per 100,000) of Sarcoma uterine cancers (SEER 12)
* Abbreviations: C = Cohort; P = Period; A = age
Figure 4d.
Percent contribution to the uterine cancer incidence rate for age, period, and cohort effects individually and combined stratified by histology
*Endometriod:
Cohort: 22.6 (95% CI: 22.0, 23.2)
Period: 14.8 (95% CI: 14.2, 15.4)
Age: 62.6 (95% CI: 61.8, 63.4)
Non-Endometriod:
Cohort: 26.6 (95% CI: 25.9, 27.3)
Period: 14.8 (95% CI: 14.2, 15.5)
Age: 58.6 (95% CI: 57.5, 59.4)
Sarcoma:
Cohort: 35.2 (95% CI: 34.5, 36.0)
Period: 11.7 (95% CI: 11.1, 12.3)
Age: 53.1 (95% CI: 52.3, 53.9)
Discussion
This study evaluated the individual and total contributions of age, period, and cohort effects to uterine cancer incidence using novel methodologies to both visually and computationally quantify their impact. The hexamap for the overall hysterectomy-corrected uterine cancer incidence displayed the increase in incidence observed in recent years3 and highlighted a cohort effect with birth cohorts around 1933 having a lower incidence compared with earlier and later birth cohorts. The hexamaps further highlighted different trends across racial and ethnic groups and histologic subtypes, with the higher rates in recent years more pronounced for non-Hispanic Black women compared with other racial and ethnic groups, and for women with non-endometrioid cancers compared with endometrioid tumors and sarcomas. These results support prior research showing non-Hispanic Black women have higher rates of non-endometrioid cancers compared with other racial and ethnic groups.3 Overall, the combined contribution of age, period, and cohort effects to hysterectomy-corrected uterine cancer incidence was 86.6%, with age effects contributing the most (65.2%), followed by cohort (20.7%) and period (14.2%) effects.
Recent research using SEER 18 data from 2000-2015 highlighted the rising incidence of uterine cancer in the U.S., which is largely driven by increases in non-endometrioid cancers3, and our study using data from SEER 12 from 1992 to 2019 supports this with the overall and histology-specific hexamaps. Further, a recent study evaluating international trends in uterine cancer incidence observed a strong cohort effect in U.S. White women using SEER 9 data from 1978-2012, showing the highest incidence in women born around the 1920s; however, the data did not have a hysterectomy-correction.22 Similarly, in our study using hysterectomy-corrected data, the hexamap for non-Hispanic White women showed the highest incidence in women born between 1913-1923.
The total contribution of age, period, and cohort effects to uterine cancer incidence of 87% that we observed suggests that approximately 13% of the total contribution is due to other factors that do not vary across age, period, or birth cohort. This aligns with research showing approximately 10% of uterine cancers are hereditary, including both familial (family history of uterine cancer) and genetic (Lynch Syndrome) factors.23 When comparing across histologies, we observed that endometrioid cancers had the highest total contribution of age, period, and cohort effects to incidence (86.5%), followed by non-endometrioid (85.3%), and sarcomas (80.2%), suggesting that there may be underlying risk of these aggressive tumors that is constant and not influenced by period or cohort effects. For example, a study by Long et al. observed a greater proportion of cancer predisposition pathogenic variants in Type II compared with Type 1 uterine cancers.24 Lastly, we observed differences in the total contribution of age, period, and cohort effects to incidence across racial and ethnic groups, with non-Hispanic White patients having the highest contribution (86.0%) and non-Hispanic AI/AN having the lowest contribution (78.1%), however, the confidence intervals were wide for the non-Hispanic American Indian/Alaskan Natives due to a smaller sample size.
Similar to most cancers, age is a strong risk factor for uterine cancer, and our study highlights its impact on uterine cancer incidence showing age effects had the largest contribution to incidence compared with cohort and period effects. The median age at uterine cancer diagnosis is 63 years, with approximately 79% of all uterine cancers being diagnosed in women aged 55 years and older.25 Further, we showed that the risk of uterine cancer increases more than 8-fold between the ages of 45 and 75 years. (Supplemental Figure 1a). Not surprisingly, age had a far greater effect on the incidence of uterine cancer than either period or cohort effects.
Historically, endometrioid uterine cancers have been thought to be estrogen-dependent and associated with obesity and other reproductive and hormonal risk factors, while non-endometrioid uterine cancers have been thought to be estrogen-independent and associated with distinct molecular abnormalities.26 However, recent research suggests that obesity may be related to both endometrioid and non-endometrioid uterine cancers.26,27 In the U.S., while obesity rates have increased over time, there are also differences across ages and birth cohorts with the highest obesity prevalence observed in women 60-69 years born in 1950, and the second highest obesity prevalences observed in women 50-59 years born in 1940 and 1960 and women 40-49 years born in 1970.28 Further, obesity rates differ by race and ethnicity with non-Hispanic Black women having the highest obesity prevalence (56.9%) and non-Hispanic Asian women having the lowest obesity prevalence (17.1%) in 2017-2018.29 Thus, obesity may be one modifiable risk factor contributing to the observed age, period, and cohort effects on the uterine cancer incidence rate.
Other hormonal risk factors for uterine cancer have changed over time and vary by race and ethnicity, including parity and use of hormonal contraceptives and postmenopausal hormone replacement therapy (HRT), and may play a role in the contribution of both period and cohort effects to uterine cancer incidence.30,31 In the U.S., the total fertility rate has decreased over time between 2010 and 2021. In 2021, Native Hawaiian or Other Pacific Islander women had the highest fertility rate, and Asian women had the lowest.32 In the U.S., oral contraceptive use peaked in the early 1970’s33 which may have contributed to the lower incidence observed in the hexamaps among the 1933 birth cohort. Overall, the use of hormonal contraceptives has declined in the U.S. between 2000 and 2017, primarily driven by a reduction in oral contraceptive use. While there has been an increase in the use of intrauterine devices, rates are still lower than oral contraceptives. Further, non-Hispanic Whites had the highest rates of hormonal contraceptive use compared to minority populations.34 Postmenopausal HRT, particularly estrogen-only formulations, have been associated with an increased risk of uterine cancer8, and trends in use have changed over time. Our finding in non-Hispanic Whites that women born between 1913-1923 had the highest uterine cancer incidence may, in part, be explained by the push for women to use unopposed estrogen for menopause in the 1960’s before research was published showing an association between unopposed estrogen use and increased risk of uterine cancer in the mid-1970’s.35,36 In the 1990’s, research then showed the risk of uterine cancer could be reduced by lowering the dose of estrogen and combining it with progesterone.36,37 However, following the early stop of the Women’s Health Initiative trial over safety concerns of estrogen-progestin therapy, there was a dramatic reduction in the prevalence and initiation of HRT.38,39 Further, HRT initiation rates vary across racial and ethnic groups with non-Hispanic Whites having the highest rates of HRT initiation both pre- and post-WHI. Further, the decline in HRT initiation post-WHI was greatest in non-Hispanic White patients and lowest in Hispanic patients.38,39
Our study had several strengths. We used data from SEER, which is a longitudinal population-based cancer registry with microscopically confirmed uterine cancer cases. We performed a hysterectomy correction on the incidence rate, which allowed us to evaluate incidence trends accurately over time and across racial and ethnic groups. We used a novel methodology to visually and empirically evaluate the total and individual contributions of age, period, and cohort effects to the uterine cancer incidence rate, which could be used in future age-period-cohort analyses. We examined our results by race and ethnicity and histologic subtype to evaluate the potential differing contributions of risk factors that vary across age, time period, and birth cohort in these different groups.
Our study also had limitations. While we used nationally representative data to obtain hysterectomy prevalence estimates for our hysterectomy correction, our incidence data came from SEER 12 which only accounts for 12.2% of the U.S. population and may not be representative of the total population.25 The hexamaps were difficult to interpret for subgroups with small sample sizes, such as American Indian/Alaskan Natives and sarcomas. Further, the percent contribution estimates for American Indian/Alaskan Natives had wide confidence intervals.
Overall, we observed differences in uterine cancer incidence rates across age, time period, and birth cohort. Further, we observed differences in the individual and combined percent contribution of age, period, and cohort effects to uterine cancer incidence overall, and across racial and ethnic groups and histologic subtypes. These findings add to and expand the limited literature on age-period-cohort analyses for uterine cancer incidence in the U.S., by including data on race and ethnicity and histology, and estimating the contribution of each effect to the incidence rate. The findings of our study can be used in several ways to reduce uterine cancer incidence. Specifically, they can be used to inform model decision-making, such as whether a uterine cancer model should be cohort-specific. Further, these findings can be used in modeling studies of uterine cancer incidence to accurately estimate the effects of interventions or public health measures that target risk factors that may vary across age groups, time periods, or birth cohorts. For example, using these results, researchers could explore key ages to target for a new screening modality or target intervention, and to see how the resulting impact may change over time. These results can help prioritize investment in research and interventions that will have the largest impact on reducing uterine cancer incidence. Further, this work highlights differences in age, period, and cohort effects on uterine cancer incidence across racial and ethnic groups and can inform disparities research. Lastly, our findings on the percent contribution of age, period, and cohort effects can be used for accurate forecasting of uterine cancer incidence.
Supplementary Material
S1a. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by age at diagnosis (SEER 12)
S1b. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by year at diagnosis (SEER 12)
S1c. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by birth cohort (SEER 12)
S2. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by birth cohort (SEER 12)
Figure 3b.
Hexamap for the uterine cancer incidence rate (per 100,000) in Non-Hispanic Black women (SEER 12)
* Abbreviations: NH = Non-Hispanic; C = Cohort; P = Period; A = age
Figure 3c.
Hexamap for the uterine cancer incidence rate (per 100,000) in Non-Hispanic American Indian/Alaska Native women (SEER 12)
*Abbreviations: NH = Non-Hispanic; AI/AN = American Indian/Alaskan Native; C = Cohort; P = Period; A = Age
Figure 3d.
Hexamap for the uterine cancer incidence rate (per 100,000) in Non-Hispanic Asian and Pacific Islander women (SEER 12)
*Abbreviations: NH = Non-Hispanic; API = Asian/Pacific Islander; C = Cohort; P = Period; A = Age
Figure 4b.
Hexamap for the incidence rate (per 100,000) of Non-Endometrioid uterine cancers (SEER 12)
* Abbreviations: NonEm = Non-Endometriod; C = Cohort; P = Period; A = age
Highlights.
Age effects had the largest contribution to uterine cancer incidence, followed by cohort and period effects.
Similar trends were noted across racial and ethnic groups and various histologies.
These findings can inform uterine cancer modeling studies on the effects of interventions.
Acknowledgements
This work was funded by the National Cancer Institute grant 5U01CA265739-02.
This study was supported by NCI 1U01 CA265739 to Jason D. Wright
Footnotes
Conflicts of Interest
Dr. Wright has received royalties from UpToDate and research support from Merck. Dr. Elkin has received research support from Pfizer. The other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and Ethnic Differences in Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic Subtype. JAMA Oncol. 2022;8(6):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. J Clin Oncol. 2019;37(22):1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open. 2021;4(4):e214708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abel MK, Liao CI, Chan C, et al. Racial disparities in high-risk uterine cancer histologic subtypes: A United States Cancer Statistics study. Gynecol Oncol. 2021;161(2):470–476. [DOI] [PubMed] [Google Scholar]
- 6.Yang YSW S; Fu WJ; Land KC The Intrinsic Estimator for Age-Period-Cohort Analysis: What It Is and How to Use It. American Journal of Sociology. 2008; 113(6):1697–1736. [Google Scholar]
- 7.Reither EN, Hauser RM, Yang Y. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the United States. Soc Sci Med. 2009;69(10):1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupper LL, Janis JM, Karmous A, Greenberg BG. Statistical age-period-cohort analysis: a review and critique. J Chronic Dis. 1985;38(10):811–830. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Plus Data, 12 Registries, Nov 2021 Sub (1992-2019) - Linked To County Attributes - Total U.S., 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission. In. [Google Scholar]
- 11.Adam EE, White MC, Saraiya M. US hysterectomy prevalence by age, race and ethnicity from BRFSS and NHIS: implications for analyses of cervical and uterine cancer rates. Cancer Causes Control. 2022;33(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simms KT, Yuill S, Killen J, et al. Historical and projected hysterectomy rates in the USA: Implications for future observed cervical cancer rates and evaluating prevention interventions. Gynecol Oncol. 2020;158(3):710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalal H, Burke DS. Hexamaps for Age-Period-Cohort Data Visualization and Implementation in R. Epidemiology. 2020;31(6):e47–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young AL, van den Boom W, Schroeder RA, et al. Mutual information: Measuring nonlinear dependence in longitudinal epidemiological data. PLoS One. 2023;18(4):e0284904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazekas AK G Optimal binning for a variance based alternative of mutual information in pattern recognition. Neurocomputing. 2023;519:135–147. [Google Scholar]
- 16.Ross BC. Mutual information between discrete and continuous data sets. PLoS One. 2014;9(2):e87357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefani AGH JB; Jardin C; Sticht H Confidence Intervals for the Mutual Information. 2013; https://arxiv.Org/pdf/1301.5942.pdf. [Google Scholar]
- 18.Jamison PM, Noone AM, Ries LA, Lee NC, Edwards BK. Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22(2):233–241. [DOI] [PubMed] [Google Scholar]
- 19.Sherman ME, Carreon JD, Lacey JV Jr., Devesa SS. Impact of hysterectomy on endometrial carcinoma rates in the United States. J Natl Cancer Inst. 2005;97(22):1700–1702. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Devesa SS, Cokkinides V, Ma J, Jemal A. State-level uterine corpus cancer incidence rates corrected for hysterectomy prevalence, 2004 to 2008. Cancer Epidemiol Biomarkers Prev. 2013;22(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temkin SM, Kohn EC, Penberthy L, et al. Hysterectomy-corrected rates of endometrial cancer among women younger than age 50 in the United States. Cancer Causes Control. 2018;29(4-5):427–433. [DOI] [PubMed] [Google Scholar]
- 22.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978-2013. J Natl Cancer Inst. 2018;110(4):354–361. [DOI] [PubMed] [Google Scholar]
- 23.Okuda T, Sekizawa A, Purwosunu Y, et al. Genetics of endometrial cancers. Obstet Gynecol Int. 2010;2010:984013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long B, Lilyquist J, Weaver A, et al. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol Oncol. 2019;152(1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. https://seer.cancer.gov/registries/data.html.
- 26.Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eakin CM, Liao CI, Salani R, Cohen JG, Kapp DS, Chan JK. The association of obesity with type I uterine cancer-is this an oversimplification? Am J Obstet Gynecol. 2022;227(3):538–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy CC, Yang YC. Use of age-period-cohort analysis in cancer epidemiology research. Curr Epidemiol Rep. 2018;5(4):418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hales CMC MD; Fryar CD; Ogden CL Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief; February 2020. 2020. [PubMed] [Google Scholar]
- 30.Yang L, Yuan Y, Zhu R, Zhang X. Time trend of global uterine cancer burden: an age-period-cohort analysis from 1990 to 2019 and predictions in a 25-year period. BMC Womens Health. 2023;23(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobson A, Hockey R, Chan HW, Mishra G. Flexible age-period-cohort modelling illustrated using obesity prevalence data. BMC Med Res Methodol. 2020;20(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osterman MJKH BE; Martin JA; Driscoll AK; Valenzuela CP . Births: Final Data for 2021. Hyattsville, MD: National Center for Health Statistics;2023. [PubMed] [Google Scholar]
- 33.Russell-Briefel R, Ezzati T, Perlman J. Prevalence and trends in oral contraceptive use in premenopausal females ages 12-54 years, United States, 1971-80. Am J Public Health. 1985;75(10):1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King LA, Michels KA, Graubard BI, Trabert B. Trends in oral contraceptive and intrauterine device use among reproductive-aged women in the US from 1999 to 2017. Cancer Causes Control. 2021;32(6):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293(23):1167–1170. [DOI] [PubMed] [Google Scholar]
- 36.Cagnacci A, Venier M. The Controversial History of Hormone Replacement Therapy. Medicina (Kaunas). 2019;55(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodruff JD, Pickar JH. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. The Menopause Study Group. Am J Obstet Gynecol. 1994;170(5 Pt 1):1213–1223. [DOI] [PubMed] [Google Scholar]
- 38.Crawford SL, Crandall CJ, Derby CA, et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women's Health Initiative Study Results. Menopause. 2018;26(6):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weissfeld JL, Liu W, Woods C, et al. Trends in oral and vaginally administered estrogen use among US women 50 years of age or older with commercial health insurance. Menopause. 2018;25(6):611–614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1a. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by age at diagnosis (SEER 12)
S1b. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by year at diagnosis (SEER 12)
S1c. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by birth cohort (SEER 12)
S2. Overall uterine cancer incidence rate (per 100,000), with and without hysterectomy correction, by birth cohort (SEER 12)
Data Availability Statement
The data analyzed in this study are available from the SEER database, which is available from the National Cancer Institute (https://seer.cancer.gov/data-software/).