Abstract
Neurodegenerative diseases are commonly classified as proteinopathies that are defined by the aggregation of a specific protein. Parkinson’s disease (PD) and Dementia with Lewy bodies (DLB) are classified as synucleinopathies since a-synuclein (a-syn)-containing inclusions histopathologically define these diseases. Unbiased biochemical analysis of PD/DLB patient material unexpectedly revealed novel pathological inclusions in the nucleus, comprised of adenosine-to-inosine (A-to-I) edited mRNAs and NONO/SFPQ proteins. These inclusions showed no colocalization with Lewy bodies and accumulated at levels comparable to a-syn. NONO/SFPQ aggregates reduced the expression of the editing inhibitor ADAR3, increasing A-to-I editing mainly within human-specific Alu-repeat regions of axon, synaptic and mitochondrial transcripts. Inosine-containing transcripts aberrantly accumulated in the nucleus, bound tighter to recombinant purified SFPQ in vitro, and potentiated SFPQ aggregation in human dopamine neurons, resulting in a self-propagating pathological state. Our data offers new insight into the inclusion composition and pathophysiology of PD/DLB.
eTOC Blurb
Belur et al identify a novel class of pathological inclusions comprised of RNA binding proteins, NONO/SFPQ, and A-to-I edited RNAs in neuronal nuclei of Parkinson’s disease and Dementia with Lewy bodies. A-to-I edited RNAs promote protein aggregation, inducing neurotoxicity by nuclear sequestration of essential transcripts encoding axon, synaptic and mitochondrial proteins.
Graphical Abstract

Introduction
The accumulation of aggregated proteins and failure of protein homeostasis (proteostasis) is thought to underlie nerve cell death in age-related neurodegenerative disorders 1. In Parkinson’s disease (PD) and Dementia with Lewy Bodies (DLB), proteinaceous inclusions termed Lewy bodies and Lewy neurites are comprised of the pre-synaptic protein a-synuclein (a-syn) that histopathologically define the disease 2,3. Lewy inclusions were initially identified as eosinophilic bodies that strongly react with anti-ubiquitin or neurofilament antibodies 4,5, however the identification of aggregation-promoting SNCA gene mutations in rare familial PD led to the discovery of a-syn as a Lewy body component and the classification of PD and DLB as synucleinopathies 2,6,7. The accumulation of filamentous a-syn and co-occurrence with neurofilament in axon terminals is considered a major pathology that is closely associated with synaptic degeneration and loss of function at nerve terminals 8–10. Early degeneration of synapses and axons is an initial key step in pathophysiology that occurs prior to cell body loss 8,9,11–13, however the mechanisms that trigger degeneration at axon terminals are not understood.
Recent genetic studies of common sporadic forms of PD and DLB have expanded our understanding of the pathophysiology beyond a-syn, establishing dysfunctional cellular degradation pathways as potential disease drivers that promote pathologic inclusions 14–17. Mutations in lysosomal GBA1 gene are the strongest link known to date 18, and other variants have been identified in additional lysosomal components with distinct degradative functions 15–17. A-Syn accumulation alone can directly disable multiple proteostasis pathways, including protein folding, maturation 19,20 and degradation by lysosomes 21–24. Thus, an intrinsic relationship exists between pathological a-syn and genetic pathways in PD and DLB, suggesting that proteostasis failure is a primary underlying cause of disease. Despite this, it is unclear how impairment of a general proteostasis pathway can result in the putative specific pathology that defines these diseases. Disruption of the proteostasis network is expected to cause dramatic and widespread aggregation of metastable proteins, including proteins near their solubility limit or that have high intrinsic aggregation propensity based on their amino acid sequence 25,26. Therefore, we sought to assess the specificity and composition of insoluble inclusions that accumulate in patient neuronal cultures and post-mortem brain.
We identify a novel pathology comprised of insoluble RNA binding proteins, NONO and SFPQ, in the nuclei of patient-derived iPSC midbrain neurons and synucleinopathy patient brains. NONO/SFPQ regulate gene expression as components of paraspeckles, punctate nuclear bodies of ~ 0.3μm in diameter that sequester mRNAs and proteins 27–32. NONO/SFPQ contain low complexity prion-like domains that allow for liquid-phase transitions into functional aggregates33 that preferentially and directly bind to inosine-containing mRNAs29. Adenosine-to-inosine (A-to-I) RNA editing occurs through an enzymatic reaction catalyzed by Adenosine Deaminase Acting on RNA (ADARs) 1 and 2, which bind and modify double stranded (ds) RNAs34. Editing converts a stable A:U base pair into a unstable I:U base pair, causing dsRNA sequences to unwind into RNAs with single-stranded (ss)-like characteristics35. As ADARs require dsRNA substrates34, the presence of inosine directly indicates a double-stranded structure in vivo. Further, A-to-I editing of dsRNA predominantly occurs within primate-specific inverted Alu repetitive elements of introns and 3’ untranslated regions (UTRs)36. Although the biological function is not completely understood, previous work has associated A-to-I editing with reduced gene expression and nuclear retention29,37–39. Under physiological conditions, a few select mRNAs can escape nuclear retention through binding competition between NONO and STAUFEN1, or CARM1 modification of NONO40,41. In addition to sequestering A-to-I edited transcripts, paraspeckles also control A-to-I editing through sequestration of soluble SFPQ, a required transcriptional enhancer of Adenosine Deaminase Acting on RNA-3 (ADAR3; ADARB2) 42,43. ADAR3 is brain specific and has no detectable catalytic activity44. Instead, ADAR3 inhibits editing by binding to dsRNA sequences in competition with the ADARs 1 and 2 44–47. By inhibiting A-to-I editing, overexpression of ADAR3 promotes dsRNA structure, while its depletion can reduce dsRNA structure, consistent with the effect of inosine on destabilizing dsRNA47. RNA editing is essential for human brain development, and axon / synaptic transcripts are heavily targeted by ADARs 48–54. A-to-I RNA editing is altered in blood and brain of PD patients, suggesting it may play a role in pathophysiology55,56. Here, we identify and characterize the downstream functional consequences of nuclear inclusions comprised of NONO/SFPQ and edited mRNA.
Results
Proteomic analysis of aggregated proteins in PD patient iPSn
To gain a comprehensive understanding of protein solubility changes in synucleinopathies, we performed sequential extractions followed by quantitative mass spectrometry 57 on established iPSC-midbrain culture lines (iPSn) derived from PD patients harboring SNCA mutation A53T and matching isogenic controls 7,19,24,58–60. PD iPSn are mature at day (d) 60 post-differentiation, and gradually accumulate insoluble a-syn between d60–90, followed by neurite degeneration after d100 19,61. Proteome aggregation was examined by searching for proteins that shifted from their normal soluble state into the insoluble fraction (Fig. 1A, quadrant 1; Supplemental Data Table 1). Most proteins showed no change in solubility (gray plots, Fig 1A) however a small subset of proteins underwent a significant shift into the insoluble state (white plots in quadrant 1, Fig. 1A). The largest shift occurred in two nuclear proteins involved in gene regulation and paraspeckle formation, NONO and SFPQ (Fig. 1A). Validation studies across multiple culture batches showed that NONO/SFPQ insolubility progressively increased with culture age between d60 and d90, revealing up to a 9-fold elevation (Fig. 1B).
Figure 1. Proteomic analysis of aggregated proteins in PD midbrain cultures.
A) Solubility analysis of day 90 cultures. Orange shade shows proteins with decreased solubility. Gray plots, non-significant; white plots, significant (FDR adjusted p-value <0.05). Proteins altered >2 fold are outside of the dashed box. See Supplemental Data Table 1. B) Quantification of protein levels obtained from SILAC labeled A53T iPSn relative to corrected lines (dashed line). Each plot represents an individual culture well (n=4, *p<0.05, FDR adjusted for multiple comparisons). C) Enrichment analysis of proteins with reduced solubility in d90 PD iPSn (orange shade in panel A) by biological process (left) or reactome (right). Nodes represent functional categories; size=number of genes/category; color=P-value (see Supplemental Data Table 2). D-G) Correlation of aggregated proteins with primary amino acid sequence or secondary structure. See Supplemental Data Table 3.
Gene ontology analysis of insoluble proteins showed most prominent changes in categories of mRNA processing, translation, and axon development (Fig. 1C, Supplemental Data Table 2). Other RNA binding proteins, including FUS and PSPC1, were also elevated in PD iPSn although not as dramatically as NONO/SFPQ (Fig 1A, B). Protein solubility changes showed specificity, since highly stable structural proteins including actin, tubulin, and the ALS-linked RNA-binding protein TDP-43 were not altered (Fig. 1B). Quantification of other essential paraspeckle proteins (RBM14, HNRNPH3, HNRNPK 27), and disease-linked HNRNPA162 showed a mild elevation at d60 (Fig. S1A). However, none of these proteins progressively accumulated with culture age as was observed with NONO/SFPQ.
To understand the basis for solubility changes in PD iPSn, we examined physicochemical properties of proteins (white plots in Fig. 1A; Supplemental Data Table 3). We found significant negative correlations between protein solubility and intrinsic disorder 63, metastable coiled-coil domains that provide structural flexibility 64, and low complexity prion-like domains 65 (Fig 1 D, E; Fig. S1B). SFPQ was previously noted to contain highly extended coiled-coil domains that are essential for its self-assembly 43. Significant positive correlations were noted with aliphatic index (a measure of thermostability 66) and alpha-helical content (Fig. 1F, G). Comprehensive analysis examined correlations with supersaturation values (a metric that incorporates both expression levels and intrinsic aggregation propensity 26), protein size, charge, isoelectric point, and beta-sheet propensity, but no significant relationships were found (Fig. S1 C-H). These data indicate that protein solubility changes in PD iPSn can be partly explained by basic physicochemical properties, where proteins with flexible, metastable domains are sensitive to aggregation.
We next tested if NONO/SFPQ aggregation occurs upon general cellular stress associated with PD, including lysosomal dysfunction 22,24 and oxidant stress 67,68. Healthy control iPSn (line 2135 from ref24) were treated with lysosomal inhibitors for 1 month, followed by quantitative MS analysis. This showed an accumulation of lysosomal LAMP1 as expected, but no change in NONO, SFPQ, FUS, or PSPC1 were observed (Fig. S1I). We next stressed iPSn with excess levels of L-DOPA to induce oxidation 69,70. L-DOPA significantly enhanced oxidation as expected 69, but had no effect on NONO or SFPQ solubility (Fig. S1J). These data suggest that solubility changes are specific to synucleinopathy patient neurons rather than generalized cellular stress.
Nuclear inclusions of NONO/SFPQ in patient iPSn and synucleinopathy patient brain.
We validated the proteomic results by western blot and immunofluorescence staining. Western blot showed an elevation of insoluble NONO, SFPQ, FUS, and PSPC1 at d60 and d90 cultures that increased over time, and a reduction in the soluble fraction (Fig. 2A, Fig. S1K, L). NONO or SFPQ mRNA levels were not different, indicating that the accumulation occurred post-transcriptionally (Fig. S1M). In comparison, the levels of insoluble a-syn were ~2 fold higher in PD iPSn (Fig. S1N). The location of NONO/SFPQ aggregates was examined by immunofluorescence staining of fixed PD cultures and nuclear / cytoplasmic fractionation. NONO/SFPQ were found in an evenly distributed, diffuse pattern in the nuclei of isogenic control neurons (Fig. 2B). In PD patient iPSn, confocal analysis showed increased NONO/SFPQ puncta in the nucleus that occurred as either large inclusions (Fig. 2B, A53T, focal plane-1), or small puncta (Fig. 2B, A53T, focal plane-2). No changes in tyrosine hydroxylase staining (white) occurred between controls and PD iPSn (Fig. 2B). Biochemical fractionation / western blot confirmed nuclear accumulation of NONO and SFPQ (Fig. 2C). Finally, analysis of a distinct synucleinopathy patient line that harbors a triplication of the wild-type (wt) SNCA locus (line 3x-1)19 revealed elevated insoluble NONO, SFPQ, FUS, and PSPC1 at day 60 compared to a healthy control (line 2135) and isogenic corrected 3x-1 (Corr), even in the absence of detectable insoluble a-syn at this time point (Fig S2A). Insolubility of these proteins persisted to day 90 in this line (Fig. S2A, right).
Figure 2. Nuclear aggregation of NONO/SFPQ in PD iPSn and synucleinopathy patient brain.
A) Sequential extraction / western blot of NONO/SFPQ in A53T patient iPSn and isogenic controls (Corr) (n=4 culture wells). CBB (Coomassie brilliant blue), GAPDH, and βiii-Tubulin are loading controls. B) Confocal microscopy iPSn (d90) of NONO/SFPQ, showing 2 focal planes of the same cell (A53T-1, −2). TH, tyrosine hydroxylase. Scale bar=10μm. Quantification represents puncta (separate or colocalized) (n=4 culture wells). C) Subcellular fractionation / western blot of d90 iPSn (n=3 culture wells). D) Sequential extraction / western blot analysis of human frontal cortex from healthy controls, Dementia with Lewy body (DLB), Alzheimer’s disease (AD) or progressive supranuclear palsy (PSP) patients (n=4 controls, n=8 DLB, AD, or PSP). See Supplemental Data Table 4 and Supplemental Figure S2B. Plots represent individual brain samples. E) Immunohistological analysis of frontal cortex. Secondary (2ary) antibody alone is a negative control. Nuclei were stained with DAPI. Scale bar=10μm. Plots represent average number of large inclusions per brain from 4 individual brains. All values are mean ± SEM, *p < 0.05; **p < 0.01; ****p < 0.0001, using student’s unpaired t-test (A, B, C, E), ANOVA with Dennett’s post-hoc test (D).
We next analyzed synucleinopathy patient brain. Biochemical analysis of cortical samples from pathologically confirmed regions of sporadic DLB and age / gender matched controls showed that insoluble NONO/SFPQ was dramatically elevated, with an average increase of 30-fold, with the most severe example showing a 100-fold increase in SFPQ insolubility (Fig. 2D; Fig S2B; Supplemental Data Table 4). Analysis of two distinct diseases involving either intermediate levels of a-syn pathology (AD with minor a-syn pathology) or the tauopathy, progressive supranuclear palsy (PSP), showed no statistical difference in insoluble NONO/SFPQ, although the average of NONO trended slightly higher in AD and PSP samples (Fig. 2D; Fig. S2B). We validated these data using a separate cohort of post-mortem tissue obtained from a distinct brain bank. Increased insoluble NONO and/or SFPQ was detected in 100% of the DLB samples (Fig. S2C, D). In comparison, ~70% of DLB samples showed an increase in insoluble a-syn (Fig. 2D, Fig. S2C, D), using the sensitive LB509 antibody (generated against purified Lewy bodies 71). Analysis of insoluble FUS showed a 4-fold elevation in DLB brain, while only a subtle change was found with TDP-43 (~10 % increase) (Fig. S2C, D).
Confocal imaging of fixed DLB brain revealed nuclear inclusions of NONO/SFPQ that occurred in a punctated pattern (Fig. 2E). Partial colocalization of NONO and SFPQ puncta was observed (Fig. 2E, arrows), although large inclusions >2um in diameter comprised of individual NONO or SFPQ proteins also commonly occurred (Fig. 2E, arrowhead). The large size of these inclusions compared to physiological paraspeckles (~0.3μm), in addition to separate NONO or SFPQ puncta, suggests that the inclusions are not paraspeckles. Quantification of inclusion diameter showed that the nuclei of DLB brains contained a higher number of large inclusions (>2um diameter) and increased total puncta per cell with a stronger intensity (Fig. 2E, S2E). These data validate the accumulation of insoluble NONO/SFPQ in synucleinopathy patient brain, supporting the relevance of the pathway in disease.
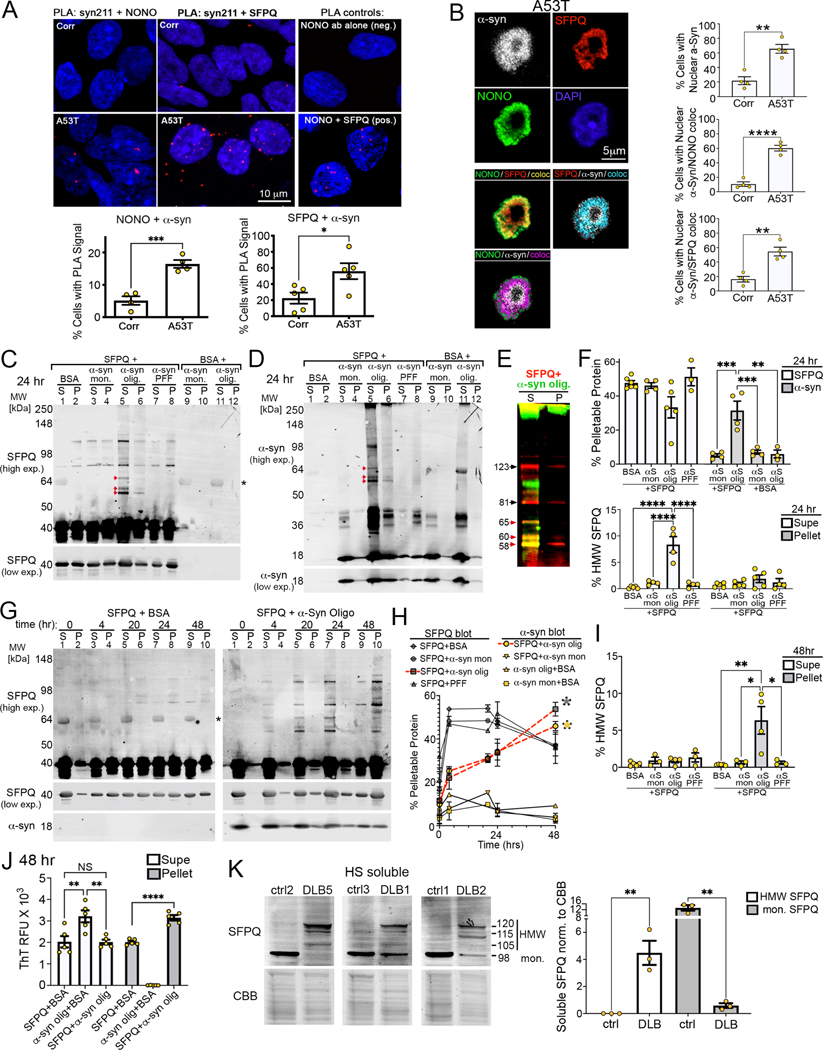
Interaction and co-aggregation of a-Syn and SFPQ in vitro and in vivo.
To gain insight into the mechanism of NONO/SFPQ aggregation, we tested if a-syn associates with NONO or SFPQ in the nucleus. This hypothesis is based on data showing a-syn is present in the nucleus in model systems 72, in synucleinopathy patient brain 73,74, and previous interactome studies show that a-syn interacts with NONO and SFPQ 75. We confirmed the interaction of a-syn with NONO/SFPQ by proximity ligation assays (PLA) (Fig. 3A), and confocal microscopy showed that ~50% of cells contained nuclear a-syn colocalized with either NONO or SFPQ (Fig. 3B, Fig. S3A). Nuclear accumulation was confirmed with three independent a-syn antibodies that react with both N and C-terminus including syn211 (residues 120–125), C20 (polyclonal c-term), and syn505 (residues 2–4) (Fig. 3B, Fig. S3B-D). Syn505 preferentially detects pathological, soluble cross-linked oligomers 76 and was generated against oxidized/nitrated a-syn epitopes 9. a-Syn and NONO/SFPQ colocalization was observed as a discrete diffuse pattern, as shown by 1um confocal slices taken throughout the nucleus of PD iPSn (Fig. 3B, Fig. S3A). In focal planes beyond the nucleus, a-syn was also detected in a punctated pattern within neuronal extensions of both controls and PD iPSn, consistent with its expected synaptic localization (Fig. S3B, D). However, colocalization with NONO/SFPQ was mainly restricted to the nucleus. We also observed colocalization within discrete puncta in ~20% of PD iPSn but not controls (Fig. S3C).
Figure 3. Interaction and co-aggregation of a-syn oligomers with SFPQ.
A) Proximity ligation assay of a-syn with either NONO or SFPQ in PD iPSn (A53T a-syn), quantified on the right (individual culture wells). B) Confocal microscopy of a-syn/NONO/SFPQ in the nucleus of A53T iPSn through a 1um focal plane, colocalization quantified on the right (n=4 culture wells). C, D) Sedimentation / western blot of recombinant SFPQ (40kDa truncated form) and a-syn mixtures (monomers (mon.); oligomers (olig.); or pre-formed fibrils (PFFs) each at 1mg/ml). Aggregation was assessed by probing for SFPQ (C) or a-syn (D) (detected on the same membrane with different secondary antibodies). Asterisk indicates residual signal from BSA. S, supernatant; P, pellet. High exposures (exp.) better reveal high molecular weight (HMW) forms. E) Co-localized SFPQ and a-syn signals from panels C and D. F) Quantification of C and D (HMW SFPQ=58–123kDa). G) Western blot of SFPQ/a-syn co-aggregation rate from 0–48 hrs. Asterisk=residual BSA signal. H) Quantification of pellet fractions from G. I) Quantification of HMW SFPQ from G. J) Thioflavin T (ThT) reactivity of SFPQ/a-syn (RFU, relative fluorescence units). K) Western blot of control and DLB brains (high salt soluble fractions). Quantification of 3 separate controls or DLB brains. All values are mean ± SEM, *p < 0.05; **p < 0.01; ****p < 0.0001, using student’s unpaired t-test for A, B, and K. ANOVA with Tukey’s test was used for F, H, I, and J.
Analysis of post-mortem DLB brain showed that a-syn colocalized with NONO/SFPQ in the nucleus, while controls showed little colocalization (Fig. S3E). Importantly, NONO/SFPQ inclusions were not detected in Lewy bodies or neurites that histopathologically define synucleinopathies (Fig. S3F). These data indicate that non-Lewy body a-syn-species accumulate within the nucleus of DLB brain and colocalize with NONO/SFPQ, suggesting that nuclear forms of a-syn may interact and trigger their aggregation in disease.
Since sequential extractions and PLA data indicated that SFPQ was elevated more dramatically compared to NONO in DLB brain (Fig. 2D; Fig. 3A), we next focused on testing if a-syn and SFPQ could directly interact and influence each other’s aggregation using purified recombinant proteins. A 40kDa truncated form of SFPQ was utilized that includes the metastable coiled-coil motif essential for polymerization, given that it was not possible to purify the full length SFPQ at quantities required for in vitro aggregation studies 43. a-Syn and SFPQ were incubated at 1mg/ml under physiological conditions (pH 7.4, 37°C) with sample agitation, and aggregation was assessed by centrifugal sedimentation / western blot analysis. In control conditions using bovine serum albumin (BSA) to control for molecular crowding, ~50% of SFPQ converted from the soluble supernatant (S) into pellet (P) fraction at 24 hours (hrs) (Fig. 3C, lanes 1, 2; Fig. 3F). Analysis of a-Syn monomers + BSA showed that ~95% of a-syn remained in the supernatant (Fig 3D, lanes 9, 10; Fig. 3F), consistent with previous studies of a-syn aggregation at 1mg/ml 77. Mixing different a-syn species with SFPQ, including a-syn monomers, oligomers (generated by lyophilization 78), or fragmented pre-formed fibrils (PFF) showed that only a-syn oligomers influenced SFPQ aggregation by inducing high molecular weight (HMW) SFPQ (ca. ~58 and 123kDa) in the supernatant fraction (Fig. 3C, lane 5; Fig. 3F). Colocalization analysis on western blots indicated that species at 58, 60, and 65 kDa contained co-migrating SFPQ and a-syn species that were not dissociated by SDS and heat (Fig. 3E, F). Even though previous work showed that a-syn oligomers generated by lyophilization are kinetically trapped in the soluble state and resist conversion into pelletable aggregates 78–81, we found that SFPQ could trigger the conversion of a-syn oligomers into pelletable aggregates, thus demonstrating a reciprocal relationship between SFPQ and a-syn (Fig 3D, lane 6). Note that the HMW smear of a-syn in the soluble fraction confirms that oligomers were generated successfully by the lyophilization protocol (Fig. 3D, lane 5). Kinetic analysis of SFPQ aggregation between 0 and 48 hrs showed that oligomeric a-syn kinetically stabilized soluble HMW SFPQ species up to 24 hr (Fig. 3G, lanes 7, 8; Fig. 3H). Between 24–48 hr, soluble HMW SFPQ oligomers were then converted to pelletable aggregates, indicating they are aggregation competent (Fig. 3G, lane 9, 10; Fig 3H, I). Consistent with a reciprocal effect on aggregation of the two proteins, the sedimentation of a-syn oligomers and SFPQ became synchronized when mixed (red dotted lines), an effect that was not observed when SFPQ was mixed with either a-syn monomers, PFFs, or BSA (Fig. 3H). We next tested if mixing a-syn oligomers and SFPQ induced conformational changes using the amyloid binding dye thioflavin T (ThT). In supernatant fractions at 48hrs, a-syn oligomers reacted slightly more with ThT compared to SFPQ and a-syn/SFPQ mixtures (Fig 3J, open columns). In contrast, pelletable a-syn/SFPQ mixtures showed higher ThT fluorescence compared to either protein alone, indicating increased amyloid content (Fig. 3J, gray columns). These data indicate that a-syn oligomers affect SFPQ aggregation by initial kinetic stabilization of soluble HMW SFPQ species followed by their aggregation into pelleted ThT-positive aggregates at 48 hr.
To determine if HMW forms of SFPQ occur in DLB brain, soluble brain extracts were analyzed by Western blot analysis. While only a single 98kDa SFPQ species was detected in control brains representing the full-length protein, DLB brains showed HMW SFPQ species migrating at 120, 115, and 105kDa (Fig. 3K). These data indicate that HMW forms of SFPQ exist in vivo and are associated with disease, suggesting that SFPQ aggregation in DLB brain may proceed through initial formation of soluble, HMW SFPQ followed by aggregation into large insoluble inclusions.
Depletion of ADAR3 and increased A-to-I editing of RNA in PD iPSn and DLB brain
We next determined the pathophysiological consequences of nuclear NONO/SFPQ inclusions. Since soluble SFPQ is a transcriptional activator of ADAR3 42,43, and NONO/SFPQ complexes bind and sequester A-to-I edited mRNAs within paraspeckles29,37–39,82 we examined the A-to-I editing pathway in more detail. We hypothesized that 1) SFPQ aggregation may cause a loss of its function by sequestering soluble, functional SFPQ, resulting in reduced ADAR3 expression and increased A-to-I editing; and 2) NONO/SFPQ inclusions may aberrantly bind and sequester A-to-I edited transcripts that are essential for neuronal health, resulting in reduced gene expression and neurodegeneration.
To address the first scenario, we determined the expression levels of ADAR3. We first confirmed that soluble SFPQ is essential for ADAR3 expression in healthy control iPSC-neurons by siRNA knock-down of SFPQ. This resulted in dramatic reduction of ADAR3 mRNA (Fig. S4A, B), which is consistent with previous findings in other non-neuronal cell types42,43. Next, we tested the promoter activity of ADAR3 in PD iPSn with a luciferase reporter and found that it was dramatically reduced (Fig. 4A). Endogenous ADAR3 mRNA and protein were also reduced by ~75% (Fig. 4B, C). This effect was specific to ADAR3, since the mRNA level of ADAR1 did not change, and the protein levels of ADARs 1 and 2 were mildly increased in PD iPSn (Fig. S4D). Analysis of DLB brain lysates by western blot showed a ~70% reduction in ADAR3 compared to healthy controls (Fig. 4D; Supplemental Data Table 5). These data suggest that insoluble aggregates of SFPQ confer loss-of-function in PD iPSn and DLB brain.
Figure 4. Reduced ADAR3 expression and increased A-to-I editing in synucleinopathy patient material.
A) ADAR3 promoter activity in isogenic corrected (Corr) or A53T iPSn by Renilla luciferase construct, normalized to SV-40 driven Firefly luciferase. Black and white plots represent two culture sets analyzed 48hrs post-transfection (n=6–10) B) ADAR3 mRNA quantified by Q-RT-PCR (n=3 culture wells). C) Western blot of ADAR3 in iPSn (cultures matching with panel B, d90) (n=3 culture wells). D) Western blot of ADAR3 levels in frontal cortex from controls (ctrl) and DLB. See Supplemental Data Table 5. E) Quantification of CYFIP2 and CADPS protein (SILAC-MS) and mRNA (RT-PCR) at d60. F) Sequencing chromatograms of CYFIP2 transcript (cDNA) and genomic DNA (gDNA) (d60). Red arrow shows A-to-I edit site (n=6 culture wells). G) Quantification of A-to-I editing of CADPS by Sanger sequencing (d60). H) Quantification of A-to-I editing from nuclei of GFP or ADAR3 lentiviral-infected A53T iPSn (d90). I) Correlation of A-to-I edited CYFIP2 with age (years) in ctrl and DLB frontal cortex. Plots represent individual patient samples. J) Quantification of CYFIP2 edits from samples in panel I. K) Quantification of CYFIP2 edits based on age (>65 years) and pathological diagnosis (BLBD- Brainstem Lewy Body Disease, DLB SNCA mt –mutant). See Supplemental Data Tables S4, 6. Values are the mean ± SEM, *p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001, using student’s unpaired t-test (A, C-H, J) or ANOVA/Tukey’s post-hoc test (B, K).
Since ADAR3 is an established regulator of A-to-I editing44–46, we next determined if RNA editing levels were altered in PD patient iPSn. Editing events are detected by comparing genomic DNA with cDNA sequences and appear as adenosine-to-guanosine (A-to-G) mismatches, since inosine is interpreted as guanosine by the sequencing machinery. We started by assessing edit levels in two select transcripts, Cytoplasmic FMR1-interacting protein 2 (CYFIP2) and Calcium-dependent secretion activator 1 (CADPS; CAPS-1), since previous work showed they have a high confidence of A-to-I editing occurrences in vivo 53 (eg. no evidence for A-G variants at the genomic DNA, A-to-I editing levels of ≥50% in cDNA from multiple brain samples, and evidence that editing in this region is conserved in evolution). Furthermore, CYFIP2 and CADPS are neuronally expressed, essential for synaptic/axonal health83–85, and our proteomic analysis showed that both proteins are reduced in PD iPSn while total mRNA levels were unchanged (Fig 4E). Sequencing revealed that the percentage of A-to-I edited CYFIP2 and CADPS transcripts were elevated in PD iPSn (Fig. 4F, G). Furthermore, restoring the expression of ADAR3 by lenti-viral infection of PD iPSn reduced CYFIP2 editing (Fig. 4H). This indicates that A-to-I editing is increased in PD patient iPSn through ADAR3 depletion.
We next measured A-to-I editing in human DLB brain. The percentage of edited CYFIP2 transcripts significantly declined with age in the cortex of healthy individuals, which is a normal physiological response and is consistent with previous findings 86 (Fig. 4I, left). However in DLB cortex, A-to-I editing remained elevated as a function of age, and the trend significantly differed from controls (Fig. 4I, right; Supplemental Data Table 6). Quantification of the combined age groups indicated a significant increase in A-to-I editing in DLB (Fig. 4J), while closer examination of the population of age 65 and older showed a more dramatic elevation in sporadic DLB and 2-fold elevation in familial PD patients that harbor SNCA mutations (Fig. 4K). In many DLB cases, the edited transcript represented ≥50% of the total proportion of expressed transcripts (Fig. S4E). Interestingly, analysis of cortical samples from early-stage sporadic patients with only brainstem Lewy bodies (BLBD) but free of cortical Lewy bodies, showed a 2-fold increase in the percentage of A-to-I edited mRNA (Fig. 4K). This suggests that increased A-to-I editing may occur during the earliest stages of pathology prior to the formation of insoluble, microscopically visible Lewy inclusions. This is consistent with immunostaining data showing colocalization of diffuse, non-Lewy body a-syn with NONO/SFPQ (Fig 3B, Fig. S3), and soluble a-syn oligomers that trigger SFPQ conversion into aggregates (Fig. 2). This suggest that increased A-to-I editing might contribute to early, initial stages of synaptic degeneration in PD 9,12,87.
We expanded our analysis of A-to-I editing events in PD iPSn and determined if global changes occur by RNA-seq. Poly-A mRNA was isolated from PD and isogenic corrected iPSn, then compared to whole genome sequencing data. Using a 300M base-pair read depth for RNA-seq, we identified 8,911 A-to-I edit sites, calculated as percent of total transcripts. Of these, 7090 sites (80%) were elevated in PD iPSn, with 3,137 sites that reached statistical significance (Fig. 5A, Supplemental Table 7). Within all sites that were significantly changed, an average increase of 21% in the conversion of A-to-I was observed in PD iPSn compared to isogenic controls (Fig. 5A, bottom). In contrast, only a small portion of edit sites (207 sites, or 2.3%) were elevated in isogenic controls (Fig. 5A, B). A heat map of the editing changes in 4 individual culture well replicates revealed robust and consistent elevations in all sites identified (Fig. 5B). Examination of the top 50 increased edited transcripts in PD iPSn revealed changes in regulators of synapse function and ion channels (GRIK2, GRIN2D, DPP6, KCNJ14), mitochondrial proteins (TOMM40, LONP1, MTIF3), endoplasmic reticulum (EMC8, LNPK, TMED6), and RNA processing proteins (TIA1, ICE2, LUC7L, UVSSA). By focusing only on significantly changed edited sites, dendrograms showed that editing was most frequently elevated within intronic (1533 sites), 3’UTR (851 sites), and intergenic regions (507 sites) in PD iPSn (Fig. 5C). By contrast, only 12 edit sites were found to be elevated in exonic regions (Fig. 5C; Supplemental Table 7). The majority of increased editing (92% or 2877 sites) occurred in human specific, inverted Alu repetitive elements. A breakdown of the Alu subtypes showed that most changes occur within more recently evolved and active Alu subtypes Y and S (combined 1944 sites), compared with older, less active AluJ sites (Fig. 5D).
Figure 5. RNA-seq of PD iPSn reveals increased A-to-I edit sites that correlate with decreased protein expression in synapse, axon, and mitochondria categories.
A) Quantification of RNA A-I edit sites that are increased in PD (A53T) iPSn (orange) or isogenic controls (Corr, blue) at day 60. Below, average % increase editing within the 3,137 sites elevated in PD iPSn. Plots represent % of total stranscript that are edited at an individual site (n=4 culture wells). B) Heat map of increased edit sites in A53T (M, left) or Corr (C, right). Each column represents an individual culture well; rows represent individual edit sites. Inset, top 50 increased edited hits in A53T iPSn with gene names shown on the left. C, D) Heat map of significantly changed RNA edit sites clustered by transcript location (C) or transcript type (D). The number of sites are indicated above each graph. E) Enrichment analysis of significantly elevated (<10%) edit sites in A53T iPSn by cell component. Nodes represent individual functional categories; size=number of genes; color= P-value. See Supplemental Tables 7, 8. F) Correlation of A-I editing levels with protein levels detected by SILAC-MS. Editing levels are from RNA-seq except for CYFIP2 and CAPDS, which were from Sanger sequencing.
To determine the biological consequences of increased RNA editing, we performed network analysis on transcripts with increased A-to-I editing that are greater than 10% higher in A53T compared to corrected iPSn, and correlated the results with proteomic data. The largest proportion of transcripts with increased editing in PD occurred within the synapse, axon/axon guidance, mitochondria, and nucleus categories (Fig. 5E, Supplemental Table 8). These categories are consistent with known sites / transcripts identified from previous studies of physiological editing in human brain 48,49,53,88. Transcript networks with increased editing showed significant overlap with protein networks that are reduced in PD iPSn, including synapse, axon guidance and nervous system development (Fig. S5A-C; Supplemental Tables 9-12). Prioritizing functional categories of reduced proteins by p-value revealed that axon guidance/nervous system development categories were the most significantly affected proteomic categories (Fig. S5D). Next, we correlated edited transcripts with their corresponding protein levels from SILAC-MS analysis. This revealed an overall negative correlation mostly driven by axon, synapse, and mitochondrial categories, (Fig 5F, yellow and red plots). In this analysis, we included both CYFIP2 and CADPS transcripts found to be edited by Sanger sequencing (Fig. 4E-G). The overall negative correlation of A-to-I editing with protein expression suggests that the biological consequence of dysregulated editing may be to aberrantly reduce expression of the synapse, axon, and mitochondrial proteins that are essential for neuron survival.
Increased A-to-I editing is associated with nuclear retention of mRNA and reduced expression of essential axon and synaptic transcripts.
A-to-I editing may regulate gene expression in multiple ways 34. Previous studies showed that NONO/SFPQ complexes preferentially bind inosine-containing RNA29 and promote nuclear retention29,37–39,82. Since we found that NONO/SFPQ inclusions accumulate in the nucleus of PD iPSn and DLB brain (Figs 1, 2), we hypothesized that increased A-to-I editing results in nuclear sequestration and reduced gene expression. To test this, we first determined the subcellular location of inosine-containing RNA. Isolation of poly-A mRNA from nuclear and cytoplasmic fractions followed by quantification of inosine showed an elevation of edited mRNA PD iPSn nuclei compared to isogenic controls, while almost no edited mRNA was found in the cytoplasm of PD iPSn (Fig. 6A). In comparison, isogenic corrected lines showed ~65% inosine-mRNA in the nucleus and ~35% in the cytoplasm. This indicates that physiologically edited mRNAs in healthy cells that lack NONO/SFPQ nuclear inclusions are exported to the cytoplasm. However under pathological conditions, where increased A-to-I editing and NONO/SFPQ inclusions co-exist, inosine-containing mRNAs accumulate in the nucleus.
Figure 6. Nuclear accumulation of A-to-I edited RNA in synucleinopathy patient samples.
A) Dot blot analysis of inosine-containing poly (A) mRNA isolated from cytosol (Cyt) or nucleus (Nuc) of A53T and isogenic corrected (Corr) iPSn (n=5 culture wells, left graph). Total input mRNA was determined by A260:280nm (right graph). B) Immunofluorescence of inosine puncta and colocalization with NONO. Average puncta values are plotted (n=4 culture wells). C) RNA-FISH analysis of polyA+ RNA. Nuclei are stained with DAPI. Scale bar=10um (n=3 culture wells). D) RNA-FISH / immunostaining of polyA+ mRNA/NONO in frontal cortex of control and DLB. NeuN, neuronal marker. White arrows indicate colocalization of NONO/polyA+ RNA foci. Secondary antibody alone is a negative control. Scale bar=10um. Colocalization analysis of individual nuclei from 3–4 different brains or average/brain (right). E, F) Quantification of nuclear / cytoplasmic mRNA ratios by RT-PCR. G) RNA-FISH of CADPS and CYFIP2. White arrows indicate large mRNA inclusions (1–2um in diameter). Right, Quantification of either individual puncta/nuclei (each color represents an individual culture well) or well average of % of cells containing large puncta. H) RNA-FISH or PRKAR2A (red) and immunofluorescence of SFPQ (green). Quantification of nuclear : cytoplasmic ratio of PRKAR2A is shown to the right (n=3 culture wells). I) Western blot validation of axon / synaptic proteins. GAPDH, actin, and CBB are loading controls (n=3–4 culture wells). Values are the mean ± SEM, *p < 0.05; **p < 0.01 and ****p < 0.0001, student’s unpaired t-test.
We confirmed elevated A-to-I edited RNAs in fixed PD cultures, which showed colocalized inosine and NONO puncta in the nucleus (Fig. 6B). The inosine signal was abolished by treatment with RNAse, indicating that it was derived from RNA (Fig 6B), and anti-inosine antibody specificity was confirmed by dot blot analysis (Fig. S6A). Nuclear mRNA retention in PD cultures was supported by fluorescence in situ hybridization (FISH) using an oligo-dT probe in vitro and in vivo. FISH showed that PD iPSn accumulated mRNA in the nucleus, while most of the mRNA was found in the cytoplasm of isogenic corrected lines (Fig. 6C). In DLB patient brain, mRNA also accumulated in the nucleus of neurons, and colocalized with NONO inclusions (Fig. 6D). We next examined the mRNA localization of specific transcripts of the synaptic / axon / neuron projection categories with no or minimal change in total mRNA but with reduced protein levels in PD iPSn including CYFIP2, CADPS, DNM1, PRKAR2A, RTN4, and CTNNA2 (Fig 4E, Fig S5B, Fig. S6B, C). Fractionation followed by mRNA purification and quantification by RT-PCR revealed that all transcripts examined were elevated in the nucleus compared to isogenic controls (Fig 6E). Analysis of mRNA transcripts that are not known to be extensively edited, including GAPDH and actin, had similar nuclear / cytoplasmic ratios (Fig. 6F). Together, this supports the conclusion that nuclear mRNA sequestration results in reduced expression of synaptic and axonal genes.
We validated the localization changes of select mRNAs by FISH in fixed PD iPSn. Using probes against CYFIP2 and CADPS mRNA, we found unusually large (1–2μm in diameter) mRNA inclusions in the nucleus of PD iPSn and depletion of cytosolic mRNA (Fig. 6G, white arrows). Analysis of PRKAR2A mRNA also confirmed increased nuclear localization in PD iPSn, and co-staining with SFPQ revealed puncta that co-localized with PRKAR2A mRNA in the nucleus (Fig 6H). We validated the proteomic data for each of these targets, showing that each protein was significantly decreased in PD iPSn by western blot analysis of iPSC culture batches that differed from those used for proteomic analysis (Fig. 6I). Control proteins including GAPDH and actin were not statistically different (Fig. 6I). Several of the axon/synaptic proteins were also decreased in DLB brain (Fig. S6D-F). These data suggest that aberrant nuclear mRNA retention of edited mRNAs may be responsible for the decreased protein expression of axon and synaptic proteins prior to neurodegeneration.
Reducing RNA editing restores the expression of axonal and synaptic proteins.
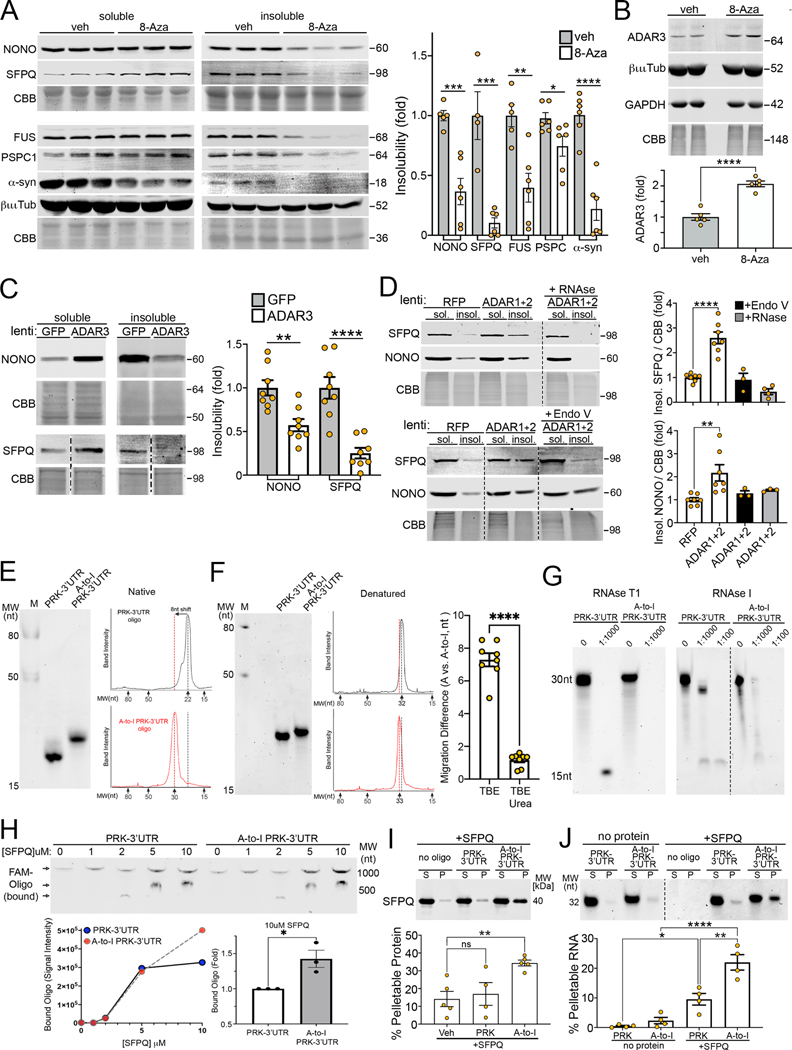
To determine if nuclear mRNA retention and reduced expression occurs through increased A-to-I editing, we reduced inosine-mRNA in PD iPSn by both pharmacological and genetic approaches. We first treated iPSn with an ADAR1 inhibitor, 8-azaadenosine (8-aza). As an analogue of its naturally occurring substrate, 8-aza-containing RNA binds with greater affinity to the ADAR active site, indicating that it can act as a competitive inhibitor89. Free 8-aza can be incorporated into polynucleotides including RNA through its triphosphate90–92, and multiple studies showed that free 8-aza, at concentrations as low as 100 nM, can reduce A-to-I editing and hence increase dsRNA structure when added to cell cultures47,93,94. We first determined if 8-aza could inhibit A-to-I editing without inducing toxicity. Using HEK cells and a previously established A-to-I editing reporter95, we found that editing was inhibited between 100 and 250nM of 8-aza (Fig. S7A), and concentrations above 1uM were toxic. Assessment of an endogenous ADAR target (CYFIP2 mRNA) showed 8-aza reduced editing by 40% in PD iPSn, which is similar to levels found in isogenic corrected lines (Fig. S7B). Next, we assessed the global levels of A-to-I editing by purifying polyA mRNA from the nucleus of vehicle and 8-aza treated PD iPSn. Inosine was detected in these samples by both immuno-dot blot and HPLC-MS. Both detection methods showed a ~50% reduction in inosine while the quantity of other nucleotides within the polyA mRNA were not changed (Fig. 7A; Fig. S7C).
Figure 7. Reducing A-to-I editing reverses nuclear retention of mRNA and restores expression of axon / synaptic proteins.
A) Quantification of inosine-containing mRNA in A53T iPSn by anti-inosine dot blot of poly (A) purified mRNA, +/− 200nM 8-aza-adenosine (8-aza), 30 days. B) Confocal analysis of polyA+RNA FISH in 8-aza treated cultures. Nuclei are stained with DAPI. Scale bar=10um. Quantification shows percent cells with polyA+ foci (n=3 culture wells). C) Western blot showing ADAR3 levels after lentiviral infection, using GFP as a control (n=4 culture wells). D) PolyA+ RNA-FISH of ADAR3 infected cultures (dpi 30) as in B. E) Nuclear/cytoplasmic mRNA ratios of axonal/synaptic transcripts quantified by RT-PCR (n=4–5 culture wells). F) Western blot of axonal/synaptic proteins from 8-aza treated cultures. CBB, GAPDH, and βiii-Tubulin are loading controls. G) Quantification of panel F (n=8–9 culture wells). H) Western blot of axonal/synaptic proteins infected with GFP control or lenti-ADAR3. I) Top, eGFP reporter plasmid containing the PRKAR2A 3’UTR. Editing levels from A53T iPSn by RNA-seq and shown. Bottom, RNA-FISH of GFP transcript (white) in HEK cells co-transfected with the GFP reporter and either vector control or ADAR1 E713Q hyperactive mutant. DAPI (nuclei) is shown in blue (n=3 culture wells). J) Western blot of A53T iPSn or isogenic controls (Corr) expressing the eGFP-3’UTR reporter, +/− 8-aza (3–4 culture wells). K) Immunostaining of 8-aza treated iPSn by PSD-95 (post-synapse) and synaptophysin (pre-synapse). β-III-Tubulin and DAPI are controls. Quantification shows PSD-95/synaptophysin colocalized puncta (avg/well, n=4). See Supplementary Figure S7. Values are the mean ± SEM, *p < 0.05; **p < 0.01, ***p < 0.001, ****p < 0.0001; student’s unpaired t-test (A-I); ANOVA with Tukey’s post-hoc test (J, K).
Having established a concentration of 8-aza that could reduce A-to-I editing in the absence of toxicity, we next analyzed mRNA localization by polyA-FISH. 8-aza reduced nuclear mRNA punctated inclusions in PD iPSn by ~50% compared to vehicle treated cultures (Fig. 7B). We confirmed this effect by overexpressing ADAR3 through lenti-viral transduction (Fig. 7C). ADAR3 also reduced nuclear polyA puncta by 75% (Fig. 7D), confirming that the rescue effect was due to reduced A-to-I editing. Next, we assessed the nuclear : cytosolic mRNA ratios of specific axon/synaptic transcripts after 8-aza treatment and found that they were decreased, reflecting more efficient export to the cytoplasm (Fig. 7E). Quantification of total mRNA showed a slight increase in PRKAR2A and CTNNA2, while no changes were found in any other transcripts, indicating that the main effect of reduced A-to-I editing was to restore nuclear : cytosolic ratios of these particular transcripts (Fig. S7D). Consistent with enhancing nuclear mRNA export, protein expression of CYFIP2, CADPS, PRKAR2A, and RTN4 was restored to control levels by 8-aza (Fig. 7F, G). We confirmed increased expression of these proteins in ADAR3 lenti-transduced PD iPSn (Fig. 7H). The protein levels of CTNNA2 were not improved by either 8-aza or ADAR3, while DNM1 protein was only rescued by ADAR3 (Fig 7 F-H). This could indicate a more effective rescue by ADAR3 compared to 8-aza, or that additional mechanisms are required to fully restore their expression. These data indicate that nuclear retention in PD iPSn is rescued by reducing A-to-I editing.
We next directly tested the role of A-to-I hyper-editing in nuclear retention and protein expression by utilizing a reporter plasmid expressing GFP fused to the editing-enriched region within the 3’UTR of PRKAR2A. This region contains 2 human-specific inverted repeat (IR) Alu regions (AluJr and AluSp) and a cluster of 5 sites that are hyper-edited in PD iPSn (Fig. 7I, Supplemental table 7). Increased editing was induced in HEK cells by expression of a hyperactive form of ADAR1 (E713Q). Expression of the reporter plasmid (GFP-PRK-3’UTR) in ADAR1 hyperactive cells resulted in nuclear retention of GFP mRNA compared to cells expressing endogenous wild-type ADAR1, while total GFP mRNA was not changed (Fig. 7I). Since the double-stranded (ds) RNA IRAlu elements are expressed equally in both vector and ADAR E713Q cells (Fig. 7I bottom graph), the effect on nuclear retention is likely due to hyper-editing as opposed to other structural characteristics of the IRAlus themselves. We confirmed this effect in PD iPSn. Expression of the GFP-PRK-3’UTR construct revealed lower GFP protein expression in PD lines compared to isogenic corrected lines (Fig. 7J). This decline was a direct result of the PRKAR2A 3’UTR, since expression of control GFP plasmids using the same promoter but lacking the 3’UTR were not different between corrected and patient iPSn (Fig. 7J). Treatment with 8-aza improved GFP expression in PD iPSn, indicating that reduced protein expression occurred from A-to-I hyper-editing (Fig. 7J). These data further support the hypothesis that increased A-to-I editing in PD iPSn contributes to nuclear retention and reduced expression of axon/synaptic proteins.
We next determined the effects of reducing A-to-I editing on neuronal health. Since PD iPSn begin to show neurite degeneration after day 100 24,60, cultures were treated with 8-aza between day 90–120 in an attempt to prevent degeneration. At day 120, synapses were quantified by immunofluorescence staining of fixed cultures by colocalization analysis of post-synaptic (PSD-95) and pre-synaptic (synaptophysin) markers (Fig. 7K, Fig. S7E). We found a decline in the number of colocalized puncta in PD iPSn compared to isogenic controls (Fig 7K, Fig. S7E). Both the staining intensity and colocalization of PSD-95 and synaptophysin were reduced in PD iPSn, while b-iii tubulin was not dramatically affected (Fig. 7K, Fig. S7E). 8-aza treatment at 200nM for one month increased the levels of individual synaptic markers and improved their colocalization, suggesting improved synapse formation (Fig. 7K, Fig. S7E). Confirmation by western blot showed that 8-aza rescued synaptophysin levels in PD iPSn (Fig. S7G). Importantly, no change was seen when isogenic corrected lines were treated with 8-aza, suggesting that the treatment is not toxic to control iPSn (Fig. S7F). We used a third assay to assess cell health by measuring the release of lactose dehydrogenase (LDH) from dying neurons. Inhibiting A-to-I editing also reduced LDH release, further supporting that neuronal health was improved (Fig S7G).
Increased RNA editing contributes to pathological aggregation of NONO/SFPQ.
We next determined if A-to-I edited RNAs play a role in nuclear protein aggregation. One month 8-aza treatment reduced insoluble NONO, SFPQ, FUS, PSPC1, and eliminated insoluble a-syn in PD iPSn (Fig. 8A). Control proteins including b-iii-tubulin and GAPDH were not changed (Fig. 8A, B). The increase of soluble SFPQ was sufficient to restore the expression of ADAR3 (Fig. 8B), suggesting that the physiological function of SFPQ was restored. We then directly overexpressed ADAR3 in PD iPSn, which also reduced insoluble NONO and SFPQ by 50% and 75% respectively and is similar to physiological levels (Fig. 8C). We further examined the mechanistic relationship between increased editing and NONO/SFPQ aggregation by overexpressing hyperactive forms of ADAR1 and 2 96 in healthy control iPSn using lentivirus, which resulted in ~2 fold overexpression (Fig. S8A). At 14 days post infection (dpi), insoluble SFPQ and NONO were elevated by hyperactive ADARs compared to RFP infected controls (Fig. 8D). Removal of edited RNA from the lysate with either RNAse, or Endonuclease V that selective degrades inosine-containing nucleic acid, reduced insoluble NONO/SFPQ aggregates (Fig. 8D). Collectively, these data indicate that increased RNA editing promotes insoluble NONO/SFPQ aggregates in midbrain cultures. This suggests that once increased editing is triggered, inosine-RNAs can further exacerbate or stabilize insoluble NONO/SFPQ aggregates, participating in a self-propagating pathological cycle.
Figure 8. A-to-I edited RNAs trigger pathological protein aggregation.
A) Sequential extraction / western blot of A53T iPSn treated with 8-aza (200nM, 30 days) (n=4–6 culture wells). B) Western blot of ADAR3 levels in 8-aza treated A53T iPSn cultures (n=5 culture wells). C) Sequential extraction / western blot of A53T iPSn infected with lenti-GFP or ADAR3 (MOI 3, dpi 14). D) Sequential extraction / western blot of control iPSn expressing hyperactive ADAR1 and ADAR2. Lysates were treated with either RNAse A or Endo V to degrade total or inosine-containing RNA, respectively. E) Native PAGE of RNA oligonucleotides (oligos) including edit site 2 of PRKAR2A 3’UTR, detected by SYBR green II. Right, oligo migration according to ssRNA molecular weight (MW) standards, in nucleotides (nt). M, Marker. F) Denaturing PAGE of RNA oligos as in E. Bar graph shows the migration difference between non-edited and edited oligos (each plot represents an individual gel lane replicate from 3 different setups). G) Denaturing PAGE of RNA oligos, digested with RNAse T1 or RNAse I. H) 100nM FAM-labeled oligos were mixed with SFPQ and analyzed by native PAGE. Quantification of the bound oligo is shown below. Arrows indicate RNA oligo bound to SFPQ at 3 possible structural states. Quantification represents 3 different setups. I) Sedimentation / western blot of 10uM SFPQ +/− RNA oligos at 1uM. Quantification represents 4 different setups. S, supernatant; P, pellet. J) Sedimentation / denaturing PAGE of RNA oligos from the same samples in panel I, +/− SFPQ, and detected by SYBR green II (n=4 different setups). Values are the mean, +/− SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.001. Student’s unpaired t-test (A- C, F, H); ANOVA with Tukey’s post-hoc test (D, I, J). Dotted lines in C, D, and G indicate cropped out lanes but are from same blot.
The mechanism of how edited RNA influences protein aggregation was examined in further detail in vitro. In silico structural analysis of the 1kb edited-enriched region of the PRKAR2A 3’ UTR using RNAfold revealed the formation of two double-stranded RNA structures formed from AluJr and AluSp. Given that this region is highly edited in iPSn, and ADAR1 can only modify dsRNA substrates, these dsRNA structures likely occur in vivo. We found that A-to-I conversion at site 2 that normally participates in A:U base pairing (Fig. S8B) disrupted the stem structure of AluJr, converting the stem structure to an open single-stranded loop (Fig. S8B, right). Conversion to inosine at the other 4 sites showed no structural changes by RNAfold, possibly because these edits are mostly within bulges and do not participate in base pairing of the dsRNA. We generated a 30 bp RNA oligonucleotide that contains site 2 to model the full length 3’UTR of PRKAR2A. Similar to the full-length 3’UTR, this adenosine-containing oligo is predicted to form a structured hairpin, while replacing A with I induces structural disorder (Fig. S8C). The oligo also contains a potential SFPQ binding site (AUCCUG)97. Structural analysis of the RNA oligo by native gel electrophoresis showed that the adenosine-RNA oligo migrated faster than predicted, at 22 nucleotides (nt), suggesting the formation of secondary structure (Fig. 8E). The inosine-RNA oligo migrated at the expected size of 30 nt (Fig. 8E). Denaturing the oligos with urea shifted the weight of the adenosine-RNA oligo to the expected size of ~30bp, while denaturation had essentially no effect on the inosine-RNA oligo (Fig. 8F). If the adenosine oligo is double-stranded, it should exhibit increased resistance to RNAse treatment, while ssRNA will be completely degraded. We found that treatment with either RNAse-T1 or RNAse-I completely digested the inosine-RNA oligo indicating it has the characteristics of a ssRNA in solution (Fig. 8G). The adenosine-RNA oligo was partially resistant, suggesting that it contains double-stranded structure (Fig. 8G). These data indicate that converting A-to-I triggers structural changes in the AluJr region of the PRKAR2A 3’UTR mRNA.
To directly test the effect of A-to-I edited RNA oligos on SFPQ, they were incubated with recombinant SFPQ followed by measurement of binding and aggregation. Binding was assessed by gel shift analysis, holding a FAM-labeled oligo at 100nM and varying the concentration of SFPQ. We found that both oligos bound to SFPQ equally at protein concentrations of 1, 2, and 5uM. However, when SFPQ was increased to 10uM, inosine-RNA oligos bound ~40% more compared to adenosine-RNA oligos (Fig. 8H). Furthermore, inosine-RNA oligos directly potentiated SFPQ aggregation in vitro, demonstrated by sedimentation / denaturing western blot analysis (Fig. 8I). RNA analysis by denaturing PAGE of the same samples showed that while the RNA oligos alone were in the supernatant fraction, the inosine-RNA oligo accumulated in the pellet fraction when mixed with SFPQ, indicating co-aggregation with SFPQ (Fig. 8J). We confirmed that inosine-RNA oligos potentiate SFPQ aggregation by a distinct native western blot assay, showing increased HMW SFPQ aggregates (Fig. S8D). Together, these data indicate that A-to-I editing promotes binding and potentiates aggregation of SFPQ directly in vitro, and is consistent with culture experiments (Fig. 8D). Increased binding of inosine-containing ssRNA to SFPQ provides a mechanistic explanation for the nuclear retention and reduced gene expression observed under conditions of elevated editing and NONO/SFPQ nuclear inclusions (Fig. 6).
Discussion
We examined how proteome composition changes in synucleinopathies by focusing on protein solubility shifts which uncovered aberrant aggregation of nuclear NONO, SFPQ, and editied mRNA. Analysis of human idiopathic DLB brain showed NONO and SFPQ aggregates in all samples analyzed and, in some cases, more abundant than insoluble a-syn, indicating its importance for sporadic synucleinopathies. Studies have identified other aggregation-prone proteins in disease beyond a-syn, amyloid-beta, tau, and TDP-43 that are commonly used to define various neurodegenerative diseases, including TMEM106B 98–101. The toxic function of Lewy inclusions has also been unclear for several decades. We show a clear mechanistic connection between NONO/SFPQ inclusions and toxicity through nuclear retention of A-to-I edited transcripts that are vital for neuron survival. Rescue experiments showed that reducing A-to-I editing alone, through either 8-aza or ADAR3, is sufficient to rescue synaptic loss and dissolve pathological aggregates in patient-derived cultures. Similarly, expression of ADAR1/2 hyperactive mutants in healthy control iPSn induced NONO/SFPQ aggregation, indicating that increased A-to-I editing contributes to inclusion formation in PD iPSn. These studies indicate that in addition to reducing the expression of vital neuronal proteins, A-to-I edited transcripts actively play a role in potentiating protein aggregation and disrupting nuclear function (Fig. S8E). Neurodegenerative diseases are classified as proteinopathies based on the aggregation of a single protein, while less attention is given to non-proteinaceous pathologies and their potential role in disease. Our studies support the notion that not one, but multiple proteins, accumulate in neurodegenerative diseases. Furthermore, non-protein containing pathologies including mRNA inclusions play an important role in pathogenesis.
Mechanistic studies showed that soluble a-syn oligomers directly influence the aggregation of purified recombinant SFPQ while insoluble a-syn fibrils (PFFs) have no effect. Consistent with immunostaining in iPSn or DLB post-mortem brain, a-syn/NONO/SFPQ was observed as nuclear puncta, but was completely devoid in cytoplasmic Lewy inclusions that are comprised mainly of fibrillar a-syn (Fig S3F). Since non-Lewy body forms of a-syn are associated with the nuclear abnormalities we describe, this suggests that neuronal dysfunction in PD/DLB brain extends beyond regions with overt a-syn pathology. Future studies that comprehensively examine the distribution and abundance of nuclear NONO/SFPQ/edited mRNA inclusions that we describe here may aid in the accuracy of diagnosis and pathological characterization of PD/DLB.
Using a region of the PRKAR2A 3’UTR as an example, our data suggest that increased editing induces structural disorder and promotes RNA that behaves more like ssRNA, which then lead to higher SFPQ binding affinity. Normally, SFPQ can bind both ds and ssRNA33,97, however it is possible that structural changes induced by A-to-I editing expose more high-affinity SFPQ binding sites in ssRNA. Nuclear retention is known to occur through hyper-editing of IRAlus, although it is possible that editing in non-Alu regions at even one site is sufficient to promote an RNA structure with more exposed SFPQ binding binding sites. This is supported by the analysis of the 3’UTR of PRKAR2A which, although is hyper-edited, only requires one A-to-I change to induce a ssRNA loop (Fig. S8B). It has been long known that A-to-I editing alters dsRNA into an RNA with more single-stranded characteristics, including sensitivity to single-stranded RNAse102 and altered migration on native PAGE gels35. Previous work also showed that A-to-I editing can promote the interaction with RNA binding proteins through destabilizing dsRNA into an RNA that is more single-stranded in nature103. These studies are consistent with the changes we observe in PRKAR2A mRNA, suggesting that the increased interaction with SFPQ occurs from ds to ssRNA conversion. Once bound, the edited RNA may serve as a scaffold that promotes the seeding and / or stabilization of insoluble SFPQ polymers (Fig. S8E). Upon surpassing a threshold, pathological inclusions form and aberrantly anchor essential neuronal transcripts with SFPQ binding sites in the nucleus and prevent their translation in the cytoplasm. Therapies that reduce editing within these essential transcripts may simultaneously restore protein expression and dissolve pathological aggregation, in turn restoring neuronal function.
Although increased editing can potentiate RNA/protein aggregation and nuclear retention under pathological conditions, physiological RNA editing diversifies the genome by altering protein coding regions, changing mRNA splicing patterns, and regulates gene expression through the editing of Alu repeats in 3’UTRs 104. Further, RNA editing has been proposed to play a role in the complex development and evolution of the human brain 88. Our studies identify a novel pathogenic feature of edited non-coding RNAs, and provide a mechanistic foundation to further examine the pathogenicity of RNAs that are extensively edited beyond the normal, physiological levels. Perturbations in this pathway and hyper-editing of human-specific Alu regions of RNA may also explain why certain pathological features of PD and DLB are unique to humans and do not occur in rodent synucleinopathy models 105. Our attempts to validate findings in immortalized cell lines and transgenic mice expressing human A53T a-syn showed no changes in NONO/SFPQ solubility (Fig. S9) and RNA editing (not shown). This may be due to the rapid cell division of cell lines, or human-specific features of the brain including the expression of IRAlus and NEAT1_2 expression that is required for paraspeckle formation. The absence of these RNAs in rodent model systems may prevent certain features of neurodegeneration including the aggregation of RNA binding proteins that associate with NEAT1_2 such as NONO and SFPQ 106. Aggregation of RNA binding proteins occurs in multiple neurodegenerative conditions including ALS and FTD 107. It will be of interest in future work to assess how A-to-I editing influences the binding and aggregation of proteins involved in these diseases including TDP-43, FUS, and HNRNPA1.
Limitations of the study
Our study utilized 8-aza to inhibit A-to-I editing, given previous work that established it as an ADAR inhibitor89,93,94. However, another cell culture study showed that the editing status of 3 transcripts (BPNT1, MRPS16, and ZDHHC20) were not affected by 8-aza108. ADARs require dsRNA to bind substrates and 8-aza was provided as a free compound in our studies and in others. However, we observed a decline in A-to-I editing using four independent assays (Fig. 7A, Fig. S7A-C). It is possible that either free 8-aza, or its incorporation into dsRNA within the cell, binds the active site of ADARs and inhibits A-to-I editing of endogenous targets through competitive binding. Indeed, previous work showed that incorporation of 8-aza into polynucleotides occurs in cells90–92. We also used 8-aza at sub-toxic levels (between 0.05 and 1uM), while others have used it at higher levels (between 1 and 10uM) with the purpose of inducing toxicity in cancerous cell lines. It is also likely that not all transcripts are equally affected by ADAR1 or 2 inhibition, due to differences in the turnover rate of individual mRNAs, the amount of time a transcript spends in the nucleus, the duration of 8-aza treatment, and the metabolic rate of individual cell types. If an mRNA has a long half-life, longer treatment times would be required to observe changes in editing. We also observed that 8-aza increased ADAR3 levels (Fig. 8B), which could contribute to reduced A-to-I editing in our neuronal models. Finally, we confirmed our 8-aza rescue studies with lenti-ADAR3 overexpression, which resulted in similar findings compared with 8-aza (Fig. 7D, H; Fig. 8C). Our attempts at simultaneously knocking down ADARs 1 and 2 resulted in neurotoxicity (not shown), which could be due to the anti-apoptotic functions of ADARs that are independent of deaminase activity109.
Although previous studies showed that A-to-I edited mRNAs are bound and retained by NONO/SFPQ complexes29,38,39, other studies concluded that physiological editing has no effect on nuclear retention, and dsRNA structure is instead responsible40,41. Therefore, it is possible that some of the effects on nuclear retention we observe occur independently of A-to-I editing. However, our data indicates that destabilizing dsRNA by introducing unstable I:U base pairs promotes SFPQ binding and aggregation directly in vitro, and that only inosine-containing ssRNA co-sediments with insoluble SFPQ aggregates (Fig. 8 E-J; Fig. S8D). Therefore, our data supports the idea the pathological nuclear retention occurs from not promoting, but destabilizing dsRNA into a structure that has a higher affinity for SFPQ. However under physiological conditions, dsRNA structure may be important for nuclear retention independent of A-to-I editing. Increased protein-RNA interactions can be elevated by editing, disrupting dsRNA, and exposing protein binding sites 103. Our data indicates that when pathological nuclear aggregates are observed, aberrant increases in A-to-I editing beyond what is observed in physiological conditions, plays an important role in both nuclear retention and protein aggregation. Presumably, if structural characteristics of IRAlu sequences alone are responsible for the pathogenic phenotypes observed here, we would not have observed a rescue by 8-aza or ADAR3 overexpression, given that the same IRAlu sequences are present and expressed in both isogenic corrected and PD iPSn.
STAR METHODS
RESOURCE AVAILABILITY
- Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Joseph R Mazzulli (jmazzulli@northwestern.edu).
-Materials Availability
All unique/stable reagents and cell lines generated in this study are available from the Lead Contact, Joseph R Mazzulli (jmazzulli@northwestern.edu) with a completed Materials Transfer Agreement.
-Data and Code Availability
- Data availability: All data reported in this paper will be shared by the lead contact upon request.
- Full length western blots are available at Mendeley (Mazzulli, Joseph (2024), “Full Western blots to accompany manuscript “Nuclear aggregates of NONO/SFPQ and A-to-I edited RNA in synucleinopathies””, Mendeley Data, V1, doi: 10.17632/pp9vnn666d.1)
- RAW files of day 60 and day 90 proteomics data. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD051673
- RAW files of RNA seq and whole genome sequencing data of A53T and isogenic corrected line are available at the Sequence Read Archive (SRA): ID: SUB14391987, BioProject ID: PRJNA1104694
Code: This paper reports original code used to identify A-to-I RNA edit sites, available at Gitbhub, https://github.com/bibb/Ato-I_pipeline_Neuron2024/tree/main, DOI: 10.5281/zenodo.11061692.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
iPSC culture and differentiation
iPSCs (induced pluripotent stem cells) were cultured on matrigel coated plates and maintained in mTESR1 media. The A53T a-syn mutation harboring iPSc and its isogenic line were kindly provided by Dr. Rudolf Jaenisch (Whitehead Institute of MIT), and were previously described and extensively characterized60. iPSC harboring SNCA triplication (lines 3x-1 and 3x-1 isogenic corrected (Corr)) was used in validation studies and has also been previously characterized19. An additional line from a healthy control subject was also used (called “Est. Ctrl” or “line 2135” from refs19,24). For differentiation into midbrain dopaminergic neurons, iPSc lines were accutased (Corning, # 25058CI) and seeded onto Matrigel (Corning, #354277) coated plates. The rest of the differentiation protocol was performed as previously described60. Cultures were exposed to differentiation media for 40 days, then switched on to neurobasal medium (ThermoFisher, # 21103049) with NeuroCult™ SM1 Neuronal Supplement (Stem cell technologies, #5711) and 1% glutamine and penicillin / streptomycin beyond 40 days until the cultures were harvest for assays. At day 60, the cultures are considered to be fully mature, non-dividing iPSC-neurons. The cultures were harvested at day 60 and day 90 for assays comparing isogenic corrected lines vs. SNCA mutant lines. For rescue experiments, the SNCA mutant cultures were treated as indicated below at day 90, then harvested at day 120. The specific culture age and harvest times are indicated below in each subsection.
Human Brain samples
De-identified postmortem frozen tissues from the frontal cortex were obtained through the Northwestern Center for Cognitive Neurology and Alzheimer’s Disease Center (CNADC) (P30 grant P30AG013854) and The Brain Bank for Neurodegenerative Disorders at Mayo Clinic, directed by Dr Dennis W. Dickson and curated by Dr Michael DeTure with support from Mayo Clinic, Rainwater Charitable Foundation, Mangurian Foundation, State of Florida Alzheimer’s Disease Initiative, and NIH grants P30 AG062677 and P01 AG003949. All samples were pathologically confirmed as synucleinopathies (DLB), AD, or PSP. Sample details are listed in Supplemental Data Table 4. We found no influence or association of sex/gender on any of the results.
METHOD DETAILS
I. iPSC-neuron culture treatment for quantitative proteomics
SILAC proteomics
The iPSC-derived neurons were labeled with light or heavy isotope containing media from day 40 for 3 – 7 weeks till the time of harvest (day 60–90). The neurons were cultured in regular neurobasal medium (light media) or custom formulation of neurobasal medium lacking L-lysine and L-arginine along with SM1-supplement. The custom neurobasal medium (heavy media) was supplemented with heavy-isotopes containing amino acids L-lysine (146mg/L) and L-arginine (84mg/L) (Cambridge isotope laboratories, # CNLM-291-H-0.5, # CNLM-539-H-0.5). The neurons were harvested and sequentially extracted into soluble and insoluble fractions as described below at day 60 and day 90. The light and heavy labeled samples from soluble and insoluble fractions were mixed at equal total protein amounts and run on an SDS-PAGE gel. The gel was stained with SimplyBlue™ SafeStain (ThermoFisher, # LC6065) and cut-out for mass-spectrometry analysis. The differential protein expression between heavy and light labeled samples were analyzed and expressed as log fold change of heavy over light for both soluble and insoluble fractions.
For Leupeptin and L-DOPA experiment, established healthy neuronal cultures were treated every other day for 30 days. The cultures were treated with vehicle (water and 0.2N Hcl in PBS) containing light isotope media or 100uM leupeptin and 50uM L-dopa heavy isotope containing media respectively. Sequential extraction was performed as described below into soluble and insoluble fractions for mass-spectrometric analysis.
II. BIOCHEMISTRY AND MOLECULAR BIOLOGY
Sequential extraction
IPSC-midbrain cultures were rinsed and harvested in cold PBS on ice. The cells were pelleted at 200xg for 5min at 4°C. The age of cultures is indicated in the figure legends or text, but was done at day 60 and day 90 for most studies. The supernatant was discarded and the pellet was used for sequential extraction of proteins. The protocol for sequential extraction was performed as previously described110. Briefly, the cell pellet was homogenized in 1% triton buffer supplemented with protease inhibitor cocktail (PIC) (Roche diagnostics, # 11–836-170–001), 1 mM PMSF, 50 mM NaF, 2 mM sodium orthovanadate. The lysate was pelleted in an ultracentrifuge at 100,000xg, 4 °C for 30 min, re-extracted in triton to wash the pellet, followed by ultracentrifugation as before. The resulting supernatant is triton-soluble (soluble) fraction. The triton-insoluble pellet is further extracted in 2% SDS lysis buffer with PIC by boiling for 10min, sonicating and pelleting the lysate at 100,000xg, 22°C for 30 min. The resulting supernatant is the triton-insoluble (insoluble) fraction. The protein concentration was determined by using micro-BCA kit (ThermoFisher, # 23235) and lysates were subjected to western blot analysis as described below.
For human brain tissue, a series of sequential extraction with intermediate washes was performed to obtain soluble and insoluble fractions as previously described19. Briefly, 500mg of tissue were homogenized in a motor-driven teflon pestle and glass vessel using high-salt buffer (HSB) (50 mM Tris-HCl pH 7.4, 750 mM NaCl, 10 mM NaF, 5 mM EDTA) with protease inhibitor cocktail, incubated for 20 min on ice and centrifuged at 100,000 x g for 30 minutes at 4 °C to obtain the soluble fraction. The pellets were then re-extracted with series of triton / sarkosyl buffers. The final sarkosyl-insoluble pellets were washed once with PBS and resuspended in PBS by sonication resulting in the insoluble fraction. The soluble and insoluble fractions were run on SDS-PAGE gel and analyzed by western blot as described under “Western blot analysis”. No differences between male and female subjects were observed.
Proteomic analysis
After SILAC labeling and sequential extraction into Triton X-100 soluble and 2% SDS soluble fractions, samples were run on SDS-PAGE and stain with Simply Blue Safe stain as described above. The gel was only run long enough for the samples to enter the gel at about 1cm in length so that small impurities were removed. Gel lanes were cut into 2-to-4 bands, and submitted to the Northwestern proteomics core for analysis, using a Q Exactive HF ultra-high field orbitrap MS. Protein identification and quantification was done using MaxQuant software, which searches spectra against a human Uniprot database linked to a decoy database where all sequences are reversed. False discovery rates (FDR) are reported as q values and only those which have <1% rate are reported. Andromeda software reports a score as −10 logarithmic probability of observing the peptide ID match by chance. MaxQuant reports integrated intensity values of heavy and light peptides, of only those proteins that have at least 2 unique peptides and passed the stringent statistics and FDR cut-offs. Heavy (H) / Light (L) ratios are used to calculate Z-score values, comparing the individual protein value to that of the median value of all proteins injected. Z-scores outside of +/− 1.96 range (outside of the 95% distribution range) are used to prioritize protein hits. P-values of 4 biological replicates (individual culture wells) were used to determine reproducibility and used with Z-scores to rank protein IDs.
Western blotting
40ug of lysate was loaded on to tris-glycine SDS-PAGE gel (10–15% based on protein of interest). To analyze proteins with high molecular weight (>120kDA), a tris-glycine gradient gel (ThermoFisher, # XP04125BOX) was used. The lysates were run at 150V for approx. 1.5hrs and then transferred onto a PVDF membrane (EMD Millipore, # IPFL00010) at 30V for 1hr. The membrane was then post-fixed in 0.4% paraformaldehyde, washed in milliQ water and then blocked in 1:1 TBS:odyssey blocking buffer (Licor # P/N 927–40003) for 1hr at room temp. The membrane was incubated with primary antibodies diluted in 1:1 ratio 0.2% TBS-tween and odyssey blocking buffer overnight at 4°C. The following day the membrane was washed with 0.2% TBS-Tween and incubated with secondary antibodies for 1 hr. The blot was washed as before and scanned on an odyssey imaging system. The western blots were analyzed using Image Studio software (licor) to quantify band intensities.
Assessment of co-aggregation of recombinant SFPQ and a-synuclein in cell free systems.
A 40kDa truncated form of recombinant SFPQ (residues 276–598) was purified as described previously 43, stored in 25% glycerol / PBS pH7.4. This region of SFPQ contains domains RRM1, RRM2, NOPS, and coiled-coil domain. Just before use, glycerol was removed from SFPQ monomers by buffer exchange into PBS pH 7.4 (3X each using 3 volumes of PBS in 10,000 MWCO filters (Millipore)). Samples were spun at 100,000x g for 1 hr to remove any pelletable material, then used for experiments. a-Syn full length monomers were isolated as described 21. Oligomers were generated by lyophilization as previously described in detail 78,79. Pre-formed fibrils (PFFs) were generated according to standardized protocols 111, by shaking / incubating monomers for 7 days, 1000 RPM, 37°C, centrifuged at 100,000 x g for 1 hour, then sonicated to generated fragmented fibrils. A-Syn forms were incubated with monomeric SFPQ with both proteins at 1mg/ml, and incubated / shaken at 1000 RPM, 37°C for the indicated lengths of time. Samples were separated into supernatant and pelletable fractions by centrifugation at 100,000 X g, 4°C, 1 hr, and 1ug protein was analyzed by western blot (using SFPQ D8 antibody (mouse) and a-syn MJFR-1 antibody (rabbit) (Abcam)). The different conditions (SFPQ + BSA, + Mon, + Olig, or + PFF) at 24 and 48 hr time points were loaded together on the same blots and detected using fluorescent conjugated secondary antibodies (Alex-647- anti-mouse; Alexa 790- anti-rabbit) for accurate quantification. For kinetic analysis, the individual time points from the same experimental condition were loaded together on the same blot, to quantify the change over time. Colocalization of SFPQ and a-syn was done on the same blot using two different colored secondary antibodies. Samples were analyzed by Thioflavin T (ThT). 10mM ThT stock was made fresh prior to each experiment by dissolving in water, then diluting to 10uM in pH 8.5 glycine buffer. 10uM ThT was added to 5 ug of total protein, incubated for 5 minutes in 96 well black fluoroplates (Nunc), and analyzed in a Molecular Devices SpectaMax M5 fluorescent plate reader (ex=450, em=485, cutoff = 470nm).
Functional analysis of the 3’UTR of PRKAR2A
A 1045bp region of the 3’UTR of PRKAR2A obtained from NM_00457.4 (starting position 2562; corresponding to Chr3+ : 48749472 – 48750516) was subcloned downstream into eGFP reporter plasmid. The cloning of EGFP with or without PRKAR2A 3’ UTR was custom ordered through VectorBuilder under Synapsin I or EF1A promoter into pLV lentiviral backbone. The 3’ UTR of PRKAR2A gene consisting of 1045 bp fragment (Chr3 + :48749472 – 48750516 ) was cloned after EGFP gene with stop codon. The lentivirus was prepared, and neurons were transduced at day 76 as described under “Lentiviral infection” at MOI3, harvested at 14 days post infection (dpi). iPSn cultures (day 90) were prepared for western blot analysis of GFP as described above. For HEK experiments, the plasmids were transfected using lipofectamine 2000 and fixed for FISH analysis 24 hours later.
Gel Electrophoresis analysis of RNA
RNA oligonucleotides (Horizon Discovery, sequences shown in Fig S8C) were reconstituted in PBS at 100uM. Oligonucleotides were prepared by diluting the samples to 500nM in water and 1X RNA loading dye (New England Biolabs (NEB), #B0363S), heating to 80°C for 15 minutes, followed by cooling at 25°C for 15–30 minutes. Native gel electrophoresis was done by loading 10ul of 500nM oligos in TBE-PAGE gels (15% acrylamide). It was essential that the gels were pre-run at 200mV for 1 hr followed by washing out of the wells prior to loading the samples. Molecular weight was estimated by the migration of bromophenol blue (15nt for a 15% TBE gel), the low ssRNA marker (NEB #N0364S) and the microRNA marker (NEB #N2102S). For denaturing electrophoresis, samples were prepared in the same way but loaded on TBE- gels containing 8M Urea. Gels were pre-run at 200V for 45 minutes-to-1 hr (this step is essential), wells were washed out, and 10ul of each sample was loaded and run for an additional 45 minutes to 1 hour. Gels were developed in 1:5000 dilution of SYBR Green II RNA stain (ThermoFisher #S7586) in 1X TBE for 20-to-45 minutes, then scanned on a Biorad gel Doc imager. Images were analyzed by Image J software to obtain peak migration in cm and integrated pixel intensity of the bands. The molecular weight (MW) in nucleotides (nt) was estimated by comparisons with the RNA ladders mentioned above and bromophenol blue). The assay was repeated 8 times, which represents 4 separate reaction tubes set up on two different days.
RNA stability assays by RNAse digestion
Oligo stocks of the same sequence used in Fig S8C were diluted to 1uM in RNA Structure Buffer (10mM Tris-Cl, pH 7.4, 100mM KCl, 10mm MgCl2), heated to 80°C for 15 minutes, and cooled to 25°C for 15-to-30 minutes. RNAse T1 (ThermoFisher # EN0541, 1000U / ul) was added at a 1:1000 dilution in a 10ul final volume reaction and incubated at 25°C for 15 minutes. The sample was mixed with 10ul of 2X RNA loading dye (NEB #B0363S) for a final volume of 20ul, and heat-killed at 100°C for 10 minutes. In parallel, 1uM oligos were digested with RNAse I (ThermoFisher # EN0601, 10U/ul) at 1:100 and 1:1000 dilutions and incubated at 37°C for 20 minutes, followed by mixing with 2X NEB loading dye and heat-kill for 10 minutes at 100°C. All 20ul of sample was loaded on TBE-UREA gels (15% acrylamide) as described above, pre-running the gels before sample loading. Gels were developed in 1:5000 dilution of SYBR Green II RNA stain (ThermoFisher #S7586) in 1X TBE for 20-to-45 minutes, then scanned on a Biorad gel Doc imager. The RNAse digestion assays were set up in 3 different reaction tubes on 2 separate days to assess reproducibility. The gels were not quantified because the inosine-containing oligos had no detectable signal after digestion.
RNA-SFPQ binding in vitro
6-FAM-labeled oligo stocks of the same sequence used in Fig S8C (Horizon Discovery) were diluted to 100nM in RNA Structure buffer (10mM Tris-Cl, pH 7.4, 100mM KCl, 10mm MgCl2), heated to 80°C for 15 minutes and cooled to 25°C for 15-to-30 minutes. 100nM RNA oligos were mixed with recombinant purified SFPQ at 1, 2, 5, and 10uM for 20 minutes at 25°C in a 10ul reaction, mixed with Novex™ Hi-Density TBE Sample Buffer (5X) (ThermoFisher # LC6678), and analyzed on TBE-polyacrylamide gels (8%). It was essential that the gels were pre-run at 200mV for 1 hr followed by washing out of the wells prior to loading the samples. After the samples were electrophoresed, gels were directly scanned on an Azure Sapphire imager using the 532nm optical module. Binding reactions were set up in 3 different reaction tubes on different days to assess reproducibility. Gels were quantified using integrated intensity signals obtained from Licor Image studio software.
RNA-SFPQ aggregation assays
Native Gel Electrophoresis:
RNA oligos were diluted to 2nM in PBS and mixed with 250nM SFPQ in a 10ul reaction volume, and incubated at 25°C for 20 minutes. Samples were mixed with Novex™ Hi-Density TBE Sample Buffer (5X) (ThermoFisher # LC6678) and the entire sample was loaded on native TBE polyacrylamide gels (6%). Gels were transferred to PVDF membranes as described under “Western Blot Analysis” and probed with anti-SFPQ antibody (D8, Santa Cruz). Gel images were scanned on Azure Sapphire Imager and quantified using Licor Image Studio software. The assay was set up three times on separate days to assess reproducibility of the aggregation. High molecular weight oligomers were defined as species that migrated slower compared to the ‘SFPQ alone’ species.
Sedimentation Analysis:
RNA oligos were diluted to 1uM in PBS and mixed with 10uM (final) recombinant SFPQ in a 10ul reaction volume. SFPQ protein was prepared in the same way as described above, “Assessment of co-aggregation of SFPQ and a-synuclein in cell free systems”, by buffer exchange in PBS to remove the glycerol present in the storage buffer immediately before setting up the assay. Samples were incubated for 10 minutes at 25°C then centrifuged at 100,000 X G, 4°C for 1 hour. Supernatant and pellet fractions were separated and analyzed by either western blot analysis for SFPQ aggregation as described above, or RNA electrophoresis by TBE-Urea gels (15% acrylamide)/ SYBR green. Quantifications of both were done using Licor Image Studio and analyzed by measuring the percent of SFPQ or RNA present in the pellet fraction. The assay was repeated 4-to-5 times in separate reaction tubes, on 2 different days to assess reproducibility.
Dual-Luciferase reporter assay
iPSc midbrain neurons at day 25 were plated on poly-D-lysine/laminin coated 96-well plate at seeding density of 80,000–100,000 cells per well and allowed to mature until day 90. For transfection, 25ul of Opti-MEM reduced serum media (Thermo Fisher, # 31985–070) was mixed with 1.2ul of lipofectamine 2000 (ThermoFisher, # 11668019 ) at RT for 5min. In a second tube, 25ul of Opti-MEM along with 500ng of pLightswitch-ADAR3 promoter-renilla luciferase plasmid (Active motif, # S703748) and 100 ng of control constitutive promoter driven pGL2-SV40-firefly luciferase plasmid (Addgene, # # 26280) were mixed at RT,5min. The reactions containing lipofectamine 2000 and plasmids were mixed, incubated at RT for 20min and co-transfected onto neurons (per one well) in neurobasal medium with sm-1 supplement. The neurons were lysed 48hrs post-transfection (at day 90) using 75 ul lysis buffer per well from Dual-Glo® Luciferase Assay System (Promega, # E2920) for 30min at RT. The luminescence of renilla and firefly luciferase was measured according to manufacturer protocol using a spectramax plate reader. The ADAR3 promoter activity was expressed as the luminescence unit of Renilla luciferase normalized to firefly luciferase.
Cytoplasmic-nuclear RNA fractionation
iPSc- midbrain neurons (day 60) at seeding density of 1–2million per well were used as starting material for fractionation. The cytoplasmic-nuclear fractionation was performed using the NE-PER kit (ThermoFisher, 78833). Briefly, the neurons were extracted using CER-I buffer with betamercaptoethanol (BME) and centrifuged to get the nuclear pellet. The nuclear pellet was washed in CER-I to prevent carry over into nuclear fraction. Once the cytoplasmic supernatant and nuclear pellet were obtained, the RNA was extracted using Sureprep nuclear or cytoplasmic RNA purification kit (Fisher scientific, # BP280550). Parallel wells for each experimental condition or genotype (n=3–4) were harvested for total RNA extraction. The RNA concentrations were measured on nanodrop 2000 (ThermoFisher). The cDNA synthesis was performed using 500–1000ng of total RNA from each cellular compartment and total fraction as described below.
RNA extraction and quantitative-RT-PCR
Total RNA from iPSC – midbrain neurons (day 60 or day 90 as indicated in the text or figure legends) was extracted using the PureLink RNA mini kit (ThermoFisher). 1000 ng of total RNA was treated with DNAseI (Thermofisher) to remove residual genomic DNA at 37 °C for 30min. Then, cDNA synthesis was performed using RevertAid First Strand cDNA Synthesis Kit (Thermofisher).Quantitative-real-time PCR (Q-RT-PCR) was performed using predesigned TaqMan probes. Please refer to the resource table for the information on the probles. The quantification was based on delta-ct method normalized to beta-actin levels as fold change. Each target mRNA was measured with n=2 technical replicates per biological replicate.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-alpha synuclein (C-20) | Santa Cruz | Cat #sc-7011-R; RRID: AB_2192953 |
| Mouse monoclonal anti-alpha synuclein (LB509) | Abcam | Cat #ab27766; RRID: AB_727020 |
| Mouse monoclonal anti-alpha synuclein (syn211) | Sigma Aldrich | Cat #S5566; RRID: AB_261518 |
| Mouse monoclonal anti-alpha synuclein (syn505) | Thermofisher Scientific | Cat # 32-8300; RRID: AB_2533225 |
| Rabbit monoclonal anti-alpha synuclein (MJFR1) | Abcam | Cat #ab138501; RRID: AB_2537217 |
| Mouse monoclonal anti-β3-tubulin | Biolegend | Cat # 802001; RRID: AB_2564645 |
| Rabbit polyclonal anti-SFPQ/PSF | Bethyl laboratories | Cat # A301320A; RRID: AB_937991 |
| Mouse monoclonal anti-SFPQ/PSF (D-8) | Santa Cruz | Cat #sc-271796; RRID: AB_10709164 |
| Mouse monoclonal anti-SFPQ/PSF B-92 | Sigma Aldrich | Cat #P2860; RRID: AB_260995 |
| Rabbit polyclonal anti-NONO | Bethyl laboratories | Cat #A300-587A; RRID: AB_495510 |
| Mouse monoclonal anti-p54/nrb A-11 | Santa Cruz | Cat #sc-166702; RRID: AB_2152178 |
| Mouse monoclonal anti-FUS/TLS (4H11) | Santa Cruz | Cat #sc-47711; RRID: AB_2105208 |
| Rabbit polyclonal anti-PSP1 | Bethyl laboratories | Cat # A303205A; RRID: AB_10952866 |
| Rabbit polyclonal anti-NSE | Polysciences | Cat #17437-100; RRID: N/A |
| Mouse monoclonal anti-ADAR3 (3.591) | Santa Cruz | Cat # sc-73410; RRID: AB_2222784 |
| Rabbit polyclonal anti-Inosine | Medical & Biological laboratories (MBL) | Cat #PM098; RRID: N/A |
| Rabbit polyclonal anti-CYFIP2 | Abcam | Cat #ab95969; RRID: AB_10679909 |
| Mouse monoclonal anti-CAPS-1 G-12 | Santa Cruz | Cat #sc-377279; RRID: N/A |
| Mouse monoclonal anti-Dynamin I (D5) | Santa Cruz | Cat #sc-12724; RRID: AB_2230650 |
| Rabbit polyclonal anti-PRKAR2A/PKA-RIIalpha | Bethyl laboratories | Cat #A301-670A; RRID: AB_1211499 |
| Rabbit polyclonal anti-alpha N-Catenin | Proteintech | Cat #14362-1-AP RRID: AB_2087961 |
| Rabbit polyclonal anti-Nogo | Novus Biologicals | Cat #NB100-56681; RRID: AB_838641 |
| Rabbit polyclonal anti-GFP | Sigma Aldrich | Cat # G1544; RRID: AB_439690 |
| Rabbit polyclonal anti-PSD95 | Cell Signaling Technology | Cat # 2507S; RRID: N/A |
| Mouse monoclonal anti-Synaptophysin 1 | Synaptic systems | Cat #101 011; RRID: AB_887824 |
| Chicken polyclonal anti-NeuN | Millipore | Cat #ABN91; RRID: AB_11205760 |
| Chicken polyclonal anti-BIII tubulin | Abcam | Cat #ab41489; RRID: AB_727049 |
| Chicken polyclonal anti-beta actin | Sigma Aldrich | Cat #SAB3500350; RRID: AB_10638013 |
| Chicken polyclonal anti-tyrosine hydroxylase (TH) | Millipore | Cat #AB9702; RRID: AB_570923 |
| Mouse monoclonal anti-GAPDH | Millipore | Cat #CB1001; RRID: AB_2107426 |
| Mouse monoclonal anti-ADAR1 (15.8.6) | Santa Cruz | Cat #sc-73408; RRID: AB_2222767 |
| Rabbit polyclonal anti-RED1 | Abcam | Cat #ab64830; RRID: AB_1141635 |
| Streptavidin, Alexa Fluor 488 Conjugate | Invitrogen | Cat #S32354; RRID: AB_2315383 |
| Secondary antibody: Goat anti-rabbit IgG secondary (H+L) HRP | Invitrogen | Cat #G21234; RRID: AB_2536530 |
| Secondary antibody: Alexa Fluor 488 Goat anti-rabbit IgG secondary (H+L) | Invitrogen | Cat #A11034; RRID: AB_2576217 |
| Secondary antibody: Alexa Fluor 488 Goat anti-mouse IgG secondary (H+L) | Invitrogen | Cat #A11029; RRID: AB_138404 |
| Secondary antibody: Alexa Fluor 568 Goat anti-rabbit IgG secondary (H+L) | Invitrogen | Cat #A11036; RRID: AB_10563566 |
| Secondary antibody: Alexa Fluor 568 Goat anti-mouse IgG secondary (H+L) | Invitrogen | Cat #A11031; RRID: AB_144696 |
| Secondary antibody: Alexa Fluor 647 Goat anti-mouse IgG secondary (H+L) | Invitrogen | Cat #A21236; RRID: AB_144696 |
| Secondary antibody: Alexa Fluor 647 Goat anti-rabbit IgG secondary (H+L) | Invitrogen | Cat #A21245; RRID: AB_141775 |
| Secondary antibody: Alexa Fluor 790 Goat anti-mouse IgG secondary (H+L) | Invitrogen | Cat #A11357; RRID: AB_2534140 |
| Secondary antibody: Alexa Fluor 790 Goat anti-rabbit IgG secondary (H+L) | Invitrogen | Cat #A11369; RRID: AB_2534142 |
| Secondary antibody: Alexa Fluor 647 Goat anti-chicken IgG secondary (H+L) | Invitrogen | Cat # A32933; RRID: AB_2762845 |
| Bacterial and virus strains | ||
| pER4 GFP lentivirus | Cuddy et al., 2019 | N/A |
| pER4 FLAG-ADAR3 lentivirus | This paper | N/A |
| pLV lentivirus | This paper | N/A |
| Biological samples | ||
| Human brain frontal cortex tissue of control, DLB patients | Northwestern University Alzheimer’s disease pathology core (CNADC). Please refer to Supplementary table 4 for more details | N/A |
| Human brain frontal cortex tissue of control, DLB, AD and PSP patients | Mayo Clinic Brain Bank (Jacksonville FL) Please refer to Supplementary table 4 for more details | N/A |
| Prnp-SNCA*A53T line M83 mouse tissue | Giasson BI et al., Neuron 2002 | Strain #:004479 RRID:IMSR_JAX:00 4479 |
| Chemicals, peptides, and recombinant proteins | ||
| Bovine serum albumin (BSA), heat shock, fatty acid free | Roche | Cat #03117057001 |
| 8-Azaadenosine | Tocris | Cat #6868 |
| L-Lysine·2HCl (13C6, 99%; 15N2, 99%) | Cambridge Isotope Laboratories | Cat# CNLM-291-H-0.5 |
| L-Arginine·HCl (13C6, 99%; 15N4, 99%) | Cambridge Isotope Laboratories | Cat# CNLM-539-H-0.5 |
| SimplyBlue™ SafeStain | ThermoFisher Scientific | Cat#LC6065 |
| Leupeptin, Hemisulfate, Synthetic | Millipore Sigma | Cat #10897610MG |
| 3,4-Dihydroxy-L-phenylalanine | Millipore Sigma | Cat #D9628 |
| RNase A Solution | Millipore Sigma | Cat #70856-3 |
| Ribonucleoside Vanadyl Complex | New England Biolabs | Cat #S1402S |
| Histoclear II | National Diagnostics | Ca t#101412-882 |
| 2X RNA loading dye | New England Biolabs | Cat #B0363S |
| Low Range ssRNA Ladder | New England Biolabs | Cat #N0364S |
| microRNA Marker | New England Biolabs | Cat #N2102S |
| SYBR Green II RNA stain | Thermofisher Scientific | Cat #S7586 |
| RNase T1 (1000U/μL) | Thermofisher Scientific | Cat #EN0541 |
| RNase I (10U/μL) | Thermofisher Scientific | Cat #EN0601 |
| Novex™ Hi-Density TBE Sample Buffer (5X) | Thermofisher Scientific | Cat #LC6678 |
| Stellaris® RNA FISH Hybridization Buffer | Biosearch Technologies | Cat #SMF-HB1-10 |
| Stellaris® RNA FISH Wash Buffer A | Biosearch Technologies | Cat #SMF-WA1-60 |
| Stellaris® RNA FISH Wash Buffer B | Biosearch Technologies | Cat #SMF-WB1-20 |
| TRIzol™ Reagent | Thermofisher Scientific | Cat #15596026 |
| Biodyne™ B Nylon Membrane | Thermofisher Scientific | Cat #77016 |
| L-glutamine | Gibco | Cat #25030081 |
| Normal goat serum (NGS) | Jackson ImmunoResearch | Cat #005-000-121 |
| Fetal bovine serum (FBS), heat-inactivated | Thermofisher Scientific | Cat #10438026 |
| Paraformaldehyde (10%, methanol-free) | Polysciences, Inc. | Cat #40181 |
| Penicillin / Streptomycin | Thermo Fisher Scientific | Cat #10378016 |
| Phenylmethylsulfonyl fluoride (PMSF) | Sigma | Cat #78830 |
| Protease Inhibitor Cocktail (PIC) | Roche | Cat #11836170001 |
| N-Lauroylsarcosine sodium salt (sarkosyl) | Sigma | Cat #L9150 |
| Sodium dodecyl sulfate (SDS) | Sigma | Cat #L4509 |
| Sodium orthovanadate (Na3VO4) | Sigma | Cat #450243 |
| Sodium fluoride (NaF) | Sigma | Cat #201154 |
| Triton X-100 | Sigma | Cat #T8787 |
| Triton™ X-100 Surfact-Amps | Thermofisher Scientific | Cat# 28314 |
| Thioflavin T (ThioT) | Sigma | Cat #T3516 |
| Clarity Max™ Western ECL Substrate | Biorad | Cat #1705062S |
| Q5® High-Fidelity DNA Polymerase | New England Biolabs | Cat #M0491L |
| ExoSAP-IT™ PCR Product Cleanup Reagent | Thermofisher Scientific | Cat #78200.200.UL |
| Nucleoside Digestion Mix | New England Biolabs | Cat# M0649S |
| Critical commercial assays | ||
| Dual-Glo® Luciferase Assay System | Promega | Cat #E2920 |
| Duolink In Situ Red Starter Kit Mouse/Rabbit | Sigma Aldrich | Cat #92101 |
| NE-PER Nuclear and Cytoplasmic Extraction Kit | Thermofisher Scientific | Cat #78833 |
| HIV1-p24 Antigen ELISA Kit | Zeptometrix | Cat #0801111 |
| Pierce BCA Protein Assay Kit | Thermofisher Scientific | Cat #23227 |
| PureLink Genomic DNA Kit | Invitrogen | Cat #K182002 |
| PureLink™ RNA Mini Kit | Invitrogen | Cat #12183025 |
| RevertAid First Strand cDNA Synthesis Kit | Thermofisher Scientific | Cat #K1621 |
| Cytoplasmic and Nuclear RNA Purification Kit | Norgen Biotek Corp | Cat #21000 |
| μMACS™ mRNA Isolation Kit | Miltenyi Biotec | Cat #130-075-201 |
| Quantitative RT-PCR: SFPQ (ID: Hs00915444_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: NonO (ID: Hs00939763_g1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: ADARB2 (ID: Hs00218878_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: CADPS (ID: Hs02846374_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: CYFIP2 (ID: Hs00910722_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: DNM1 (ID: Hs01074761_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: CTNNA2 (ID: Hs01093122_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: RTN4 (ID: Hs00199671_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: PRKAR2A (ID: Hs00177760_m1) | Thermofisher Scientific | Cat #4351372 |
| Quantitative RT-PCR: ACTB (β-actin) (ID: Hs99999903_m1) | Thermofisher Scientific | Cat #4351372 |
| Deposited data | ||
| Full Western Blots | This Paper, Mendeley Data | Mendeley doi: 10.17632/pp9vnn666d.1 |
| Raw proteomics data files | This Paper | ProteomeXchange Consortium via the PRIDE partner repository PXD051673 |
| RNA-seq and whole genome sequencing of A53T and Corr lines | This Paper | Sequence Read Archive (SRA) ID: SUB14391987, BioProject ID: PRJNA1104694 |
| Experimental models: Cell lines | ||
| GM15010 (SNCA Triplication, 3x-1) | Stojkovska et al.,2022 | N/A |
| GM15010 (SNCA Triplication, 3x-1 corr) | Stojkovska et al.,2022 | N/A |
| Healthy control (Est Ctrl or 2135) | Mazzulli et al., 2011, Mazzulli et al., 2016; Somatic cells originally from: Hedrich et al., 2006 | N/A |
| A53T alpha-synuclein and isogenic control | Soldner et al., Cell, 2011 | N/A |
| Experimental models: Organisms/strains | ||
| Oligonucleotides | ||
| See Supplemental Table 13 for primers,siRNA, RNA-FISH probes and RNA oligos | This Paper, and siRNA sequence is from Imamura K et al., Mol Cell 2014 | N/A |
| Recombinant DNA | ||
| pLightswitch-ADAR3 promoter-renilla luciferase | Active Motif | Cat #S703748 |
| pGL2-SV40-firefly luciferase | Addgene, Hanna J et al., PNAS 2010 | Cat #26280 |
| pLV-eGFP-control | This paper, VectorBuilder | Vector ID : VB220726-1331jqn |
| pLV-eGFP-PRK-3UTR (under synapsin promoter) | This paper, VectorBuilder | Vector ID : VB220726-1330tvv |
| pLV-ADAR1 E713Q | This paper, VectorBuilder | Vector ID : VB220529-1079awp |
| pLV-ADAR2 E488Q | This paper, VectorBuilder | Vector ID : VB220529-1077xcc |
| GluRB RNAG reporter | Dr. Michael F. Jantsch, Garncarz W et al., RNA Biol 2013 | N/A |
| pER4-GFP | Cuddy et al., 2019 | N/A |
| pER4-FLAG-ADAR3 | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism V6.0 software | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ / Fiji V1.0 software | National Institutes of Health | https://imagej.net/software/fiji/ |
| MATLAB2021b | The Mathworks Inc. | https://www.mathworks.com/products/new_products/release2021b.html |
| Airlocalize | Github | https://github.com/timotheelionnet/AIRLOCALIZE |
| Odyssey software (Image Studio V3.1.4) | Li-Cor Biosciences | https://www.licor.com/bio/image-studio/ |
| Snapgene V5.3 software | Snapgene | https://www.snapgene.com/ |
| PLAAC database | Lancaster et al., Bioinformatics 2014 | http://plaac.wi.mit.edu/ |
| PASTA 2.0 database | Walsh,I et al., Nucleic acids Res 2014 | http://old.protein.bio.unipd.it/pasta2/) |
| Protean 3D software | DNASTAR | https://www.dnastar.com/software/lasergene/protean-3d/ |
| Quality Check (QC) Module | Thermofisher cloud | apps.thermofisher.com |
| FASTQC v.0.11.5 | Babraham Institute | http://www.bioinformatics.babraham.ac.uk/projects/fastqc |
| BWA v.0.7.17 | Li,H et al., Bioinformatics 2009 | https://github.com/lh3/bwa |
| BCFTools v.1.10.0 | Danecek, P et al., Gigascience 2021 | https://www.htslib.org. |
| GATK RNAseq short variant discovery pipeline | Broad Institute | https://github.com/gatk-workflows/gatk3-4-rnaseq-germline-snps-indels |
| STAR v.2.7.5 | Dobin,A et al., Bioinformatics 2013 | https://github.com/alexdobin/STAR/releases |
| Morpheus | Broad Institute | https://software.broadinstitute.org/morpheus/ |
| Imaris v9.9 | Oxford Instruments | https://imaris.oxinst.com/ |
| Cytoscape V3.9.0 | Cytoscape Consortium | https://cytoscape.org/ |
| Original Code to identify A-to-I RNA edit sites | This Paper | Github DOI: 10.5281/zenodo.11061692 |
| Other | ||
| DAPI Fluoromount mounting media | Southern Biotech | Cat #0100-20 |
| Intercept blocking buffer | Li-Cor Biosciences | Cat #927-70001 |
| Lenti-X concentrator | Clontech | Cat #631232 |
| Lipofectamine 2000 | Thermofisher Scientific | Cat #11668019 |
| Matrigel | Fisher | Cat #CB-40234 |
| mTeSR1 media | StemCell Technologies | Cat #85850 |
| Neurobasal SM1 media | Thermo Fisher Scientific | Cat #21103-049 |
| NeuroCult SM1 supplement | StemCell Technologies | Cat #05711 |
| Amicon Ultra-0.5 Centrifugal Filter Unit | Millipore Sigma | Cat #UFC501096 |
| PVDF transfer membrane, 0.45 mm pore size | Millipore Sigma | Cat #IPFL00010 |
| X-tremeGENE HP DNA Transfection Reagent | Roche | Cat #6366236001 |
| Accutase Cell Detachment Solution | Corning | Cat #25058CI |
| Opti-MEM™ Reduced Serum Medium | Gibco | Cat #31985-070 |
| Prolong diamond antifade mountant | Invitrogen | Cat # P36970 |
| DNA Retardation Gels (6%), 1.0 mm | Invitrogen | Cat #EC6365BOX |
For Nuclear-cytoplasmic ratio, RNA from day 60 iPSn was fractionated as described above and parallel wells were harvested for total RNA extraction for each experimental condition. Delta Ct method was used to calculate the RNA levels of cytoplasmic or nuclear fractions relative to total (cytoplasmic+nuclear) target transcript levels and represented as fold change.
For RNA extraction from human brain frontal cortex, 40–50mg of brain tissue per sample was used and Total RNA was extracted with TRIzol™ Reagent (ThermoFisher, #15596026). The aqueous phase was used and mixed with equal volumes of 70% ethanol. The rest of the extraction was performed using the PureLink RNA mini kit (ThermoFisher) and cDNA synthesis was performed as mentioned above. No differences between male and female subjects were observed.
RNA Dot-blot
Total RNA or Cyto/nuclear RNA was isolated from neurons as described above from day 60 iPSn. For polyA+ RNA isolation, total RNA or fractionated RNA was used and subjected to purification using μMACS™ mRNA Isolation Kit (Miltenyi Biotec, cat# 130-075-201) according to manufacturer’s protocol. 30 ng of RNA in RNAse free water was heated to 95C in a PCR tube for 3min and chilled briefly on ice. 2ul of sample was blotted onto Biodyne™ B Nylon Membrane (Thermofisher ,cat# 77016), followed by UV-crosslinking at 125mJ/cm2 for 20–40 s. The membrane was washed in 10mls of 0.1%TBS-tween (TBST) for 5 min RT and block in 0.1%TBT + 2% BSA for 1hr RT. Anti- Inosine antibody (MBL life science, cat# PM098, 1:1000) was added in blocking solution and incubated at 4C overnight. The membrane was washed 3 times with 0.1%TBST and incubated with HRP-conjugated secondary (1:5000) 1hr RT, followed by 3 washes with 0.1%TBST and developed using Clarity Max™ Western ECL Substrate (Biorad,cat# 1705062S). The membrane was imaged in Biorad Chemidoc imaging system.
Assessment of inosine content in polyA mRNA by HPLC-MS analysis.
Total polyA mRNA was extracted using μMACS™ mRNA Isolation Kit (Miltenyi Biotec, cat# 130–075-201) from day 60 iPSn. 500ng of polyA RNA was digested using Nucleoside Digestion Mix (NEB, cat#M0649S). Samples were submitted to the metabolomics core at the Metabolite Profiling Core Facility at the Whitehead Institute of the Massachusetts Institute of Technology (https://biology.mit.edu/tile/metabolite-profiling-core-facility ). The samples were analyzed by HPLC-MS and elution profiles were compared to purified standards for each nucleotide. Raw peak areas were used to quantify the relative amount of each nucleotide and expressed as fold change compared to the isogenic control line. The assay was repeated in 4 individual culture well replicates. For quality control (QC) analysis, a “pool” of sample consisting of a mixture of several uL from each of the biological samples was run, which created a representative sample that was run multiple times to get a measure of technical reproducibility for each nucleotide. A CV (standard deviation / average) was calculated for these technical replicates and metabolites with a CV < 0.25 were considered reliably detected. Also, a larger injection volume was run with 0.3-fold and 0.1-fold dilutions of the pooled sample, which indicated whether samples fall in the linear range of detection for each metabolite, or there was a detector saturation or nearing the lower limit of detection.
gDNA extraction
Genomic DNA from day 60 iPSC-neurons was extracted using PureLink Genomic DNA extraction kit (ThermoFisher). For the human brain tissue, the organic phase from the trizol extraction above was utilized and 400ul of ethanol was added. The samples were centrifuged at max speed to pellet gDNA. The supernatant was discarded and rest of the extraction was performed using the PureLink Genomic DNA kit. The concentration and purity of genomic DNA was measured on nanodrop 2000 (ThermoFisher).
A-to-I editing and Sanger sequencing
For detection of A-to-I site in day 60 iPSc-neurons or human brain tissue, 100ng of cDNA or gDNA was used as template for amplification of target gene using touchdown PCR. The primers used to amplify cDNA or gDNA regions can be found in Supplemental Table 13. The PCR amplification was performed using Q5 polymerase (NEB) in 20ul reaction with following protocol: 98 °C 30s ; 12 cycles : 98 °C 10s, 65 °C-55 °C 30s( −1 °C/cycle),72 °C 15s ; 18 cycles : 98 °C 10s, 55 °C 30s,72 °C 15s; final extension 72°C 1min. The PCR products were then treated with ExoSAP-IT™ PCR Product Cleanup Reagent (ThermoFisher, # 78200.200.UL). The PCR products were run on an agarose gel to confirm the size and specificity of amplified product before submitting to Sanger sequencing.
The raw Sanger sequencing chromatogram files (.ab1 file) were opened in Quality Check (QC) Module on thermocloud platform (ThermoFisher). The peak traces were exported as .csv files to obtain the peak heights of the base call. The peak heights for bases A and G at edit site were obtained and editing percentage was calculated as: Peak height of “G” /(Peak height of “A” + peak height of “G”) X 100.
Lactate Dehydrogenase (LDH) cytotoxicity assay
To measure neuron viability in iPSc-neurons, we used CyQUANT™ LDH Cytotoxicity Assay to measure LDH activity according to manufacturers’ protocol. This was done at day 120, and the iPSn were treated with 8-aza starting at day 90 (30-day treatment). Briefly, 50 ul media from vehicle or 8-aza 30 day treated neurons was incubated with 50ul of reaction mixture in 96-well microplate for 30min at room temperature. The reaction was stopped and absorbance was measured at 490nm and 680nm in a molecular devices plate reader. The LDH activity was determined by subtracting the absorbance at 680nm from absorbance at 490nm and expressed as fold change.
III. IMAGE AQUISTION AND ANALYSIS
Immunocytochemistry
For SFPQ/NONO staining, iPSC midbrain cultures at day 90 were grown on poly-D-lysine/laminin coated glass coverslips. Cells were quickly rinsed with cold PBS to remove residual culture media and fixed in 4% paraformaldehyde in PBS for 15 min at room temperature (RT). The fixed cells were washed 3 times with PBS and permeabilized/blocked in 0.1% Triton X-100 with 5% normal goat serum (Jackson Immunoresearch, # 005000121) and 2% BSA for 1hr at RT. Primary antibodies NONO rabbit polyclonal dilution 1:200 (Bethyl labs, # A300–587A), SFPQ mouse monoclonal antibody (sigma, # P2860) dilution 1:200 and Tyrosine hydroxylase chicken polyclonal (EMD Millipore, AB9702 ) dilution 1:500 were added in 0.1% PBS-tween with 5%NGS and 2%BSA and incubated overnight at 4 °C. The following day, coverslips were washed 3 times in 0.1% TBS-tween and goat Alexa fluor secondary antibodies (ThermoFisher) dilution 1:750 were added and incubated for 1hr at RT, washed as before and mounted on glass slides with DAPI Fluoromount –G (southern biotech, # 10020) for confocal microscopy. The images were acquired on a Leica confocal microscope (CTR4000 / DMI4000B) with 0.3 um Z-step size covering the focal plane of the nuclei.
For NONO/Inosine staining, neurons were fixed in 4% paraformaldehyde in PBS for 15 min at RT. For the Rnase treated condition, cells after fixation were treated with Rnase (1mg/ml, Sigma cat#70856) for 30min, RT and rinsed with 1X PBS 3 times. Cells were permeabilized with 0.5% Triton with 200mM RVC(NEB) for 10min RT, washed 3 times with 1x PBS+ 2mM RVC, followed by blocking with 1xPBS +2%BSA+2mMRVC for 1hr, RT. Primary antibodies for NONO (Santacruz,1:200) and Inosine (MBL, 1:200) in blocking solution were added and incubated at 4C overnight, washed 3 times with 0.1%tween in 1x PBS 3 times. Secondary antibodies (Invitrogen, 1:750) were added and incubated for 1hr at RT, followed by 3 washes with 1X PBS+ 0.1% tween. The cells were mounted with Prolong diamond antifade mountant (Thermofisher) and imaged.
Proximity Ligation Assay in iPSC midbrain neurons
Duolink® In Situ Red Starter Kit Mouse/Rabbit (Sigma, cat# DUO92101) was used according to manufacturer’s protocol with minor changes. iPS-derived neuronal cultures on coverslips were fixed at day 90, permeabilized as described above. The cells were blocked in Duolink blocking solution and incubated with primary antibodies rabbit anti-SFPQ (Bethyl labs,1:200) / anti-NONO(Bethyl labs,1:200) with mouse anti-synuclein syn211 (Sigma,1:100) in antibody diluent overnight at 4C. For negative control, one of the primary antibody was omitted.The next day 1X Wash buffer A and B were equilibrated to RT for all subsequent wash steps. Cells were washed with Wash A and the anti-mouse minus and anti-rabbit plus PLA probes were added and incubated for 1 hour at 37 °C. Ligation was carried out for 45min and amplification reaction for 2hrs with wash steps and dilutions according to manufacturer’s protocol. After the final wash, the cells were mounted using Duolink DAPI mountant and imaged using confocal microscope.
Immunohistochemistry
Paraffin-embedded tissue sections on slides from human frontal cortex of healthy control and dementia with Lewy bodies (DLB) patients were de-paraffinized using Histoclear II (101412–882, National Diagnostics), 3 x times immersed in coplin jars for 10min each. The slides were then immersed in descending ethanol concentration: 100% ethanol. 3 x times 5min; 95% ethanol 2 x times 3min; 70% ethanol 1 × 2min and allowed to sit in deionized water. For SFPQ/NONO staining, the sections were treated with 88% formic acid for 1 min and washed with water for 5min. Antigen retrieval was performed by placing the slides in decloaking solution (Biocare medical, # CB910M) and placed in pressure cooker for 20min, 22psi. The sections were removed and washed in running tap water for 5min and allowed to sit in deionized water for 2min. The tissue sections were immersed in blocking solution of 0.3% tritonX-100 in PBS with 3% BSA for 30min, RT. Primary antibodies NONO 488-conjugated mouse antibody dilution 1:100 (Santacruz biotechnology, #sc-166702 AF488), SFPQ rabbit polyclonal antibody dilution 1:200 (Bethyl labs, # A301320A ), were added in blocking solution and incubated overnight at 4 °C. The next day sections were washed 3 times in TBS-tween for 5min each and secondary goat Alexa fluor antibodies (ThermoFisher) were added in blocking buffer dilution 1:100 for 30min at RT. The sections were washed as before and mounted onto slides with DAPI Fluoromount –G for image acquisition on a Leica confocal microscope.
For NONO nuclear spot count analysis, Z-Stacked images of Z-step size 1um were captured using Leica confocal microscope (CTR4000 / DMI4000B). The maximum intensity Z projection of 6 slices were used for analysis. In figure 1E, the spots were counted by Imaris Software by quantifying the number of puncta per nuclei, then calculating an average per individual brain sample. A total of 4 controls and 4 DLB brains were analyzed. For Figure S3E, the images were analyzed using MATLAB2021b through the Airlocalize software 112 as a separate validation method. Briefly, the max intensity projection images for each condition were opened through Airlocalize and the spots to be detected were sampled randomly for a local Gaussian fit. The detection threshold value = 45 was set for positive spot signal. The spots outside the nucleus were excluded and average integrated intensity was used for analysis. No differences between male and female subjects were observed.
Human brain nuclear inclusion analysis
Images acquired by confocal microscopy with 1um Z-step size were imported into Imaris 9.9 and analyzed using the cell module with the DAPI channel as cell and NONO/SFPQ inclusions as two vesicle types with respective channels. The Cell Background Subtraction Width was set to 5–7.00 μm and estimated vesicle diameter to 0.7 μm. Non-nuclear objects were manually discarded. The Vesicle Background Subtraction and enable region growing for each vesicle type was selected and the filter quality above automatic threshold was applied. The intensity mean was manually thresholded for each channel and respective vesicle type. Vesicles outside nucleus were manually discarded and only vesicles within the nucleus were analyzed. The creation parameters were saved for batch process to apply for different control and DLB groups. The statistic attributes were exported through vantage plot and calculated vesicle diameter for each vesicle type per image or field of view was used for plotting the graph. No differences between male and female subjects were observed.
RNA-Fluorescent in situ hybridization (FISH)
For iPSc-neurons at day 90, the cells on coverslips were fixed in 4% PFA in PBS for 20min at RT. The PFA was quenched for 10min by adding 125mM Glycine with 10mM Ribonucleoside Vanadyl Complex (RVC) (New England Biolabs, # S1402S). The cells were washed 3 times in PBS with 2mM RVC and permeabilized with 0.1% triton in RNAse free water with 2mM RVC for 15min at RT. Stellaris RNA-FISH reagents (LGC biosearch technologies) were used to perform the polyA+ RNA-FISH. Briefly, the permeabilized cells were then incubated with wash buffer A with 10% formamide for 30min at RT. For hybridization and detection of polyA+ RNA, a custom Stellaris biotin conjugated oligo(dt)30 probe was used at 125nM final concentration, 37 °C, overnight in a humidified chamber. Next day, the cells were washed with washed buffer A with 10% formamide twice for 30min each, followed by wash buffer B for 5min.The cells were then blocked in 2%BSA in PBS with 2mM RVC for 30min RT and streptavidin secondary antibody in block solution (ThermoFisher) was added for 1hr at RT. The cells were washed with 0.1% PBS-tween 3 times and mounted with DAPI Fluoromount –G for image acquisition on a confocal microscope (Z-step of 0.5um). Images were than imported to ImageJ for analysis and converted into binary image and intermodes thresholded to count for polyA+ nuclei.
For analysis of mRNA inclusions of CADPS and CYFIP2, images from 3-to-4 separate culture wells were analyzed for the number of cells per field of view that contained total puncta as well as puncta of >1um in diameter and normalized to DAPI signal. Data were plotted as both individual cells containing nuclear mRNA puncta (of all sizes) and an average of the cells that only contained the large puncta (defined as >1um in diameter).
For polyA+ RNA FISH after ADAR3 overexpression, iPSn at day 90 were infected with lentiviral particles as described under “Lentiviral transduction” at MOI3, and harvested at dpi 30.
For RNA-FISH with IHC in human frontal cortex tissue paraffin sections, the tissue was deparaffinized and subjected to antigen retrieval as described above (formic acid treatment was excluded). The tissue sections were then permeabilized in 0.3% TritonX-100 in PBS with 10mM RVC for 15min RT, washed 5min in 1X PBS +RVC. The tissue sections were then blocked for biotin using endogenous biotin blocking kit (ThermoFisher, # E21390) and subjected to stellaris RNA-FISH protocol as mentioned above using biotin conjugated-oligo(dt)25 probe for overnight hybridization. The next day, the sections were washed with wash buffer A and B as before, followed by incubation of primary antibody anti-NONO rabbit polyclonal dilution 1:200 (bethyl labs), anti-NeuN mouse monoclonal dilution 1:1000 (EMD Millipore, # ABN91) and streptavidin secondary for oligo-dt RNA probe in PBS with 0.3% with 3% BSA at 4°C overnight. The next morning, tissue sections washed 3 times with 0.1% PBS-tween for 5min and goat secondary Alexa fluor antibodies (ThermoFisher) were added for 30min at RT. The sections were washed as before and mounted with DAPI Fluoromount –G for imaging using a Leica confocal microscope. The colocalization analysis for NONO and polyA+ RNA was done using coloc2 plugin in ImageJ software on NeuN positive nuclei using the triangle threshold. No differences between male and female subjects were observed.
Synaptic Puncta Colocalization Analysis
Immunocytochemistry of day 120 iPSC-derived mid-brain neuronal cultures were performed as described above (cultures were treated with 8-aza for 30 days, starting at day 90). Primary antibodies for pre-synaptic marker mouse monoclonal Synaptophysin-1 (Synaptic systems #101 011) dilution 1:250, post-synaptic marker rabbit polyclonal PSD-95 (Synaptic systems #342 403) dilution 1:250 and chicken polyclonal neuronal specific-beta III Tubulin (Abcam #ab41489) dilution 1:500 was used. Z-stacked Images with step-size of 0.5um were acquired using the Leica confocal microscope (CTR4000 / DMI4000B). The standard deviation Z-projection of 8 slices was used for image analysis using the Synapse Counter plugin in ImageJ software113. Images were converted into binary with default setting in ImageJ software for analysis. The colocalization analysis of pre- and post- synaptic puncta was based on previously experimentally determined synaptic puncta size4.To set the puncta size, the default parameters of minimum = 10 px2; maximum=400 px2 were used for pre-synaptic and post-synaptic compartment size. The rolling ball radius=5 was set for local background subtraction of the puncta. The total number of puncta for each compartment, and colocalized pre- and post-synaptic puncta was obtained through the output window. The colocalized puncta is automatically defined as pre- and post-synaptic compartments having overlap of 33–100%. The number of synaptophysin-PSD95 colocalized positive puncta was normalized to total synaptophysin puncta number per image and represented as fold change.
IV. PHARMACOLOGICAL TREATMENT OF CULTURES
Treatment of iPSC-neurons with ADAR inhibitor 8-Aza-adenonsine.
IPSC-neurons were treated from day 60 or day 90 in culture for 30 days with vehicle (DMSO) or 200nM of ADAR inhibitor 8-aza-adenosine (8-aza) (Tocris, #6868). The vehicle or 8-aza was diluted in neurobasal media with sm-1 supplement and added onto cultures three times per week until the day of harvest for use in downstream assays.
To test 8-aza in HEK cells, we utilized an RFP – editing site linker-GFP reporter construct, a gift from Dr. Michael F. Jantsch (University of Vienna)95. The stem-loop linker belonging to established editing substrate glutamate receptor B (GluR-B) contains the edit site encoding for stop codon (UAG). In the presence of A-I editing, the stop codon (UAG) is converted into W (UGG) leading to downstream GFP being transcribed. The ratio of GFP / RFP provides a measure of A-I editing activity in transfected cells. HEK cells were transfected with 250ng of reporter plasmid using Xtreme gene HP DNA transfection reagent (Sigma, #6366236001). The cells were then treated with DMSO, 50nM, 100nM and 250nM 8-aza for 5 days. The cells were fixed and imaged using Leica confocal microscope (CTR4000 / DMI4000B).
V. LENTIVIRAL TRANSDUCTION
Plasmid and lentiviral particle preparation
For ADAR hyperactive mutants ADAR1 E713Q and ADAR2 E488Q the CDS were custom cloned into pLV lentiviral backbone (VectorBuilder) under synapsin promoter. Flag-tag-ADAR3 was cloned into pER4 lentivector at BmtI/PciI site under the PGK promoter. The lentiviral vectors were then packaged as previously described (Cuddy et.al 2019). The lentivirus was concentrated using LentiX concentrator (Takarabio) and titered with HiV1-p24 ELISA kit (Zeptometrix). The 2135 line of iPSC-neurons (day 60) were transduced with hyperactive ADARS at MOI 3 at assay timepoint and harvested 14 days post infection for western blot analysis. For ADAR3 overexpression in A53T iPSn, cultures were infected at MOI3 and harvested at 30 days post infection for ADAR3 oligo-dT FISH assay and CYFIP2 A-to-I RNA editing by Sanger sequencing.
VI. COMPUTATIONAL ANALYSES
Computational analysis of RNA secondary structure
A 1045 bp region of the 3’UTR of PRKAR2A obtained from NM_00457.4 (starting position 2562; corresponding to Chr3+ : 48749472 – 48750516) was analyzed by RNA fold (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi ) 114. Results from the MEF and Centroid secondary structures were used to determine structural changes in the 5 hyper-edited sites identified from RNA-seq. Each adenosine identified to be hyper-edited in PD iPSn by RNA-seq was changed to inosine either individually or in combination, and used as the input for RNAfold. Only constructs where site 2 (position 2656 of NM_00457.4) was converted to inosine resulted in any predicted changes in structure. The Centroid structures are shown in Figure S8B. The same procedure was used to analyze the 31bp oligonucleotide region, which starts at position 2650 in NM_00457.4. Structural alterations were confirmed by gel electrophoresis and RNAse protection assays.
Gene Ontology and Enrichment analysis of Proteomics and RNA-seq data
The data from proteomics was ranked according to fold change and significance (FDR q<0.05) and imported into g:Profiler (Raudvere et al., 2019) as ordered query. The significance (p<0.05) and threshold parameters were set as default setting. The terms having less than 4 genes or more than 500, redundant parent terms were discarded. The reactome pathway and gene ontology (GO) terms were exported as .gem files. The enrichment map was generated using cytoscape (v3.9.0) and Enrichment map plugin (Merico et al.,2010). The size of the node indicates the number of proteins, color represents the p-value and the name around the clusters represents the major pathway.
Computational analysis for solubility correlations
The proteins with significant solubility changes from day 90 A53T iPSn proteomics data set was used for studying physicochemical and other intrinsic parameters. The canonical FASTA protein sequences were obtained as bulk through Retrieve/ID mapping feature on uniprot database (https://www.uniprot.org/uploadlists/). The PLAAC database was used to study prion-like amino acid composition (http://plaac.wi.mit.edu/) using the default parameters65. The output LLR (log-likelihood ratio) was used for analysis. For supersaturation analysis, previously published folded and unfolded supersaturation score were used and plotted against solubility115. Secondary structure parameters of percent coiled-coiled, alpha-helix, beta-sheet and intrinsic disorder regions were analyzed through the PASTA 2.0 database (http://old.protein.bio.unipd.it/pasta2/)116. Basic intrinsic properties such as isoelectric point, charge and aliphatic index were calculated using the Protean 3D software (https://www.dnastar.com/software/lasergene/protean-3d/). Simple linear regression analysis was performed between the above parameters and decreased solubility to study correlations using GraphPad Prism software.
VII. RNA-SEQ AND A-TO-I EDITING ANALYSIS.
Detection of A-to-I editing using RNA-seq and Whole genome sequencing
Total RNA and genomic DNA from day 60 iPS neurons were extracted using Purelink RNA mini kit and Purelink genomic extraction kit (Thermofisher). The Quality control check, library preparation and sequencing were performed using services provided by Novogene Co, Ltd. (https://www.novogene.com/us-en/). For A-to-I editing RNA-seq, total RNA of all samples was analyzed using bioanalyzer and 1.5–2 ug of total RNA with RIN score 9.7 −10 was used for downstream polyA+ mRNA library preparation and sequencing. Deep sequencing was performed using Human mRNA Sequencing (WOBI) service (Novogene) to obtain 300M reads PE150 per sample with Novaseq 6000 platform. The whole genome sequencing (WGS) of A53T iPSn and isogenic control was performed using Human Whole Genome Sequencing (WOBI) service (Novogene) to obtain PE150 using Novaseq 6000 platform. The subsequent analysis was performed in-house as described below.
Whole-genome sequencing variant calling
Quality control (QC) for whole-genome sequencing (WGS) reads was performed using FASTQC v.0.11.5 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc). Read alignment to the human reference genome build hg38 was done using the Burrows-Wheeler Aligner mem option (BWA v.0.7.17) 117. Germline variants were called using the Genome Analysis ToolKit best practices pipeline version 4.1.8 118. Briefly, aligned reads in bam format were marked for duplicates, sorted by coordinates and recalibrated using the base quality scores (BQSR). Next, the HaplotypeCaller tool was used to call variants in gVCF format for each chromosome separately, then BCFTools v.1.10.0 119 concat option was used to concatenate each gVCF into a single file containing all chromosomes. GenomicsDBimport and GenotypeFromDB were used to merge sample-wise gVCFs and call raw variants, respectively. Hard filtering and Variant Quality Score Recalibration (VQSR) were used to filter bad quality variants. Post-GATK QC’s were done using BCFTools v.1.10.0, removing variants without PASS in the FILTER field, with read depth (DP) < 10, genotype quality (GQ) < 20, indel left-normalization, and multiallelic splitting.
RNA-Seq variant calling
RNAseq reads were similarly analyzed for QC using FASTQC v.0.11.5 as WGS reads. We followed GATK directions using the RNAseq short variant discovery pipeline (https://gatk.broadinstitute.org/hc/en-us/articles/360035531192-RNAseq-short-variant-discovery-SNPs-Indels-). Briefly, exon junction-aware alignment to the human reference genome build hg38 was performed using the STAR v.2.7.5 120 with the 2-pass mode, followed by mark duplicates, splitting reads with N cigar markers, and base quality recalibration. Sample-wise VCFs were generated using HaplotypeCaller, including non-variant sites to compare genotypes across samples. Variant filtering was done with RNAseq specific settings to obtain good quality variants. Sample-wise filtered VCFs were merged using BCFTools v.1.10.0 merge command.
Detection of RNA A-to-I editing sites
VCF files from WGS and RNASeq variant calling were merged using BCFTools v.1.10.0 merge command. Custom text editing scripts were made (available at Github, DOI: 10.5281/zenodo.11061692) to select the following variants from RNASeq per sample: i) The canonical editing from A to G in the forward strand; and ii) T to C in the reverse strand. For (i), we compared genotypes of each site to the genotypes obtained in the WGS variant call set, and we kept variants if there were homozygous references (0/0 for A/A or T/T). We also selected sites if the WGS genotypes were homozygous alternatives (1/1 for A/A or T/T), and the RNAseq genotypes were either heterozygous or homozygous containing the edited bases G or C. The set of candidate editing sites were annotated with ANNOVAR 121, including variant localization (exonic, intronic, intergenic, etc.) and distance to nearby genes. The A-I RNA editing database REDI 122 was used to annotate the candidate editing sites with known A-I changes, including overlap with Alu and repeat regions. Read abundance ratio for editing sites was performed on each sample taking the alternative allele depth divided by the site allele depth. Two-tailed t-tests were done using the t.test function in R, to statistically compare the read abundance ratios between the mutated (M) and corrected (C) samples. Multiple testing correction was done using a false-discovery rate of < 0.05, using the Benjamini-Hochberg method.
A-to-I RNA editing heatmap analysis
Differentially expressed A-I edit sites from RNAseq of day 60 neurons were imported into Morpheus analysis tool (https://software.broadinstitute.org/morpheus/) in a form of excel spreadsheet matrix with editing values for each genotype as data matrix, gene names as row annotations and location/transcript type of edit sites as column annotations. The dendrogram was generated using hierarchical clustering of column annotations. The heatmap was saved and exported as .png or .pdf file.
QUANTIFICATION AND STATISTICAL ANALYSIS
Quantification methods of western blots and images have been described above. In each quantification, a single plot point indicates a separate biological replicate (individual culture well), taken from at least two distinct iPSC passages / differentiation batches. The value of n and what n represents is indicated in each figure legend. Analyzed data was plotted and tested for statistical significance using the GraphPad Prism software. Statistical significance between two samples was determined using a paired or unpaired t-test. For more than two conditions, significance was determined using a one-way ANOVA with Tukey’s multiple comparison test. A p-value of <0.05 was considered to be significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). For each quantification, the type of error bar used and statistical test is specified in the figure legends. For RNA-seq, Two-tailed t-tests were done using the t.test function in R, to statistically compare the read abundance ratios between the mutated (M) and corrected (C) samples. Multiple testing correction was done using a false-discovery rate of < 0.05, using the Benjamini-Hochberg method.
Supplementary Material
List of significant and non-significant changes in protein solubility at 90 A53T iPSn. Values plotted in figure 1A are indicated in the gray shaded columns G and I.
Proteins with decreasing solubility analyze by GO terms Biological process (Tab A) or Reactome (Tab B).
Proteins with decreasing solubility were analyzed for Prion-like domains, supersaturation, amyloid aggregation propensity, and hydrophobicity.
Patient information for samples analyzed by NONO, SFPQ, FUS, and TDP-43 western blots.
Patient information and raw integrated values for western blot.
Patient information and raw quantification values of CYFIP2 RNA editing in brain samples.
Gene name, chromosome location, repeat type, gene location, and edited transcript (% of total transcripts) are shown.
Edited transcripts that are increased in A53T iPSn by <10% were analyzed by GO and shown in their respective categories. Tab A) Cell Component, Tab B) Reactome.
Total protein levels were analyzed by combining both soluble and insoluble fractions of day 60 and day 90 samples. Tab A) Decreased proteins in A53T iPSn at day 60, Tab B) Increased proteins in A53T iPSn at day 60, Tab C) Decreased proteins in A53T iPSn at day 90, Tab D) Increased proteins in A53T iPSn at day 90.
GO analysis showing individual decreased proteins in each Reactome category.
GO analysis showing individual decreased proteins in each Reactome category
GO analysis showing individual decreased proteins in each Cell Component category
Table S13. List of Oligonucleotides used in this study, linked to Figures 4 and 8 and STAR Methods. Sequences of oligonucleotides used in this study.
Highlights.
Insoluble NONO and SFPQ inclusions accumulate in the nuclei of PD/DLB patient neurons
Elevated A-to-I RNA editing occurs by reduced transcription of editing inhibitor ADAR3
NONO/SFPQ inclusions sequester edited RNAs encoding axon/synapse/mitochondria proteins
Aberrantly edited RNAs potentiate SFPQ aggregation, resulting in a pathogenic cycle
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke and the National Institute On Aging of the National Institutes of Health under Award Number R01NS118824 and R21NS107768 to JRM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Proteomics services were performed by the Northwestern Proteomics Core Facility, generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center, instrumentation award (S10OD025194) from NIH Office of Director, and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569. We thank the Northwestern Center for Cognitive Neurology and Alzheimer’s Disease Center (CNADC) (P30 grant P30AG013854) and The Brain Bank for Neurodegenerative Disorders at Mayo Clinic, directed by Dr Dennis W. Dickson and curated by Dr Michael DeTure with support from Mayo Clinic, Rainwater Charitable Foundation, Mangurian Foundation, State of Florida Alzheimer’s Disease Initiative, and NIH grants P30 AG062677 and P01 AG003949. We thank Willayat Y. Wani, Naomi Murata, and John Palucki for technical support.
Footnotes
Declaration of Interests
The Authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
All data required for re-analysis will be provided by the corresponding author, JRM, upon request.
References
- 1.Lansbury PT, and Lashuel HA (2006). A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 443, 774–779. 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, and Goedert M. (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839–840. 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, and Braak E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, and Ihara Y. (1988). Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol 75, 345–353. 10.1007/BF00687787. [DOI] [PubMed] [Google Scholar]
- 5.Goldman JE, Yen SH, Chiu FC, and Peress NS (1983). Lewy bodies of Parkinson’s disease contain neurofilament antigens. Science 221, 1082–1084. 10.1126/science.6308771. [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 7.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. (2003). alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841. 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Sandmann-Keil D, Gai W, and Braak E. (1999). Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett 265, 67–69. 10.1016/s0304-3940(99)00208-6. [DOI] [PubMed] [Google Scholar]
- 9.Duda JE, Giasson BI, Mabon ME, Lee VM, and Trojanowski JQ (2002). Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol 52, 205–210. 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 10.Tanji K, Mori F, Mimura J, Itoh K, Kakita A, Takahashi H, and Wakabayashi K. (2010). Proteinase K-resistant alpha-synuclein is deposited in presynapses in human Lewy body disease and A53T alpha-synuclein transgenic mice. Acta Neuropathol 120, 145–154. 10.1007/s00401-010-0676-z. [DOI] [PubMed] [Google Scholar]
- 11.Lin L, Lesnick TG, Maraganore DM, and Isacson O. (2009). Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends Neurosci 32, 142–149. 10.1016/j.tins.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soukup SF, Vanhauwaert R, and Verstreken P. (2018). Parkinson’s disease: convergence on synaptic homeostasis. EMBO J 37. 10.15252/embj.201898960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HC, Ulane CM, and Burke RE (2010). Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67, 715–725. 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia R, Sabir MS, Bandres-Ciga S, Saez-Atienzar S, Reynolds RH, Gustavsson E, Walton RL, Ahmed S, Viollet C, Ding J, et al. (2021). Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet 53, 294–303. 10.1038/s41588-021-00785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, International Parkinson’s Disease Genomics, C., andMe Research, T., Kerchner GA, Ayalon G, et al. (2017). A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 49, 1511–1516. 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein AD, and Mazzulli JR (2018). Is Parkinson’s disease a lysosomal disorder? Brain 141, 2255–2262. 10.1093/brain/awy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, International Parkinson’s Disease Genomics, C., Heutink P, and Shulman JM (2017). Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 140, 3191–3203. 10.1093/brain/awx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, et al. (2009). Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361, 1651–1661. 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojkovska I, Wani WY, Zunke F, Belur NR, Pavlenko EA, Mwenda N, Sharma K, Francelle L, and Mazzulli JR (2022). Rescue of alpha-synuclein aggregation in Parkinson’s patient neurons by synergistic enhancement of ER proteostasis and protein trafficking. Neuron 110, 436–451 e411. 10.1016/j.neuron.2021.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313, 324–328. 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, and Krainc D. (2011). Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52. 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, and Sulzer D. (2004). Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295. 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 23.Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, et al. (2013). Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science 342, 983–987. 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzulli JR, Zunke F, Isacson O, Studer L, and Krainc D. (2016). alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A 113, 1931–1936. 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiti F, Stefani M, Taddei N, Ramponi G, and Dobson CM (2003). Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424, 805–808. 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 26.Ciryam P, Kundra R, Morimoto RI, Dobson CM, and Vendruscolo M. (2015). Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol Sci 36, 72–77. 10.1016/j.tips.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox AH, Nakagawa S, Hirose T, and Bond CS (2018). Paraspeckles: Where Long Noncoding RNA Meets Phase Separation. Trends Biochem Sci 43, 124–135. 10.1016/j.tibs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Bond CS, and Fox AH (2009). Paraspeckles: nuclear bodies built on long noncoding RNA. J Cell Biol 186, 637–644. 10.1083/jcb.200906113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, and Carmichael GG (2001). The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell 106, 465–475. 10.1016/s0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 30.West JA, Mito M, Kurosaka S, Takumi T, Tanegashima C, Chujo T, Yanaka K, Kingston RE, Hirose T, Bond C, et al. (2016). Structural, super-resolution microscopy analysis of paraspeckle nuclear body organization. J Cell Biol 214, 817–830. 10.1083/jcb.201601071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souquere S, Beauclair G, Harper F, Fox A, and Pierron G. (2010). Highly ordered spatial organization of the structural long noncoding NEAT1 RNAs within paraspeckle nuclear bodies. Mol Biol Cell 21, 4020–4027. 10.1091/mbc.E10-08-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, and Lamond AI (2002). Paraspeckles: a novel nuclear domain. Curr Biol 12, 13–25. 10.1016/s0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 33.Knott GJ, Bond CS, and Fox AH (2016). The DBHS proteins SFPQ, NONO and PSPC1: a multipurpose molecular scaffold. Nucleic Acids Res 44, 3989–4004. 10.1093/nar/gkw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishikura K. (2016). A-to-I editing of coding and non-coding RNAs by ADARs. Nat Rev Mol Cell Biol 17, 83–96. 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner RW, Smith JE, Cooperman BS, and Nishikura K. (1989). A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci U S A 86, 2647–2651. 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Athanasiadis A, Rich A, and Maas S. (2004). Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol 2, e391. 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, and Spector DL (2005). Regulating gene expression through RNA nuclear retention. Cell 123, 249–263. 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Chen LL, DeCerbo JN, and Carmichael GG (2008). Alu element-mediated gene silencing. EMBO J 27, 1694–1705. 10.1038/emboj.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LL, and Carmichael GG (2009). Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell 35, 467–478. 10.1016/j.molcel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elbarbary RA, Li W, Tian B, and Maquat LE (2013). STAU1 binding 3’ UTR IRAlus complements nuclear retention to protect cells from PKR-mediated translational shutdown. Genes Dev 27, 1495–1510. 10.1101/gad.220962.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu SB, Xiang JF, Li X, Xu Y, Xue W, Huang M, Wong CC, Sagum CA, Bedford MT, Yang L, et al. (2015). Protein arginine methyltransferase CARM1 attenuates the paraspeckle-mediated nuclear retention of mRNAs containing IRAlus. Genes Dev 29, 630–645. 10.1101/gad.257048.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirose T, Virnicchi G, Tanigawa A, Naganuma T, Li R, Kimura H, Yokoi T, Nakagawa S, Benard M, Fox AH, and Pierron G. (2014). NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol Biol Cell 25, 169–183. 10.1091/mbc.E13-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee M, Sadowska A, Bekere I, Ho D, Gully BS, Lu Y, Iyer KS, Trewhella J, Fox AH, and Bond CS (2015). The structure of human SFPQ reveals a coiled-coil mediated polymer essential for functional aggregation in gene regulation. Nucleic Acids Res 43, 3826–3840. 10.1093/nar/gkv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CX, Cho DS, Wang Q, Lai F, Carter KC, and Nishikura K. (2000). A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 6, 755–767. 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oakes E, Anderson A, Cohen-Gadol A, and Hundley HA (2017). Adenosine Deaminase That Acts on RNA 3 (ADAR3) Binding to Glutamate Receptor Subunit B Pre-mRNA Inhibits RNA Editing in Glioblastoma. J Biol Chem 292, 4326–4335. 10.1074/jbc.M117.779868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan MH, Li Q, Shanmugam R, Piskol R, Kohler J, Young AN, Liu KI, Zhang R, Ramaswami G, Ariyoshi K, et al. (2017). Dynamic landscape and regulation of RNA editing in mammals. Nature 550, 249–254. 10.1038/nature24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, You Q, Lyu R, Qian Y, Tao H, Zhang F, Cai Y, Jiang N, Zheng N, Chen D, and Wu Z. (2023). Folate metabolism negatively regulates OAS-mediated antiviral innate immunity via ADAR3/endogenous dsRNA pathway. Metabolism 143, 155526. 10.1016/j.metabol.2023.155526. [DOI] [PubMed] [Google Scholar]
- 48.Li JB, and Church GM (2013). Deciphering the functions and regulation of brain-enriched A-to-I RNA editing. Nat Neurosci 16, 1518–1522. 10.1038/nn.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang T, Park CK, Leung AK, Gao Y, Hyde TM, Kleinman JE, Rajpurohit A, Tao R, Shin JH, and Weinberger DR (2016). Dynamic regulation of RNA editing in human brain development and disease. Nat Neurosci 19, 1093–1099. 10.1038/nn.4337. [DOI] [PubMed] [Google Scholar]
- 50.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, and Seeburg PH (2000). Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406, 78–81. 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 51.Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, and Sprengel R. (1995). Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science 270, 1677–1680. 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 52.Palladino MJ, Keegan LP, O’Connell MA, and Reenan RA (2000). A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell 102, 437–449. 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- 53.Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, and Church GM (2009). Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science 324, 1210–1213. 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- 54.Mladenova D, Barry G, Konen LM, Pineda SS, Guennewig B, Avesson L, Zinn R, Schonrock N, Bitar M, Jonkhout N, et al. (2018). Adar3 Is Involved in Learning and Memory in Mice. Front Neurosci 12, 243. 10.3389/fnins.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Wu H, Li J, Wang Z, Cai M, Liu X, and Liu G. (2023). Transcriptomic analysis reveals associations of blood-based A-to-I editing with Parkinson’s disease. J Neurol. 10.1007/s00415-023-12053-x. [DOI] [PubMed] [Google Scholar]
- 56.Pozdyshev DV, Zharikova AA, Medvedeva MV, and Muronetz VI (2021). Differential Analysis of A-to-I mRNA Edited Sites in Parkinson’s Disease. Genes (Basel) 13. 10.3390/genes13010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, and Mann M. (2002). Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1, 376–386. 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 58.Soldner F, Laganiere J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, et al. (2011). Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146, 318–331. 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway KA, Harper JD, and Lansbury PT (1998). Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4, 1318–1320. 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 60.Cuddy LK, Wani WY, Morella ML, Pitcairn C, Tsutsumi K, Fredriksen K, Justman CJ, Grammatopoulos TN, Belur NR, Zunke F, et al. (2019). Stress-Induced Cellular Clearance Is Mediated by the SNARE Protein ykt6 and Disrupted by alpha-Synuclein. Neuron 104, 869–884 e811. 10.1016/j.neuron.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cuddy LK, Wani WY, Morella ML, Pitcairn C, Tsutsumi K, Fredriksen K, Justman CJ, Grammatopoulos TN, Belur NR, Zunke F, et al. (2019). Stress-Induced Cellular Clearance Is Mediated by the SNARE Protein ykt6 and Disrupted by alpha-Synuclein. Neuron. 10.1016/j.neuron.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, et al. (2013). Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473. 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh I, Martin AJ, Di Domenico T, and Tosatto SC (2012). ESpritz: accurate and fast prediction of protein disorder. Bioinformatics 28, 503–509. 10.1093/bioinformatics/btr682. [DOI] [PubMed] [Google Scholar]
- 64.Fiumara F, Fioriti L, Kandel ER, and Hendrickson WA (2010). Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell 143, 1121–1135. 10.1016/j.cell.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lancaster AK, Nutter-Upham A, Lindquist S, and King OD (2014). PLAAC: a web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 30, 2501–2502. 10.1093/bioinformatics/btu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikai A. (1980). Thermostability and aliphatic index of globular proteins. J Biochem 88, 1895–1898. [PubMed] [Google Scholar]
- 67.Fahn S, and Cohen G. (1992). The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol 32, 804–812. 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 68.Sulzer D. (2007). Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci 30, 244–250. 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Mazzulli JR, Burbulla LF, Krainc D, and Ischiropoulos H. (2016). Detection of Free and Protein-Bound ortho-Quinones by Near-Infrared Fluorescence. Anal Chem 88, 2399–2405. 10.1021/acs.analchem.5b04420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, and Zecca L. (2000). Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A 97, 11869–11874. 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, and Iwatsubo T. (1998). Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152, 879–884. [PMC free article] [PubMed] [Google Scholar]
- 72.Maroteaux L, Campanelli JT, and Scheller RH (1988). Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8, 2804–2815. 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koss DJ, Erskine D, Porter A, Palmoski P, Menon H, Todd OGJ, Leite M, Attems J, and Outeiro TF (2022). Nuclear alpha-synuclein is present in the human brain and is modified in dementia with Lewy bodies. Acta Neuropathol Commun 10, 98. 10.1186/s40478-022-01403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, Weston LJ, Owen N, Weissman TA, Luna E, et al. (2019). Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci Rep 9, 10919. 10.1038/s41598-019-47227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hallacli E, Kayatekin C, Nazeen S, Wang XH, Sheinkopf Z, Sathyakumar S, Sarkar S, Jiang X, Dong X, Di Maio R, et al. (2022). The Parkinson’s disease protein alpha-synuclein is a modulator of processing bodies and mRNA stability. Cell 185, 2035–2056 e2033. 10.1016/j.cell.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, Toker NJ, Jeon S, Fredriksen K, and Mazzulli JR (2018). Reversible Conformational Conversion of alpha-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron 97, 92–107 e110. 10.1016/j.neuron.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, and Lee VM (2003). Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 300, 636–640. 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 78.Chen SW, Drakulic S, Deas E, Ouberai M, Aprile FA, Arranz R, Ness S, Roodveldt C, Guilliams T, De-Genst EJ, et al. (2015). Structural characterization of toxic oligomers that are kinetically trapped during alpha-synuclein fibril formation. Proc Natl Acad Sci U S A 112, E1994–2003. 10.1073/pnas.1421204112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Froula JM, Castellana-Cruz M, Anabtawi NM, Camino JD, Chen SW, Thrasher DR, Freire J, Yazdi AA, Fleming S, Dobson CM, et al. (2019). Defining alpha-synuclein species responsible for Parkinson’s disease phenotypes in mice. J Biol Chem 294, 10392–10406. 10.1074/jbc.RA119.007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, et al. (2012). Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell 149, 1048–1059. 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fusco G, Chen SW, Williamson PTF, Cascella R, Perni M, Jarvis JA, Cecchi C, Vendruscolo M, Chiti F, Cremades N, et al. (2017). Structural basis of membrane disruption and cellular toxicity by alpha-synuclein oligomers. Science 358, 1440–1443. 10.1126/science.aan6160. [DOI] [PubMed] [Google Scholar]
- 82.Kumar M, and Carmichael GG (1997). Nuclear antisense RNA induces extensive adenosine modifications and nuclear retention of target transcripts. Proc Natl Acad Sci U S A 94, 3542–3547. 10.1073/pnas.94.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, and Giangrande A. (2003). CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron 38, 887–898. 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- 84.Abekhoukh S, and Bardoni B. (2014). CYFIP family proteins between autism and intellectual disability: links with Fragile X syndrome. Front Cell Neurosci 8, 81. 10.3389/fncel.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jockusch WJ, Speidel D, Sigler A, Sorensen JB, Varoqueaux F, Rhee JS, and Brose N. (2007). CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131, 796–808. 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Nicholas A, de Magalhaes JP, Kraytsberg Y, Richfield EK, Levanon EY, and Khrapko K. (2010). Age-related gene-specific changes of A-to-I mRNA editing in the human brain. Mech Ageing Dev 131, 445–447. 10.1016/j.mad.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burke RE, and O’Malley K. (2013). Axon degeneration in Parkinson’s disease. Exp Neurol 246, 72–83. 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paz-Yaacov N, Levanon EY, Nevo E, Kinar Y, Harmelin A, Jacob-Hirsch J, Amariglio N, Eisenberg E, and Rechavi G. (2010). Adenosine-to-inosine RNA editing shapes transcriptome diversity in primates. Proc Natl Acad Sci U S A 107, 12174–12179. 10.1073/pnas.1006183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veliz EA, Easterwood LM, and Beal PA (2003). Substrate analogues for an RNA-editing adenosine deaminase: mechanistic investigation and inhibitor design. J Am Chem Soc 125, 10867–10876. 10.1021/ja029742d. [DOI] [PubMed] [Google Scholar]
- 90.Spremulli EN, Crabtree GW, Dexter DL, Chu SH, Farineau DM, Ghoda LY, McGowan DL, Diamond I, Parks RE Jr., and Calabresi P. (1982). Biochemical pharmacology and toxicology of 8-azaadenosine alone and in combination with 2’-deoxycoformycin (pentostatin). Biochem Pharmacol 31, 2415–2421. 10.1016/0006-2952(82)90538-x. [DOI] [PubMed] [Google Scholar]
- 91.Montgomery JA, Elliott RD, and Thomas HJ (1975). The synthesis and evaluation of azapurine nucleosides as cytotoxic agents. Ann N Y Acad Sci 255, 292–305. 10.1111/j.1749-6632.1975.tb29237.x. [DOI] [PubMed] [Google Scholar]
- 92.Bennett LL Jr., and Allan PW (1976). Metabolism and metabolic effects of 8-azainosine and 8-azaadenosine. Cancer Res 36, 3917–3923. [PubMed] [Google Scholar]
- 93.Zipeto MA, Court AC, Sadarangani A, Delos Santos NP, Balaian L, Chun HJ, Pineda G, Morris SR, Mason CN, Geron I, et al. (2016). ADAR1 Activation Drives Leukemia Stem Cell Self-Renewal by Impairing Let-7 Biogenesis. Cell Stem Cell 19, 177–191. 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramirez-Moya J, Baker AR, Slack FJ, and Santisteban P. (2020). ADAR1-mediated RNA editing is a novel oncogenic process in thyroid cancer and regulates miR-200 activity. Oncogene 39, 3738–3753. 10.1038/s41388-020-1248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garncarz W, Tariq A, Handl C, Pusch O, and Jantsch MF (2013). A high-throughput screen to identify enhancers of ADAR-mediated RNA-editing. RNA Biol 10, 192–204. 10.4161/rna.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Havel J, and Beal PA (2015). A Phenotypic Screen for Functional Mutants of Human Adenosine Deaminase Acting on RNA 1. ACS Chem Biol 10, 2512–2519. 10.1021/acschembio.5b00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, Gueroussov S, Albu M, Zheng H, Yang A, et al. (2013). A compendium of RNA-binding motifs for decoding gene regulation. Nature 499, 172–177. 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Copley KE, and Shorter J. (2022). Flying under the radar: TMEM106B(120–254) fibrils break out in diverse neurodegenerative disorders. Cell 185, 1290–1292. 10.1016/j.cell.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schweighauser M, Arseni D, Bacioglu M, Huang M, Lovestam S, Shi Y, Yang Y, Zhang W, Kotecha A, Garringer HJ, et al. (2022). Age-dependent formation of TMEM106B amyloid filaments in human brains. Nature 605, 310–314. 10.1038/s41586-022-04650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang YX, Cao Q, Sawaya MR, Abskharon R, Ge P, DeTure M, Dickson DW, Fu JY, Ogorzalek Loo RR, Loo JA, and Eisenberg DS (2022). Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. Nature 605, 304–309. 10.1038/s41586-022-04670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang A, Xiang X, Wang J, Lee C, Arakhamia T, Simjanoska M, Wang C, Carlomagno Y, Zhang G, Dhingra S, et al. (2022). Homotypic fibrillization of TMEM106B across diverse neurodegenerative diseases. Cell 185, 1346–1355 e1315. 10.1016/j.cell.2022.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rebagliati MR, and Melton DA (1987). Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell 48, 599–605. 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- 103.Stellos K, Gatsiou A, Stamatelopoulos K, Perisic Matic L, John D, Lunella FF, Jae N, Rossbach O, Amrhein C, Sigala F, et al. (2016). Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat Med 22, 1140–1150. 10.1038/nm.4172. [DOI] [PubMed] [Google Scholar]
- 104.Nishikura K. (2010). Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem 79, 321–349. 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dawson TM, Ko HS, and Dawson VL (2010). Genetic animal models of Parkinson’s disease. Neuron 66, 646–661. 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakagawa S, Naganuma T, Shioi G, and Hirose T. (2011). Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice. J Cell Biol 193, 31–39. 10.1083/jcb.201011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harrison AF, and Shorter J. (2017). RNA-binding proteins with prion-like domains in health and disease. Biochem J 474, 1417–1438. 10.1042/BCJ20160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cottrell KA, Torres LS, Dizon MG, and Weber JD (2021). 8-azaadenosine and 8-chloroadenosine are not selective inhibitors of ADAR. Cancer Res Commun 1, 56–64. 10.1158/2767-9764.crc-21-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sakurai M, Shiromoto Y, Ota H, Song C, Kossenkov AV, Wickramasinghe J, Showe LC, Skordalakes E, Tang HY, Speicher DW, and Nishikura K. (2017). ADAR1 controls apoptosis of stressed cells by inhibiting Staufen1-mediated mRNA decay. Nat Struct Mol Biol 24, 534–543. 10.1038/nsmb.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stojkovska I, and Mazzulli JR (2021). Detection of pathological alpha-synuclein aggregates in human iPSC-derived neurons and tissue. STAR Protoc 2, 100372. 10.1016/j.xpro.2021.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Polinski NK, Volpicelli-Daley LA, Sortwell CE, Luk KC, Cremades N, Gottler LM, Froula J, Duffy MF, Lee VMY, Martinez TN, and Dave KD (2018). Best Practices for Generating and Using Alpha-Synuclein Pre-Formed Fibrils to Model Parkinson’s Disease in Rodents. J Parkinsons Dis 8, 303–322. 10.3233/JPD-171248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lionnet T, Czaplinski K, Darzacq X, Shav-Tal Y, Wells AL, Chao JA, Park HY, de Turris V, Lopez-Jones M, and Singer RH (2011). A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat Methods 8, 165–170. 10.1038/nmeth.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dzyubenko E, Rozenberg A, Hermann DM, and Faissner A. (2016). Colocalization of synapse marker proteins evaluated by STED-microscopy reveals patterns of neuronal synapse distribution in vitro. J Neurosci Methods 273, 149–159. 10.1016/j.jneumeth.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Gruber AR, Lorenz R, Bernhart SH, Neubock R, and Hofacker IL (2008). The Vienna RNA websuite. Nucleic Acids Res 36, W70–74. 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, and Vendruscolo M. (2013). Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep 5, 781–790. 10.1016/j.celrep.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walsh I, Seno F, Tosatto SC, and Trovato A. (2014). PASTA 2.0: an improved server for protein aggregation prediction. Nucleic Acids Res 42, W301–307. 10.1093/nar/gku399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, and Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, and DePristo MA (2010). The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20, 1297–1303. 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, and Li H. (2021). Twelve years of SAMtools and BCFtools. Gigascience 10. 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang K, Li M, and Hakonarson H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38, e164. 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lo Giudice C, Tangaro MA, Pesole G, and Picardi E. (2020). Investigating RNA editing in deep transcriptome datasets with REDItools and REDIportal. Nat Protoc 15, 1098–1131. 10.1038/s41596-019-0279-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of significant and non-significant changes in protein solubility at 90 A53T iPSn. Values plotted in figure 1A are indicated in the gray shaded columns G and I.
Proteins with decreasing solubility analyze by GO terms Biological process (Tab A) or Reactome (Tab B).
Proteins with decreasing solubility were analyzed for Prion-like domains, supersaturation, amyloid aggregation propensity, and hydrophobicity.
Patient information for samples analyzed by NONO, SFPQ, FUS, and TDP-43 western blots.
Patient information and raw integrated values for western blot.
Patient information and raw quantification values of CYFIP2 RNA editing in brain samples.
Gene name, chromosome location, repeat type, gene location, and edited transcript (% of total transcripts) are shown.
Edited transcripts that are increased in A53T iPSn by <10% were analyzed by GO and shown in their respective categories. Tab A) Cell Component, Tab B) Reactome.
Total protein levels were analyzed by combining both soluble and insoluble fractions of day 60 and day 90 samples. Tab A) Decreased proteins in A53T iPSn at day 60, Tab B) Increased proteins in A53T iPSn at day 60, Tab C) Decreased proteins in A53T iPSn at day 90, Tab D) Increased proteins in A53T iPSn at day 90.
GO analysis showing individual decreased proteins in each Reactome category.
GO analysis showing individual decreased proteins in each Reactome category
GO analysis showing individual decreased proteins in each Cell Component category
Table S13. List of Oligonucleotides used in this study, linked to Figures 4 and 8 and STAR Methods. Sequences of oligonucleotides used in this study.
Data Availability Statement
- Data availability: All data reported in this paper will be shared by the lead contact upon request.
- Full length western blots are available at Mendeley (Mazzulli, Joseph (2024), “Full Western blots to accompany manuscript “Nuclear aggregates of NONO/SFPQ and A-to-I edited RNA in synucleinopathies””, Mendeley Data, V1, doi: 10.17632/pp9vnn666d.1)
- RAW files of day 60 and day 90 proteomics data. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD051673
- RAW files of RNA seq and whole genome sequencing data of A53T and isogenic corrected line are available at the Sequence Read Archive (SRA): ID: SUB14391987, BioProject ID: PRJNA1104694
Code: This paper reports original code used to identify A-to-I RNA edit sites, available at Gitbhub, https://github.com/bibb/Ato-I_pipeline_Neuron2024/tree/main, DOI: 10.5281/zenodo.11061692.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
All data required for re-analysis will be provided by the corresponding author, JRM, upon request.