SUMMARY
Electric fields affect the activity of neurons and brain circuits, yet how this happens at the cellular level remains enigmatic. Lack of understanding of how to stimulate the brain to promote or suppress specific activity significantly limits basic research and clinical applications. Here, we study how electric fields impact subthreshold and spiking properties of major cortical neuronal classes. We find that neurons in the rodent and human cortex exhibit strong, cell-class-dependent entrainment that depends on stimulation frequency. Excitatory pyramidal neurons, with their slower spike rate, entrain to both slow and fast electric fields, while inhibitory classes like Pvalb and Sst (with their fast spiking) predominantly phase-lock to fast fields. We show that this spike-field entrainment is the result of two effects: non-specific membrane polarization occurring across classes and class-specific excitability properties. Importantly, these properties are present across cortical areas and species. These findings allow for the design of selective and class-specific neuromodulation.
In brief
Lee et al. study the impact of oscillatory electric fields on neuronal activity across different cell types, brain regions, and species. Electric fields strongly entrain both cellular subthreshold responses and spike timing and phase, with entrainment properties being stimulation frequency and cell class specific across areas.
INTRODUCTION
Delivery of exogenous electric fields through stimulation devices to alter brain activity has a rich history in neuroscience. Since the early work by Fritsch and Hitzig mapping the brain region during surgery,1,2 extracellular electric stimulation (ES) has been used to study the functional connectivity, excitability, and response of the brain. In therapeutic interventions, ES alleviates symptoms of neurological disorders such as Parkinson’s disease,3–5 dystonia,6 depression,7–9 epilepsy,10,11 and others.12 However, there is still a lack of understanding at the basic cellular level of what happens when brain matter is electrically stimulated to promote or suppress certain activity patterns.13–17 This has limited the application of ES as a basic science tool and, importantly, for effective therapeutic intervention.
Brain circuits consist of multiple distinct and diverse cell classes, yet how ES affects these classes at a cellular level remains unknown. Most studies of ES focus on the excitatory glutamatergic neurons in the neocortex,17–19 hippocampus,20–23 and elsewhere.24–26 Although inhibitory GABAergic cells are less abundant, they are diverse and play a critical role in structuring and regulating brain circuit activity.27–31 Given such diversity, the extent to which cell classes may respond distinctly16,32 remains unclear. As a result, ES protocols applied in both animal models and humans do so without considering the remarkable cellular diversity comprising brain circuits.
Here, we study the direct impact of extracellular ES in two cortical brain regions, the visual cortex and CA1 hippocampus, using simultaneous intracellular (whole-cell patch-clamp) and extracellular recordings from identified single neurons of major excitatory and inhibitory cell classes. We observe strong, differential, and cell-class-specific spike-field entrainment. Excitatory neurons entrain already at slow ES frequencies (8 Hz), while inhibitory neurons only entrain for fast ES frequencies (30–140 Hz). We also show that the class-dependence of spike entrainment is not the result of a differential subthreshold (i.e., non-spiking) mechanism, as cortical neurons across classes, brain regions, and, in fact, species exhibit ubiquitous, robust, and frequency-independent membrane coupling to ES oscillations. We see similar entrainment properties in human excitatory cortical neurons suggesting widely present biophysical mechanisms in which the unique ion channel-composition of each cell class dictates class-specific entrainment to ES. The latter opens the door to the design of new ES waveforms with improved selectivity and control.
RESULTS
To simultaneously stimulate and record intracellular and extracellular signals close to identified neurons, we use a customized 8-pipette multi-patch brain slice electrophysiology setup to examine cellular and cell-class-specific effects of the ES-generated field. This system enables simultaneous extracellular stimulation, intracellular current injection, whole-cell patch-clamp recordings, and extracellular recordings within 50–120 μm of the ES site (Figure 1A). ES effects are assessed at the single compartment (soma) and membrane level, i.e., on the extracellular , intracellular somatic , and the resulting membrane voltage (, calculated as ) around the patch-clamped soma. To study the impact of sinusoidal ES (delivered via an extracellular pipette) on , , and of single neurons, we first estimated the electric field and resulting -deflection around the soma as a function of distance (Figures 1A–1C). The highest ES amplitude (200 nA) induced a amplitude of 1.1 ± 0.5 mV (mean ± SD) and an extracellular field of 22.6 ± 8.9 mV/mm (mean ± SD) close (approx. 15 μm) to the soma. The -distance relationship was captured by the point-source approximation for a purely resistive and homogeneous medium.19,33 We conclude that the ES amplitudes in our study are relatively weak (compared with the spiking threshold) and within the physiological range measured in rodents and humans in vivo.16,34
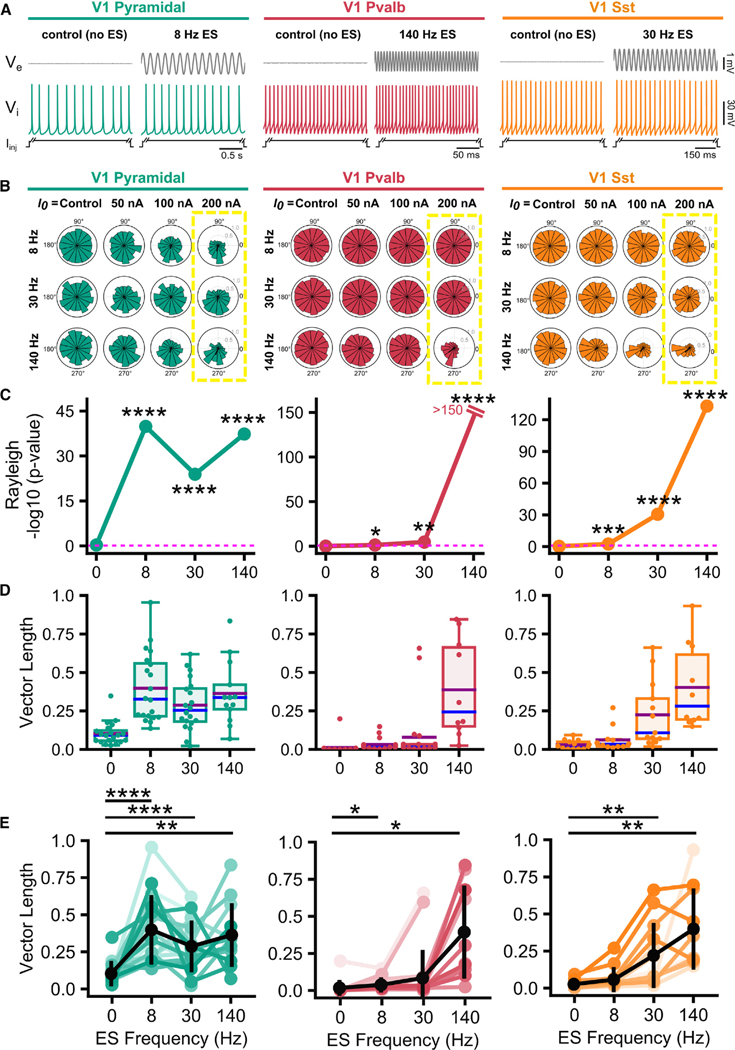
Figure 1. Cellular and cell-class-specific characterization of ES effects.
(A) Top: simultaneous ES, , and recordings from multiple electrodes near the recorded cell. Bottom: experiment in mouse cortical slice (yellow, extracellular ES electrode; green, patched soma; blue, intracellular electrode; red, extracellular electrodes recording ).
(B) amplitude as function of distance between ES and recording electrodes (ES: 25–200 nA at 8 Hz; circles: mean amplitude; error bars: SD) Trendlines: least-squares fit of the point-source approximation.
(C) and electric field amplitude elicited by ES at the extracellular electrode closest to the recorded soma (~15 μm). Blue, amplitude for each experiment (n = 59); black, mean and SD.
(D) Sample fluorescent images, cellular morphology, electrophysiology, and spike frequency vs. current input (f-I) curves from identified mouse neocortical cell classes (colored lines, individual neurons; black, median).
(E) Experiments in pyramidal, Pvalb, and Sst neurons during simultaneous ES (200 nA at 8, 30, and 140 Hz) and intracellular DC current injection to elicit spiking. Top, closest to the soma; bottom, of a spiking neuron responding to (5 s shown).
(F) Introduction of spike time analysis and quantification of spike-field entrainment.
(G) Top: polar plots of spike-phase distributions for a pyramidal, Pvalb, and Sst cell in the absence (control) and presence of ES (200 nA; 8, 140, and 30 Hz ES for pyramidal, Pvalb, and Sst cells, respectively). The population vector length (black line) and skewed spike-phase distribution reflects the degree of entrainment. Number of spikes (control, ES): Pyr (52, 56), Pvalb (702, 864), and Sst (216, 218).
We used acute slices of mouse primary visual cortex (V1), focusing on excitatory pyramidal cells and the two largest GABAergic cell classes in cortical layer 5 (L5), the parvalbumin (Pvalb)- and somatostatin (Sst)-expressing cells comprising approximately 54% and 25% of the L5 GABAergic cell population, respectively35 (Figure 1D). We used several modalities (fluorescent markers in transgenic animals, electrophysiology, and cellular morphology) to identify individual neurons from the three cell classes (Figure 1D). Excitatory pyramidal neurons were initially identified in transgenic mice (driven by L5-specific Tlx3 or Sim1 promoters) or by their pyramidal morphology, and confirmed by their characteristic regular-spiking firing and slower spike frequency vs. current input (f-I) curve in patch-clamp recordings.36 Inhibitory Pvalb and Sst neurons were identified using transgenic mice and confirmed by their fast-spiking firing and steep f-I curves36 (Figure 1D). The recorded cells were filled with biocytin, for post hoc processing to reveal their morphology and confirm classification.
In principle, even weak ES can impact the cellular membrane,19 but whether and how it does so for different cell classes remains speculative. To address this, we stimulated (via intracellular DC injection) and recorded from pyramidal, Pvalb, and Sst neurons while delivering sinusoidal ES 50 μm away from the soma, at amplitudes from 25 to 200 nA. Intracellular current up to 2-fold rheobase was injected to elicit spiking during control and ES (Table S1; 154.5 ± 64.9 pA for pyramidal neurons; 259.5 ± 99.8 pA for Pvalb; 191.5 ± 102.8 pA for Sst). The ES itself (Figures 1A–1C) never elicited action potentials in the absence of other intracellular inputs18,19 and did not alter the spike frequency (Figure S1). ES frequency was varied from 8 to 140 Hz, covering a range used in ES applications (Figure 1E). We mapped the spikes during ES to their respective spike phase along the sinusoid (reference: ; Figure 1F). Next, we assessed spike-field entrainment of the different cell classes via population vector analysis (Figure 1G). Increased spike-field entrainment due to the ES-induced field results in an inhomogeneous spike-phase distribution with a distinct, preferred spike phase and, therefore, a larger population vector. A weak ES effect on spike timing or decreased entrainment, in turn, is marked by a homogeneous spike-phase distribution and a small population vector length (e.g., control experiments where no ES is delivered; Figure 1G). We found a diversity in the entrainment profile of cortical cell classes that depends on ES parameters as well as cell class properties and activity (Figure 1G).
Rich, diverse, and cell-class-specific spike-field entrainment to ES
To understand the origin of differential electric field entrainment, we first looked at the influence of ES on the spiking cellular membrane. Hitherto, the assumption has been that ES has the same effect on the neural membrane across various classes as long as experimental parameters (distance of the ES electrode, the induced field, etc.) are controlled for. To test this, we conducted experiments where we analyzed the spiking activity of cells in the presence vs. absence of ES. To isolate the pure ES effect, experiments were conducted under synaptic blockers. To elicit spiking, we injected intracellular DC current into the soma (duration: 9 s). This was repeated under two conditions: in the absence of any ES (“control”) and then, the same intracellular current was injected while concurrently applying ES at various frequencies (, and 140 Hz) and amplitudes (25 to 200 nA) (Figure 2A).
Figure 2. Cell-class-specific entrainment of spiking to ES.
(A) Example and traces, with spiking induced by intracellular current injection during control (no ES) or sinusoidal ES application.
(B) Spike-phase distribution for V1 cell classes for varying ES parameters. Rows, ES frequency (top to bottom); columns, ES amplitude (left to right). Control experiments show a homogeneous spike-phase distribution, i.e., no inherently preferred spike phase, while increasing ES amplitude skews the spike-phase distribution, showing increasing spike-phase entrainment. Different ES frequencies exhibit a distinct, cell-class-specific effect on the spike-phase entrainment.
(C–E) Summary statistics for the highest ES amplitude (200 nA) in (B) (yellow boxes). (C) Spike entrainment to ES assessed by Rayleigh’s test, with values plotted as a function of ES frequency. Dashed pink line: p = 0.05. Pyramidal spiking shows statistically significant spike phase entrainment to slow (8 Hz), medium (30 Hz), and fast (140 Hz) ES frequencies, while Pvalb and Sst are most strongly entrained by high ES frequencies (140 Hz) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Table S2). (D) The population vector length plotted for each cell (circles) within each class. Purple and blue lines: mean and median values, respectively; whiskers: remaining distribution. (E) The population vector length for each cell (from D) across each ES frequency compared against control conditions to assess degree of entrainment (paired t test, false discovery rate [FDR]-corrected for multiple comparisons: *p < 0.05, **p < 0.01, ****p < 0.0001). Pyramidal: n = 21 cells (8 and 30 Hz), n = 13 (140 Hz); Pvalb: n = 22 (8 and 30 Hz), n = 12 (140 Hz); Sst: n = 13 for (8 and 30 Hz), n = 10 (140 Hz).
To what extent does ES impact spiking? Notably, ES did not alter the spike frequency elicited by intracellular DC injection (Figure S1). We next tested whether ES affected the temporal patterning of spikes. Because the imposed ES and the recorded in the proximity of the recorded cells is sinusoidal, spike times can be mapped to spike phases (Figures 1E and 1F; reference signal: closest to the recorded neuron; spike phase: 0°–360°). To test whether ES altered the temporal patterning of spike times, we looked at the spike-phase distribution as function of cell class and ES parameters using circular statistics.19,21 Specifically, the population vector length (range 0–1) and the Rayleigh test (null hypothesis: spike phases are uniformly distributed along the unit circle, exhibiting no preferred phase), serve as a metric of spike-phase coordination. Without ES, the lack of spike-phase coordination results in a uniform spike-phase distribution, small vector length, and a statistically insignificant value of the Rayleigh test. In contrast, the presence of spike-phase coordination due to ES gives rise to a non-uniform spike-phase distribution (with spike phases mapped based on the local signal dominated by the ES), large vector length, and a statistically significant value of the Rayleigh test. To control for spurious correlations, we devised the following control experiment: adopt the signal from the subsequent experiment where ES is present, map spike times to spike phases, and run the same statistics for this “virtual” ES experiment. As for spike-phase coordination due to ES, a time-locked but ES-independent mechanism at any of the tested ES frequencies will result in an inhomogeneous spike-phase distribution under control conditions.
We first looked at pyramidal neurons, where longer current injections lead to temporally irregular spiking.19,37 Indeed, for control experiments, homogeneous spike-phase distributions possess very small vector lengths, exhibiting no preference in terms of spike-phase (Figures 2B and 2C; Table S2). With increasing ES amplitude, spike-phase entrainment appears, as seen in decreasing values (Rayleigh test) and increasing vector lengths (Figures 2C–2E and S2; Table S2). Furthermore, spike-field entrainment of pyramidal neurons is ubiquitous: while pyramidal neurons exhibit preferred spike-field entrainment at 8 Hz ES, they also exhibit strong spike-field coordination even for fast ES frequencies above 100 Hz (Figures 2B and 2C).
We next looked at the spike-field entrainment of major cortical inhibitory cell classes. We ran similar suprathreshold experiments (control vs. ES) with Pvalb and Sst interneurons and performed identical analyses calculating the same entrainment metrics. In sharp contrast to pyramidal neurons, we found that both Pvalb and Sst entrain their spiking predominantly to high ES frequencies: Sst entrain for >30 Hz whereas Pvalb exhibit strong spike-field coordination for 140 Hz and much weaker coordination for slower ES of the same amplitude (Figures 2C–2E and S2; Table S2). Again, the spike-field entrainment observed in Pvalb and Sst is solely attributed to the ES: control experiments (no ES) show no coordination between spiking and any effect in a similar timescale to ES for any cell class. It is only when ES is imposed at higher frequencies for each inhibitory class that Pvalb and Sst spiking is entrained (Figures 2B and S2). Notably, even at the lowest ES amplitude of 25 nA, strong spike-field coordination occurs across their relevant entrainment frequencies for all classes tested (Figure S2). We conclude that while Pvalb and Sst interneurons exhibit strong spike-field entrainment to ES, this depends on the ES parameters, with cells exhibiting a preference for fast ES (>30 Hz for Sst and 140 Hz for Pvalb).
Surprised by the richness of the spike-field entrainment characteristics, we sought to reproduce our findings in another brain region. We repeated our experimental protocols in the hippocampus, recording from pyramidal and Pvalb neurons in CA1 (Figure S3). As with V1 neurons, we found that in CA1 neurons weak ES causes strong pyramidal spike-phase coordination, resulting in increasing spike-field entrainment that remains strong even for fast ES frequencies (>100 Hz; Figure S3). Hippocampal inhibitory Pvalb, on the other hand, like their V1 counterparts, exhibit a much more nonlinear spike-field entrainment profile with a clear preference for fast ES (Figures S3F–S3I; Table S3). Therefore, the rich and diverse patterns of spike-field entrainment to ES seen in V1 are also observed in hippocampal CA1. We conclude that ES spike-phase coordination is robust, brought about by common biophysical mechanisms shared across cortical areas.
Ubiquitous and robust subthreshold entrainment across cell classes
We asked whether ES impacts the non-spiking (subthreshold) membrane in a class-specific manner that then leads to the observed spike entrainment. We analyzed pyramidal, Pvalb, and Sst neurons by either holding them at their resting potential (Table S1) or at various subthreshold polarization levels and delivered sinusoidal ES of varying amplitude (25–200 nA) and frequency (1–100 Hz) 50 μm from the cell soma. To assess the effects of ES on the subthreshold neurons, we analyzed the resulting amplitude and phase deflections of , , and (Figure 3).
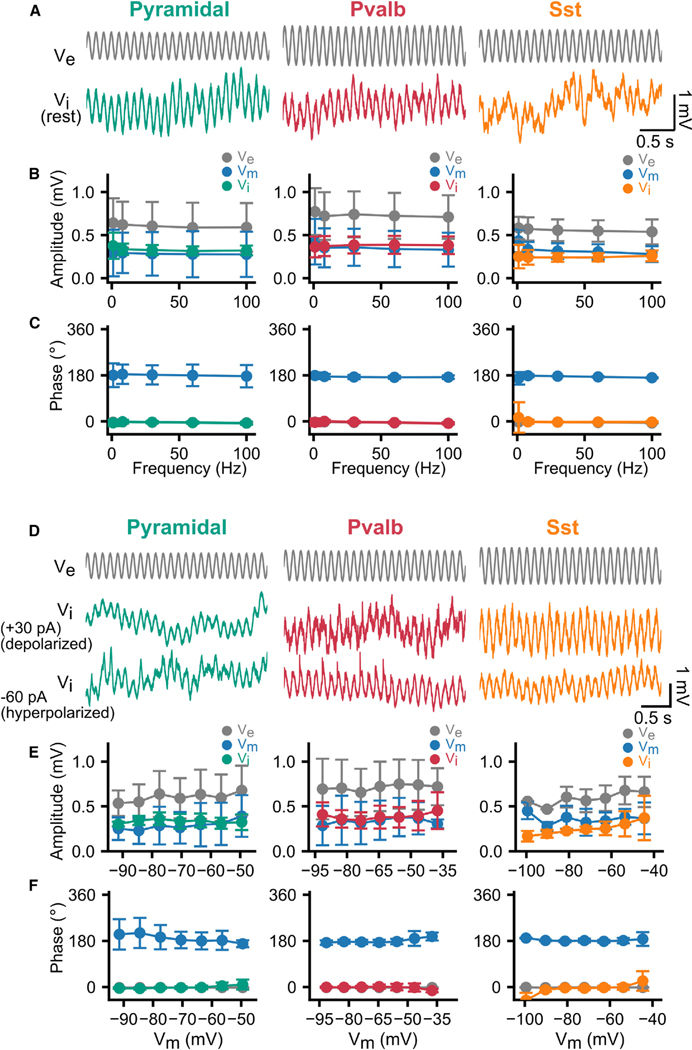
Figure 3. Non-specific, ES-frequency-independent subthreshold entrainment of all cell classes to ES.
(A) Entrainment of to ES for cells at rest. Gray traces, at the closest extracellular location (15 μm) from the soma. Subthreshold ES (8 Hz and 100 nA) is delivered 50 μm from soma.
(B and C) ES effect on neurons at rest (ES: 100 nA, 1–100 Hz). Amplitude (B) and phase (C) of the ES-induced (gray), (green, red, or orange), and (blue) for each cortical cell class (circles: mean; error bars: SD). All cell classes exhibit ES frequency independence with induced , , and amplitude and phase remaining constant for ES frequencies ranging from 1 to 100 Hz (one-way ANOVA, p > 0.05 for Pyr, Pvalb, and Sst).
(D) ES effect on hyper- and depolarized neurons held at a range of membrane potentials via intracellular during simultaneous sinusoidal ES (8 Hz and 100 nA). (E and F) Amplitude (E) and phase (F) of the ES-induced (gray), (green, red, or orange), and (blue) for each cortical class for hyper- and depolarized potentials (circles, mean; error bars, SD). n = 24, pyramidal; n = 22, Pvalb; n = 13, Sst cells.
In the presence of synaptic blockers and without intracellular current injection, the , , and response (amplitude and phase) to ES measures the pure subthreshold ES effect on the cellular membrane (Figures 3A–3C). All cell classes readily entrained to the sinusoidal ES, irrespective of ES amplitude and frequency (Figure 3A). The ES induced a local oscillation at the soma that remained unaltered in amplitude and phase (Figures 3B and 3E). We found that the and potential at the soma of all cell classes followed all tested ES frequencies up to 100 Hz without any membrane filtering (Figures 3B and 3C). Our findings are consistent with AC electrodynamics theory, predicting that the cell membrane voltage polarization induced by an external ES drive remains constant, even for very high frequencies.38,39 It follows that ES is a fundamentally different stimulus than intracellular stimulation, where membrane resistor-capacitor (RC), circuit-like filtering attenuates higher frequencies and allows lower frequencies to shape the membrane response. We also note that the amplitude of the pure ES effect induced was a fraction of 1 mV (: 0.29 ± 0.26 mV, Pyr; 0.36 ± 0.23 mV, Pvalb; 0.34 ± 0.11 mV, Sst; Figure 3B).
Is such unimpeded membrane entrainment across cell classes a result of the membrane residing at rest? To address this, we depolarized and hyperpolarized all cells relative to their resting potential via intracellular DC injections (from −90 to 90 pA, duration: 5 s) to induce subthreshold-level changes in membrane polarization while applying extracellular ES (8 Hz with 100 nA). We then assessed the impact of ES on , , and (Figures 3D–3F). Once again, the subthreshold, ES-induced effect remained robust and unchanged across cell classes in terms of the and amplitude and phase, even when membrane polarization varied by 40–50 mV (Figures 3D–3F). In summary, we find that, at subthreshold, rest, and polarized levels, the cell membrane can follow the applied ES and does so robustly across all cell classes.
Spike-field entrainment strongly correlates with the spike frequency of the cell
Although cortical classes show strong, class-specific spike entrainment to ES, the same neurons exhibit indistinguishable entrainment characteristics to the same sinusoidal ES when not spiking: pyramidal neurons exhibit a combination of non-specific and frequency-specific (8 and 140 Hz) spike-field entrainment, inhibitory Pvalb neurons only entrain to high frequencies (140 Hz), while inhibitory Sst entrain to medium-to-high frequencies (>30 Hz). How can these observations be reconciled? To investigate whether cellular properties can explain ES entrainment, we considered four intrinsic properties—spike threshold, firing rate, input resistance, and resting voltage.36 Looking at the intrinsic properties vs. the resulting spike-field strength (expressed by vector length), we found that these properties do not explain within-class ES entrainment (Figure S4). Therefore, ES is paramount for the emergence of spike-field entrainment even when cell classes differ in intrinsic properties and do not explain the strong frequency dependence seen in the spike-field coordination.
We next looked at the temporal structure of spiking, seeking mechanisms that explain the ES frequency dependence. To do so, we first looked at the temporal structure of spiking by calculating the interspike interval (ISI; Figure 4A). Consistent with other studies,36 the instantaneous firing-rate (1/ISI) distributions show the typical slow and irregular firing that pyramidal neurons are known for (spike rate: 8.5 ± 3.0 Hz; Figure 4B) as well as the fast, more stereotyped firing of Pvalb (133.4 ± 35.7 Hz; Figure 4B). Finally, Sst present an intermediate and more diverse case, with faster spike rates than Pyr though slower than Pvalb and with a considerably wider firing-rate distribution (57.4 ± 37.8 Hz; Figure 4B). Closer examination suggests that at least a portion of the highly entrained spikes stem from neurons with spike frequencies similar to the ES frequency, implying that for a given spike j, when (ES frequency) ≈ 1/ISI_j, the coordination between sinusoidal ES and spike j is enhanced. To test this hypothesis, we separated spikes based on whether their ISI was within a certain window of the ES frequency. For example, we looked at the spike-field coherence of pyramidal neurons with a spike frequency (1/ISI) of 8 Hz (“center frequency”) ± (frequency window) when ES frequency is also 8 Hz (Figure 4C). We then varied the frequency-window width and looked at whether the resulting population vector length changed within each bin. If no measurable difference is observed in terms of the vector length, then entrainment occurs equally strongly across spike rate bins with no preference (in terms of ISI). If, on the other hand, spikes whose inverse ISI is close to the ES frequency are strongly entrained compared with the rest, then this supports a frequency-dependent mechanism like resonant ionic conductances where subpopulations of neurons are candidates to be highly entrained to a particular ES frequency, depending on their ISI. The results from pyramidal neurons provide support for both phenomena (Figures 4C and 4D), i.e., while a subset of spikes (with 1/ISI ≈ ES frequency) are strongly entrained to the ES (Figure 4D), there is still substantial entrainment across pyramidal neurons (compared with control) and especially to ES frequencies much higher than pyramidal 1/ISI.
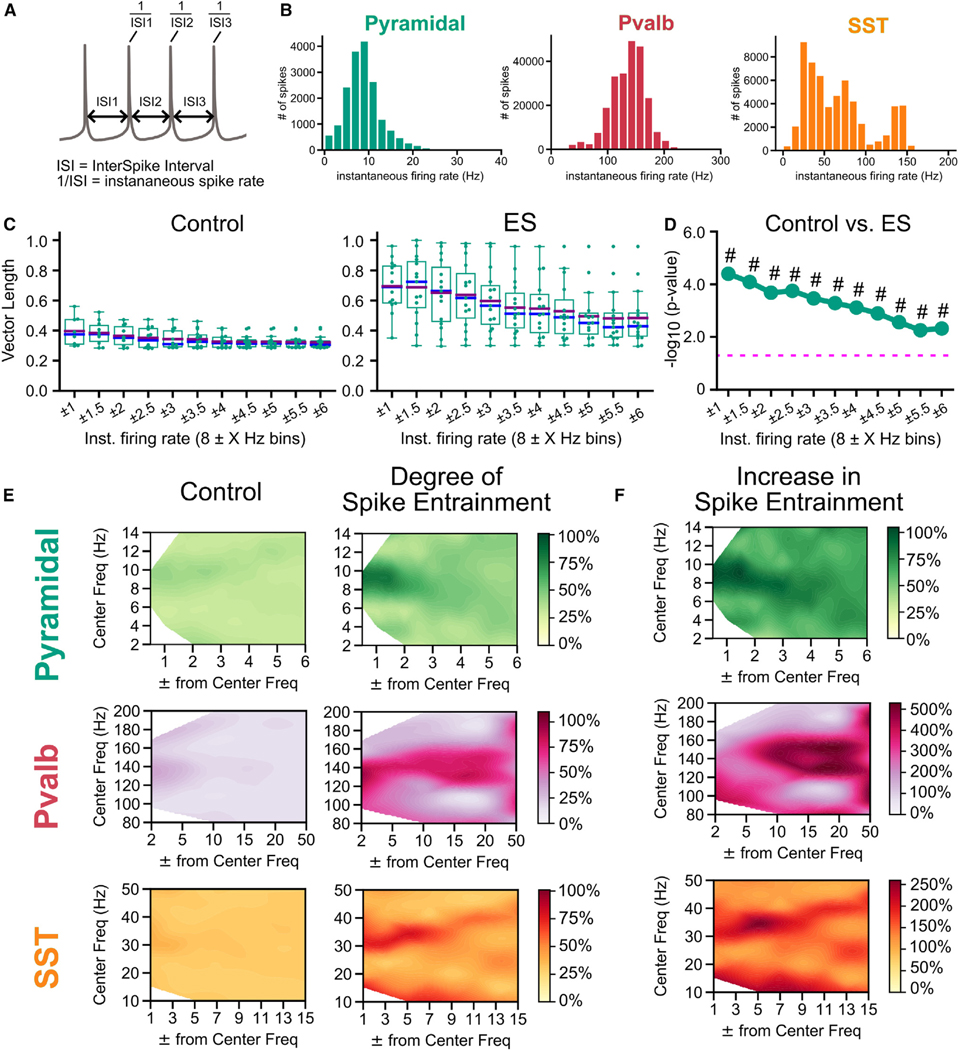
Figure 4. Cell-class-specific ES entrainment of spiking correlates with spike-rate properties of the individual classes.
(A) Instantaneous spike rate for each spike, calculated as the inverse of the interspike interval (ISI, time between a spike and the next consecutive spike).
(B) Histogram of instantaneous spike rate distributions for all spikes recorded in each class. (Pyramidal: n = 21; Pvalb: n = 22; Sst: n = 13 cells).
(C) Degree of spike entrainment (as evaluated by population vector length) to ES (8 Hz and 200 nA) for each recorded cell’s spikes in the pyramidal class containing only spikes within a specific spike-rate range/bin (vector length means bootstrapped for 10,000 trials). Bin size (boundaries) are designated by the spike rate to be analyzed (a “center” frequency) within a frequency-window range (ranging from ±1 to ±6 Hz). Tighter spike-frequency ranges are on the left, with the range widening toward the right. Boxplots, quartiles; purple and blue lines, mean and median values; whiskers, remaining distribution.
(D) Plot of −log10 values (Welch’s t test) for comparison between the same-spike-range bins (e.g., 8 ± 1 Hz control vs. 8 ± 1 Hz ES bins) between control and ES in (C). Dashed pink line: p = 0.05; #: effect size (Cohen’s d) where d > 0.8.
(E) Degree of spike entrainment (expressed as % of the normalized vector length, 0–1) to control (left) or ES for spikes within specific spike-rate bins for the three classes. Results shown for ES (200 nA) at 8 Hz (Pyr), 140 Hz (Pvalb), and 30 Hz (Sst).
(F) Percentual increase in spike entrainment (vector length) in ES vs. control for Pyr, Pvalb, and Sst. Pyramidal: n = 21 for ES frequencies 8 and 30 Hz and n = 13 for 140 Hz; Pvalb: n = 22 for 8 and 30 Hz and n = 12 for 140 Hz; Sst: n = 13 for 8 and 30 Hz and n = 10 for 140 Hz.
To what extent do such highly entrained subpopulations of spikes exist in other cell classes? We used the same principle to look at spike-field entrainment as function of 1/ISI by varying the window (width) around the center frequency. The main difference between Pvalb and Sst compared with pyramidal neurons is their discrepancy in spike rate (Figure 4B). For Pvalb, the condition 1/ISI ≈ ES frequency can only be met for ES frequency 140 Hz because Pvalb typically spike around 100–200 Hz. For Sst, the same condition holds for ES frequency around 30 Hz because they tend to spike around 20–100 Hz (Figure 4B). Spike-field entrainment of spikes at various ES frequencies is significantly increased when 1/ISI ≈ ES frequency across cell classes (Figures 4D and 4E). Although this principle holds in general, the specifics of the entrainment profile differ between cell classes. For example, for pyramidal neurons at 8 Hz, spikes where 1/ISI deviates from the ES frequency by approx. 20% exhibit a decrease in their degree of entrainment (vector length) by more than 50% (Figure 4E). On the other hand, for Pvalb at 140 Hz, 1/ISI can deviate by more than 35% (1/ISI ≈ 90 Hz) without spikes experiencing an appreciable decrease in their entrainment. Finally, Sst at 30 Hz form an intermediate case, where spikes off by approx. 33% (1/ISI ≈ 21 Hz) still exhibit 50%–75% of the entrainment (Figure 4E). These observations are compatible with what is known about the cell classes explored in our study. Despite the entrainment of pyramidal neurons to subthreshold stimuli across ES frequencies, their irregular spiking due to a plethora of active and co-activated membrane mechanisms is a constant source of variability counteracting a tight entrainment window around the center frequency. On the other hand, once Pvalb with their fast and less adaptive spike-related mechanisms are entrained, there is little source of variability to counteract field entrainment, resulting in a wide entrainment window around the center frequency. We conclude that class-specific spike-field coordination is the result of two effects: first, a non-specific membrane polarization ubiquitously present across cells and classes; second, class-specific excitability and activity properties that allow spikes of a similar timescale to the ES to entrain particularly strongly.
Spike-field entrainment is dictated by the balance between membrane conductances but not by a specific conductance
The analyses pursued so far about the origin of the spike-field coupling point to a generic mechanism that cannot be explained only by a specific ion channel or morphology. Even within the same brain area, the three cell classes we investigated have a different biophysical setup to each other, different expression profiles in many key ion channel genes (e.g., Kv3.1, HCN1–3, etc.), and different morphologies (pyramidal neurons are extended, Pvalb are more compact, and Sst are in-between).
We tested the hypothesis that the class-specific combination of conductances dictates the spike rate and thus the spike-field coupling to specific ES frequencies, as opposed to a particular ionic conductance or morphology feature. To do so, we used biophysically realistic, computational single-cell models of excitatory and inhibitory V1 classes. Briefly, we recently developed a multi-objective single-cell generation workflow using whole-cell patch-clamp recordings (responding to step currents) and morphological reconstructions to generate and validate class-specific single-cell models.40 Importantly, the evolutionary optimization procedure results in the 40-best single-cell models per experiment, termed “hall of fame” (or hof; Figure S5). The fact that a population of models is produced for each acquired cell (as opposed to a single “best” model) that generalize equally well allows us to assess mechanistic details as well as robustness—the same perturbation can be tested both across multiple models representing the same experiment/cell (“within cell”), across cells/experiments representative of a class (“within class”) and between cell populations that are members of different classes (“across class”).
We emulated the experimental setup by simulating the extracellular ES using a point-source approximation (Figure 5) with sinusoids of 8, 30, and 140 Hz combined with intracellular step currents (Figure 5A). The intracellular stimulation elicits spiking, with an overall spike rate broadly unaffected by simultaneous ES (Figure S6) but a spike-phase distribution strongly affected by the presence vs. absence (control) of ES across frequencies for both pyramidal and Pvalb models (Figure 5A: control vs. ES rose plots for three ES frequencies). Specifically, the pyramidal model, with a mean spike rate of approx. 8 Hz, is entrained across ES frequencies (Figure 5A) whereas the Pvalb model, with a mean spike rate larger than 100 Hz, is only entrained for an ES frequency at 140 Hz (Figure S6). Therefore, the computational models capture the basic properties of spike-phase coupling observed in our experiments. We also tested how the spike frequency of a particular cell model affects spike-phase coupling to ES. Here, we used the population hof models for the pyramidal and Pvalb cells and swept across the intracellular current step amplitude (pyramidal models, 165–200 pA; Pvalb models, 490–630 pA; Figure S6), keeping the resulting spike rate within a physiological range. As with the in vitro experiments, we saw that when the spike frequency of each hof model closely matches the ES frequency, the degree of spike-phase entrainment is maximized, irrespective of the exact current injection amplitude, with the extent of entrainment varying across hof models (Figure 5B, bottom row).
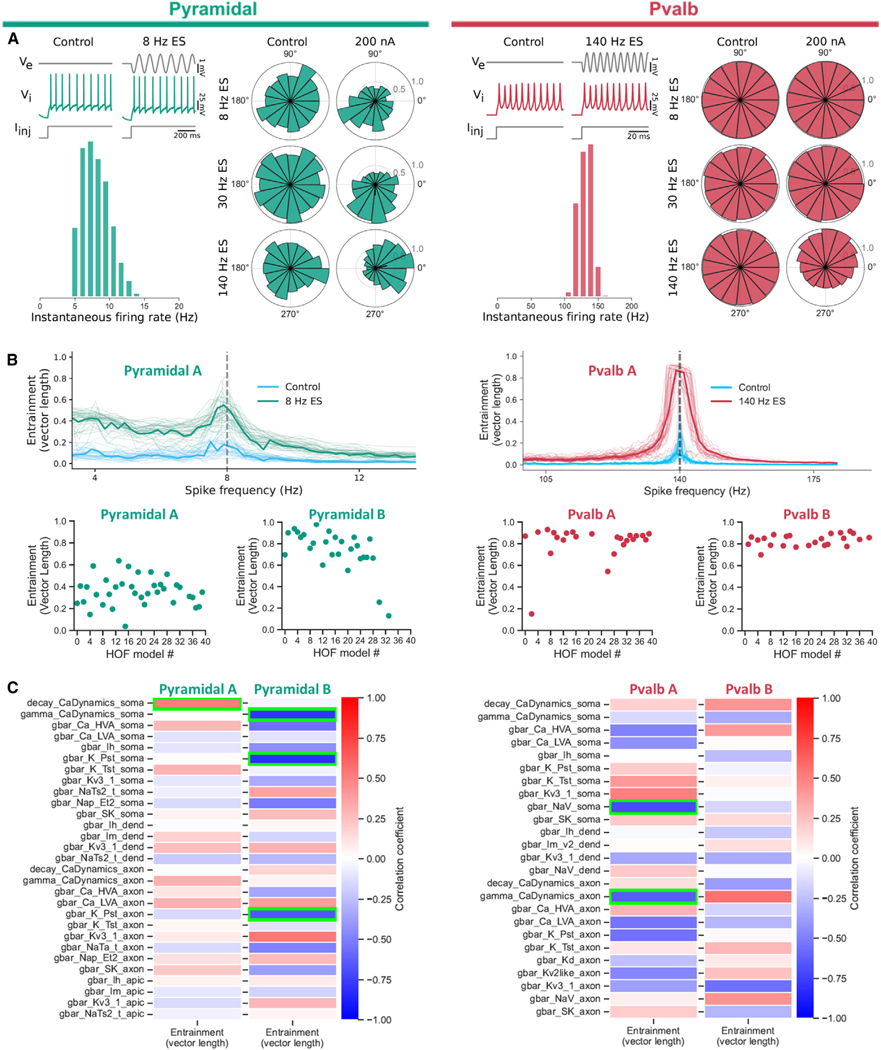
Figure 5. Computational modeling of mouse V1 neurons suggests spike-rate differences rather than individual conductances as the major contributor to class-specific spike-field coupling.
(a) (Top left) A bio-realistic model of a pyramidal neuron (cell ID: 488698341) is used to emulate the experimental setup accounting for the sinusoidal ES. Intracellular DC current is combined with weak sinusoidal ES of various frequencies (8, 30, and 140 Hz) such that the spike rate remains unperturbed by the ES. (Bottom left) Model ISI distribution (control). (Right) Spike-phase relationship for the simulations (ES at 200 pA, top to bottom: 8, 30, and 140 Hz ES). Weak ES gives rise to strong spike-phase coupling in the presence, but not absence, of ES. (Top right) Same setup as for (A) but using a bio-realistic inhibitory Pvalb model (cell ID: 569998790; see also Figure S5). The Pvalb model shows preferential entrainment to fast ES, while the pyramidal model readily entrains to both slow and fast ES (Figure S6).
(B) Top: hall of fame (hof) models of the pyramidal (left) and Pvalb (right) cell from (A) exhibit the robustness of the spike-field coupling (40 hof models per cell; Figure S5). Identical setup like in (A) for each hof model (spike-field entrainment, population vector length; thin lines, vector length for each hof model; thick line, mean vector length across hof models; cyan, control, no ES). With Pvalb spiking faster than pyramidal models (spike rate distributions in A), ES strongly entrains all hof models when ES frequency matches the spike rate. Bottom: spike-phase entrainment at the preferred ES frequency (pyramidal: 8 Hz; Pvalb: 140 Hz) across hof models for two pyramidal (pyramidal A: 488698341; pyramidal B: 354190013) and two Pvalb cells (Pvalb A: 569998790; Pvalb B: 471077857).
(C) Correlation between model conductances and spike-phase entrainment at the preferred ES frequency (for pyramidal neurons: 8 Hz; Pearson correlation across hof models; 40 hof models per cell; B, bottom row). Green boxes, statistically significant correlations (p < 0.0017 for pyramidal and p < 0.002 for Pvalb models).
Do specific conductances dictate the spike-field coupling or does the combination of conductances dictate the spike rate that, in turn, affects entrainment to specific ES frequencies in a cell-class-specific manner? To address this question, we looked at how individual conductances of the pyramidal and Pvalb hof models correlated with the degree of ES entrainment. For this analysis, we included a second pyramidal and Pvalb cell with their hof models (40 hof models per cell). We adjusted the intracellular current injection of each hof model to match the spike frequency with the ES frequency and found a distribution of spike-phase coupling strengths across hof models. To test whether specific conductances correlate with increased entrainment, we looked at the correlation between each hof model conductance and the spike-phase coupling (Figure 5C). We found that, for each cell, different conductances correlate with ES entrainment (Figure 5C, green boxes), even though all hof models clearly show strong spike-field entrainment (Figure 5B). Although these simulations do not completely exclude specific conductances as key contributors of spike-field entrainment, they suggest that the ES coupling in our experiments is not the result of a single conductance activated at a specific ES frequency but, instead, that the spike rate (i.e., the result of a balance between numerous conductances) of each cell class and its relationship to the ES frequency is a much stronger predictor of spike-field entrainment.
Spike-field entrainment of human neocortical excitatory neurons to ES
To what extent do the ES effects seen in the rodent brain translate to human neurons? Although human pyramidal neurons share properties with their rodent counterparts,41,42 they also possess many distinct characteristics.43,44 This, in turn, might affect how human vs. rodent neurons ought to be stimulated to achieve specific activity patterns. We used our setup to investigate the subthreshold and spiking ES effects of human cortical neurons isolated from neurosurgical specimens.45
We imposed the same, weak sinusoidal ES field across human pyramidal neurons and measured how , , and are affected by the ES strength and frequency (Figure 6A) under two regimes: spiking (Figures 6C–6E) and subthreshold non-spiking (Figure 6F). For subthreshold ES, we found that human pyramidal cells entrain to subthreshold ES unperturbed, from low to high frequencies (up to 100 Hz) without any apparent filtering. Also, we found that at subthreshold, rest, and polarized levels (across a 40-mV range), the human cell membrane readily follows the applied ES (Figure 6G).
Figure 6. Subthreshold and spiking ES entrainment of human neurons.
(A) Top (left): human cortical slice obtained via neurosurgical resections. (Right) Electrophysiology trace of a human pyramidal neuron during intracellular current injections. Bottom (left): human slice with electrodes applying ES, recording a neuron (white), and at multiple locations. (Middle) Human pyramidal cell morphology. (Right) f-I curves of recorded cells.
(B) Left: amplitude as function of distance between ES (25–200 nA and 8 Hz) and recording electrodes (circles: mean amplitude; error bars: SD). Trendlines: least-squares fit of point-source approximation.
(C) ES-induced and electric field amplitude at the extracellular electrode closest to the soma (~15 μm). Blue, amplitude for each experiment (n = 5); black, mean and SD.
(D) Sample trace of a human pyramidal neuron showing entrainment of (green) to subthreshold ES (8 Hz and 100 nA) delivered 50 μm from soma, (gray) measured closest (15 μm) to the soma.
(E) Example and traces with spiking induced by intracellular DC stimulus during control (no ES) and sinusoidal ES.
(F) Spike rate distribution for all spikes recorded from human neurons (n = 4).
(G) Subthreshold ES effect (top) at rest and (bottom) at hyper- and depolarized potentials via intracellular current injection (−90 to 90 pA; ES: 100 nA at 1–100 Hz). (gray), (green), and (blue outlined circle) amplitude (left) and phase (right) are shown (circles mean; error bars, SD).
(H) Spike-phase distribution for varying ES parameters. Rows, ES frequency; columns, ES amplitude.
(I–K) Summary statistics for ES amplitude = 200 nA (yellow box in H). (I) Spike-phase entrainment of human neurons to ES assessed via Rayleigh’s test (****p < 0.01; dashed pink line: p = 0.05). (J) The population vector length for each cell. Purple and blue lines: mean and median values; whiskers: remaining distribution.
(K) The population vector length for each cell (from J, each line represents a cell) across ES frequencies (paired t test, false discovery rate [FDR]-corrected for multiple comparisons: *p < 0.05, Table S4).
(L) Spike entrainment (vector length) in control (no ES, left) and ES (8 Hz at 200 nA) experiments (right) for each recorded cell, containing only spikes within a specific spike-rate range/bin. Bootstrapped means for each cell (bootstrapping across 10,000 trials). Bin size (boundaries) are designated by the spike rate (a center frequency) within a frequency-window range (from ±1 to ±3 Hz).
(M) The spike-phase entrainment quantified via −log10 values (Welch’s t test) for comparison between the same-spike-range bins (e.g., 8 ± 1 Hz control vs. 8 ± 1 Hz ES bins) between control vs. ES in (K). Dashed pink line: p = 0.05; #: effect size (Cohen’s d) where d > 0.8.
How about ES entrainment under spiking conditions? We injected intracellular current to elicit spiking simultaneously during ES application. This did not affect their spike rate (paired t test, p > 0.05 for control vs. 8, 30, and 140 Hz for all amplitudes) validating that, compared with the intracellular stimulation, the impact of ES on excitability (or number of spikes) is comparatively weak. Moreover, without ES (control), spikes are not entrained at the frequencies related to ES (Rayleigh testing, p > 0.1; Figures 6G–6K). We found that weak ES readily entrains spiking in human pyramidal neurons and the degree of entrainment increased with ES amplitude (Figures 6H–6K). Unlike in rodents, human pyramidal neurons exhibit the strongest spike-field entrainment for high ES frequencies (>100 Hz) despite their slower spiking (spike rate: 7.5 ± 0.8 Hz). We also looked at the extent to which spikes with 1/ISI ≈ (ES frequency) entrain more strongly than the rest (Figures 6L and 6M). Given the slow spiking of human pyramidal neurons, this condition can only be satisfied for 8 Hz. We found that spikes with 1/ISI ≈ 8 Hz entrain particularly strongly compared with spikes with a more disparate spike frequency, with inclusion of latter spikes resulting in the quick deterioration of coordination (Figures 6L and 6M). Thus, there is a subset of highly coordinated spikes where 1/ISI ≈ 8 Hz. On the other hand, the very high entrainment of human excitatory spiking with fast ES frequencies—much faster than pyramidal cell firing—and the subthreshold entrainment properties of their membrane to fast stimuli also point to a mechanism very similar to the non-specific membrane polarization observed in rodent neurons. Similar trends are also observed even for weaker ES amplitudes. We conclude that, similar to that in rodents, spike-field coordination in human excitatory neurons is a combination of two effects: non-specific membrane polarization presents across cells and a spike-activity-related effect that allows spikes of similar timescale to the ES to entrain particularly strongly.
DISCUSSION
We found that membrane entrainment to a relatively weak (in amplitude) sinusoidal extracellular stimulus (ES) occurs across all cell classes (excitatory and inhibitory), multiple cortical areas (V1 and hippocampus) and species (rodent and human), pointing to a fundamental property of individual neurons across all classes in our study. Although intracellular current injections generally result in strong membrane filtering (i.e., higher frequencies are greatly attenuated by the membrane capacitance),46 membrane response to ES remains essentially unfiltered. Yet, this general property is superimposed onto a rich, diverse, class-specific spike-field coordination. Specifically, we found that pyramidal neurons, with their slower spike rate, entrain spiking to both slow and fast (>100 Hz) ES, while inhibitory classes like Pvalb and Sst, with their faster spiking, predominantly phase-lock to fast but not to slow ES. A resonant mechanism that purely relies on a match between spike rate and ES frequency may explain pyramidal coupling to 8 Hz ES as well as Pvalb coupling to 140 Hz but cannot explain the pyramidal and, to a lesser extent, Sst coupling to very fast ES. We note that the class-specific spike-field coordination stems from the use of sinusoidal ES rather than alternative waveforms (e.g., pulses), where the fundamental frequency of the stimulus is obscured and the response occurs at multiple timescales.
Our experiments point to class-specific spike-field coordination resulting from two effects. The first is a non-specific membrane polarization present across all cells, exemplified in the subthreshold ES experiments where membrane ES entrainment is universal and does not depend on the specifics of class or area. The second effect is class-specific, where spikes elicited at similar timescales to the ES strongly phase-lock to the external field. Although some ionic conductances have been linked to resonant coupling at different frequencies (e.g., the m-current, Im,47,48 and the hyperpolarization-activated h-current, Ih42,49), the spike output of neurons is ultimately the result of interactions between multiple ion channels distributed along their complex morphologies. Our experiments and computational models show that distinct cortical classes with different conductance and spiking profiles exhibit ubiquitous and robust coordination to ES across frequencies. This suggests that membrane entrainment to ES is a fundamental property rather than mediated by a specific ion conductance. Still, because the cellular response (from the spike waveform to the excitability profile and output spike rate) is crucially affected by its ionic setup,47,48 a cell’s active properties are implicitly reflected in the ES entrainment. For example, while V1 pyramidal neurons typically spike slowly, their spike rate can increase drastically during bursting (i.e., 200–300 Hz), mediated by a host of somadendritic mechanisms, suggesting that their ionic setup, in principle, supports fast activity and locking to fast ES.50–55 Thus, while ES entrainment is ubiquitous, the characteristics of spike-field entrainment for each cortical class ultimately reflects its morpho-electric properties and ionic setup.
Our results in V1 L5 pyramidal and hippocampal CA1 neurons of adult mice bear some similarities with observations made for somatosensory L5 pyramidal neurons in the juvenile rat19: subthreshold sinusoidal ES coupling is ubiquitous, unfiltered, and robust, while small-amplitude ES potently biases the spike phase (but not spike frequency). Both juvenile and adult pyramidal neurons exhibit preferred spike coupling to 8 Hz ES vs. 30 Hz ES, compared with control. But here we also report how fast ES beyond 100 Hz leads to strong spike-field coupling in pyramidal neurons, a surprising observation given the slower (compared with the ES frequency) spike rate of pyramidal neurons in our study. It is conceivable that pyramidal neurons in juvenile rats vs. adult mice may entrain differently to fast ES due to age-related differential expression of individual ion channels impacting the spike response even if subthreshold responses remain similar.19
In contrast to rodent excitatory neurons, human L5 pyramidal neurons entrained across ES frequencies with particularly strong phase-locking at fast ES (100 + Hz). Where does such strong spike-field coordination come from? Notably, the subthreshold response of human pyramidal neurons is not different to mouse V1 or CA1 neurons, so their increased entrainment to fast ES cannot be explained purely through the subthreshold results. It has been suggested that human neurons have lower capacitance filtering compared with rodent neurons.43 Decreased filtering may contribute to strong spike coupling for fast ES as well as enhanced integration of fast synaptic input.56,57 Ih exhibits higher expression in human vs. mouse neocortical excitatory neurons, with increased Ih expression counteracting dendritic filtration.42 Finally, the voltage-gated potassium conductance at the soma of L5 human pyramidal neurons is smaller than in rodents and can contribute to spike repolarization and timing.58–60 Although our computational models did not identify a specific role for these conductances in rodent neurons, they may contribute in spike-field coupling of human neurons and their preferential phase-locking at fast ES.
Our findings shed light on cellular ES effects and are relevant for the design of next-generation neuromodulation technologies using customized ES waveforms for the selective coordination of specific cell classes in vivo. The main parameter exerting such selectivity based on our results is ES frequency, with slow ES facilitating coordination of excitatory classes and fast ES facilitating coordination of both excitatory and inhibitory populations. Spike-field coordination in various frequency bands is the substrate of many cognitive functions and therapeutic interventions.34,61–63 Beyond local ES effects near the stimulation site, class-specific ES protocols can impact spatially distributed brain regions further away. For example, in humans, cortical structures have reliable monosynaptic connections to the subthalamic nucleus, a major target for deep brain stimulation in movement disorders.4,6,64–68 Beyond invasive intracranial stimulation, we expect the same effects to apply to noninvasive stimulation, as long as they impose an electric field of certain spatiotemporal properties across the membrane of neurons (e.g., Grossman et al.69). Our findings form the basis for designing such selective, class-specific ES interventions.
Coordinated circuit activity often produces an endogenous and measurable extracellular field, especially so in cortical structures.70 The resulting field is often not confined locally and can be spatially diffuse, e.g., hippocampal theta oscillations are volume-conducted and measured in adjacent neocortical regions.71 Because the field strength in our experiments (~1–25 mV/mm) is comparable to that measured in vivo, our results can also shed light on cellular entrainment due to endogenous fields, i.e., via ephaptic coupling72 or far-field effects from other regions, with the spatial coherence and geometry of the sources impacting the spatial extent of the field.73 The ubiquitous nature of sinusoidal ES coupling suggests that slower (e.g., alpha, theta, and beta) and faster endogenous oscillations (gamma and ripples) are felt and followed by the membrane across cortical neurons. To what extent is spiking affected by such oscillations in a manner that constitutes an alternative, non-synaptic communication between neurons? In the absence of synaptic drive that sufficiently depolarizes neurons and leads to spiking, endogenous fields are rarely strong enough to trigger spiking under physiological conditions. But, in the presence of such a synaptic drive that also gives rise to a measurable extracellular field,70 the field can modulate spike timing and enhance the coordination between local spiking and various bands of the local field potential. For example, Purkinje cells in the cerebellum coordinate their spiking with millisecond precision through ephaptic coupling.26 Fast coordination (within milliseconds) between pyramidal neurons has also been observed in the neocortex,53 despite local synapses only supporting slower spike coordination. The spike-field coupling we see across classes for fast ES (140 Hz) can explain such fast coordination even in the absence of very fast synapses and, in such a manner, provide an alternative, energy-efficient, non-synaptic paradigm for circuit coordination across timescales in the brain.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to Costas Anastassiou (costas.anastassiou@cshs.org).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
The code used to analyze and process the physiology data is available at https://github.com/anastassiou-team/celltype_brainstim_invitro
The code to setup, run and analyze the simulations is available at https://github.com/anastassiou-team/celltype_brainstim_insilico
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Mouse and human tissue were prepared with slice preparation reagents and protocols standardized at the Allen Institute74 and described below. Tissue was extracted from the temporal or frontal lobe of patients undergoing neurosurgery for epilepsy or tumors (mean age: 27 ± 13.8 years, 3 donors). The tissue specimens were distal to the core pathological site and was deemed not to be of diagnostic value.
Acute mouse brain slices, from both female and male mice at ages P40-P50, were prepared as described in 75. All mouse procedures were approved by the Allen Institute’s Institutional Care and Use Committee.
METHOD DETAILS
Acute slice preparation
Mice were anesthetized with 5% isoflourane and transcardially perfused with ice-cold, oxygenated artificial cerebrospinal fluid solution (ACSF.I74): in mM: 0.5 CaCl2·2H2O, 25 D-glucose, 98 HCl, 20 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 MgSO4·7H2O, 1.25 NaH2PO4, 3 myo inositol, 12 N-acetylcysteine, 96 N-methyl-D-glucamine (NMDG), 2.5 KCl, 25 NaHCO3, 5 Na-L-ascorbate, 3 Na-pyruvate, 0.01 taurine, 2 thiourea). Parasagittal cortical slices (350 μm) were prepared in ACSF.I with a Compresstome (Precisionary Instruments) or VT1200S Vibratome (Leica Biosystems), and the slicing angle was set to 17 degrees relative to the sagittal plane to preserve apical dendrites of pyramidal cells. Hippocampal slices were sliced in the coronal plane. Slices were kept in a recovery chamber containing oxygenated ACSF.I for 10 minutes at 34°C. They were then transferred to a holding chamber containing oxygenated ACSF.IV,74 consisting of the following (in mM): 2 CaCl2·2H2O, 25 D-glucose, 20 HEPES, 2 MgSO4·7H2O, 1.25 NaH2PO4, 3 myo inositol, 12.3 N-acetyl-L-cysteine, 2.5 KCl, 25 NaHCO3, 94 mM NaCl, 5 Na-L-ascorbate, 3 mM Na-pyruvate, 0.01 taurine, 2 thiourea. Slices were kept in the holding chamber (at least one hour) at room temperature until time of recording.
Acute human cortical slices were prepared as described in Campagnola et al.75 All procedures involving human tissue are reviewed by the review boards at the hospitals performing the surgeries before beginning the study, and all patients provided informed consent. The specimens were placed in a sterile container filled with pre-chilled, carbogenated aCSF VII74 (in mM: 0.5 CaCl2·2H2O, 25 D-glucose, 92 HCl, 20 HEPES, 10 MgSO4·7H2O, 1.2 NaH2PO4, 92 NMDG, 2.5 KCl, 30 NaHCO3, 5 Na-L-ascorbate, 3 mM Na-pyruvate, 2 thiourea). Specimens were transported from the surgical site to the laboratory within 10–40 min, where they were trimmed and mounted for best preserving intact cortical columns (pial surface to white matter) during slicing. Specimen were sliced in aCSF VII using a Compresstome or Vibratome and transferred to a holding chamber containing oxygenated aCSF VII at 34°C for 10 min, then moved and kept in aCSF IV74 at room temperature for a minimum of one hour before recording began.
Electrophysiology and analysis
Rig setup
Recordings were performed in a customized multi-patch system, as described in Campagnola et al.75 The multi-patch system consists of 8 headstages with pipettes arranged in a semi-circular organization, as seen in Figure 1A, and allows for simultaneous intra- and extracellular stimulation and recordings at multiple locations close by cell soma. One pipette was used for the intracellular stimulation and recordings, one for extracellular stimulation, and the rest were placed within 50–120 μm3 of each cell soma to record the extracellular voltage. The electrode distance and placement (within +/− 5mm) was measured with Acq4 software,76 which was connected to the microscope and micromanipulators for providing accurate tracking of the hardware and cell position. The pipette holders were equipped with customized metal shields to reduce crosstalk artifacts. Each headstage was controlled independently through modified triple-axis motors (Scientifica; PatchStar). Intracellular and extracellular stimulation was applied through Multiclamp 700B amplifiers (Molecular Devices). (Intracellular and extracellular stimulation was applied through different amplifers). Data acquisition was conducted using Multi-channel Igor Electrophysiology Suite (MIES; https://github.com/AllenInstitute/MIES), custom software written in Igor Pro (WaveMetrics). Recorded signals were amplified with a Multiclamp 700B amplifier and digitized at 50–200 kHz with ITC 1600 DAQs (Heka). Pipette pressure was regulated using both electro-pneumatic control valves (Proportion-Air; PA2193) under control of MIES software, and through manual (via mouth) pressure. Slices were visualized through an upright microscope (BX61WI, Olympus) with oblique infrared illumination (WI-OBCD condenser) equipped with 4X and 40X objectives, on a custom motorized stage (Scientifica), and images were taken with a digital CMOS camera (Hamamatsu; Flash 4.0 V2). Acq4 software76 was also used for imaging, and subsequent image analysis.
Cell class identification and characterization
In mouse recordings, individual neuronal cell classes were identified by the following methods: Excitatory pyramidal cells were identified with Cre- or Flp-dependent transgenic mice with fluorescent reporters (TdTomato or eGFP) specific for Layer 5 excitatory neurons (through Tlx3 or Sim1 promoters) or by their pyramidal morphology (in V1 and hippocampus). They were confirmed by their regular-spiking firing pattern (in response to intracellularly injected current steps) in patch-clamp recordings. Inhibitory Pvalb and SST cells were identified with Cre- or Flp-dependent transgenic mice with fluorescent reporters (TdTomato or eGFP) specific for Pvalb or SST cell classes (though Pvalb or SST promoters) and confirmed by their fast-spiking firing pattern. The intracellular recording pipette was filled with dye (Cascade Blue) to confirm overlap between the recorded cell and fluorescence to ensure proper cell class targeting. In human neuron recordings, pyramidal classes in Layer 5 were targeted by their location (approximately 1800 mm from pia42), large size and clear apical dendrites, and then confirmed by their regular-spiking firing pattern (in response to intracellularly injected current steps) in patch-clamp recordings.
After recordings, the slices underwent post-hoc processing to confirm cortical layer boundaries (via 4’,6-diamidino-2-phenylindole (DAPI) staining) and to reveal their cellular morphology (via biocytin labeling). Slices were first processed for immunohistochemistry by fixation in 4% paraformaldehyde and 2.5% glutaraldehyde for at least 40 hours at 4°C, then transferred and washed in phosphate buffer saline (PBS) for 1–7 days. Slices were then placed in 5 μM DAPI in PBS for 15 min at room temperature and then washed three times in PBS. Slices were then transferred to 1% hydrogen peroxide (H2O2) in PBS to extinguish endogenous peroxidases for 30 min, and then washed three times in PBS. For biocytin labeling, a 3,3’-diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories) was used. Slices were mounted and dried for approximately 2 days before imaging on an AxioImager Z2 microscope (Zeiss) equipped with an Axiocam 506 monochrome camera (Zeiss) at 20X, with images acquired via the Zeiss Efficient Navigation software.
Computer simulations
Single cell simulations were carried out in NEURON (Version 8.2.2),77 using the BMTK Python framework (BMTK Version 1.0.7 – Python Version 3.10.11).78 Bio-realistic, conductance-based mouse V1 single-cell models developed in40 were adopted. Briefly, we recently developed a workflow to generate biophysically and morphologically detailed cortical single-cell models at scale. It relies on a parallel multi-objective optimization framework deployable in high-performance computer architectures.40,79,80 From patch-clamp experiments, electrophysiology responses to a battery of standardized current stimuli (1 s-long DC current injections of increasing amplitude) are analyzed, resulting in a set of subthreshold and spiking features for each experiment (e.g., spike timing, amplitude, width, etc.) The workflow extracts 11 electrophysiology features from in vitro experiments, and their mean and standard deviation (st.d.) are computed for a particular stimulation waveform. For every feature, an absolute standard score is calculated with the current feature value measured from the output traces of the models and , being the experimentally measured mean and st.d., respectively.81 The second data modality required for the biophysically realistic single-cell models is the morphology (soma and dendritic compartments, axonal ones are not required but can also be used). Axonal reconstructions were replaced with a stub using the Import3d_SWC_read() NEURON function.
We replicated experiments by simulating 10 s-long segments and evaluated data from 1 to 9 s (for Pvalb cells) or by simulating 20s-long segments to evaluate data from 1 to 19 s (for Pyramidal cells), with intracellular current step injections of varying amplitude. The ES electrode was positioned 50 μm from the soma in agreement with the in vitro experiments. Intracellular and extracellular current injections were configured using the IClamp and Xstim BMTK modules respectively. Two cell classes (pyramidal and Pvalb) and two different cells from each class were simulated. Corresponding cell IDs: Pyramidal A, 488698341; Pyramidal B, 354190013; Pvalb A: 569998790; Pvalb B, 471077857. In addition to the intracellular DC injection, we also added continuous background noise (Gaussian distribution) with an amplitude optimized to replicate the experimental spike rate distribution statistics. Specifically for Figures 5A and S6A, multiple simulations of hof 0 for Pyramidal A and Pvalb A models were performed (Pyramidal: 16 simulations 165–200 pA; Pvalb: 48 simulations 490–630 pA; N = 2,453 spikes for each Pyramidal rose plot, N = 48,942 spikes for each Pvalb rose plot). For the simulations in Figures 5A and 5B, the st.d. of the intracellular noise injection was 125 pA for the Pyramidal and 110 pA for the Pvalb models leading to a spike rate standard deviation of 1.5 Hz and 5 Hz, respectively. For the simulations in Figure 5C, we adjusted the injected DC stimulus and noise distribution, with the pyramidal models having a spike rate of 8±1.5 Hz (mean±st.d.) and the Pvalb models having a spike of 140±5 Hz (mean±st.d.) The target spike rate for the models sought to replicate the experimental spike rate across models of the same class (Table S1). The maximum accepted error offset was ± 0.1 Hz. Models which failed to satisfy these criteria were not used for the correlation analysis (Figure 5).
Electrophysiological recordings
Electrophysiological recordings were performed in slices (for both mouse and human slices) at 3234 °C perfused under carbogenated recording ACSF (aCSF.IX74 (in mM: 1.3 CaCl2·2H2O, 25 D-glucose, 1 MgSO4·7H2O, 1.2 NaH2PO4, 3 KCl, 18 NaHCO3, 126 NaCl, 0.16 Na-L-ascorbate). To examine the effects of ES without potentially confounding factors of synaptic activity, recordings were conducted in the presence of synaptic blockers. Synaptic transmission blockers kynurenic acid (1 mM, Sigma) and gabazine (10 μM, Tocris) were added to the aCSF to block fast glutamatergic and GABAergic synaptic activity, respectively. Whole-cell patch-clamp recordings were performed with internal solution containing 130 K-gluconate, 10 HEPES, 0.3 ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA), 3 KCl, 0.23 Na2GTP, 6.35 Na2Phosphocreatine, 3.4 Mg-ATP, 13.4 Biocytin, and 50 μM Cascade Blue dye (excited at 490 nm), 280–295 mOsm and pH 7.2–7.3. (Electrophysiological values are reported without liquid junction potential correction). Pipettes for extracellular stimulation and voltage recordings contained aCSF IX. Patch pipettes were pulled from borosilicate glass capillaries (Sutter Instruments) with a DMZ Zeitz-Puller (Zeitz), with tip resistances of 4–6 MΩ for whole-cell recordings and 1–2 MΩ for extracellular stimulation and voltage recordings.
QUANTIFICATION AND STATISTICAL ANALYSES
Electrophysiology
Whole-cell patch-clamp recordings were performed on neurons that had a stable seal (>1 GΩ) and successful break-in. Bridge balance was monitored and did not exceed 15 MΩ. Resting membrane potential (RMP) was measured at 1–2 minutes after break-in of the cell, and only neurons with RMP over −53 mV were recorded. Bias current was injected within the MIES software package for the remainder of the experiment to maintain the determined resting membrane potential. To examine the cell-class-specific firing pattern and intrinsic properties of the recorded cell, and to generate the F-I (frequency-current) curve, intracellular current steps were injected into the cell at current-clamp mode. 20 pA steps (1 second) from −160 pA up to 200 pA were applied to the cell for all cell classes), and in further steps up to 400 pA for cells with a higher spike threshold. The rheobase was determined by the minimum current amplitude needed to generate an action potential (AP). The interspike interval (ISI), the difference in time between an APl and the next subsequent AP, was used to calculate the cell firing rate. AP threshold was calculated by first identifying the membrane voltage deflection point at which the first-order derivative of the membrane potential (dV/dt) exceeded 20mV/ms, and adjusted to where the dV/dt was 5% of the average maximal dV/dt. Input resistance was calculated by first plotting the voltage vs. the injected current at the hyperpolarizing steps from −120 pA to 20 pA, and then taking the slope of the resulting fit.
Subthreshold entrainment and analysis
To analyze the effect of ES on subthreshold cellular dynamics, we recorded from neurons under current-clamp mode, while delivering ES of varying amplitude (25–200 nA) and frequency (1–100 Hz) for 5 seconds. Neurons were either held at their resting potential, or at varying subthreshold polarization levels (through intracellular current delivered through the pipette 30 pA steps, from −90 pA to 90 pA). To calculate the resulting amplitude, frequency, and phase of the extracellular , intracellular somatic , and the resulting membrane voltage (, calculated as ), we first divided the raw unfiltered trace into [0, T)-timed intervals, where T (in seconds) represents the period of the ES as defined by T = 1/frequency. (e.g. for an 8 Hz stimulus, T = 0.125 s (1/8 Hz), which results in 40 intervals (5 s / T) for a 5 second stimulus duration). We then aligned these intervals to determine the mean waveform by calculating the mean of the aligned interval traces from 0 to T. The trace used was from extracellular electrode closest to the cell soma (15 μm away), and the trace was from the intracellular recording electrode. The amplitude, frequency, and phase of the mean waveform of each neuron from each recording was extracted for the analysis of subthreshold neuronal entrainment in Figure 3.
Spike entrainment and analysis
To examine the effects of ES on the suprathreshold properties of neurons, we injected intracellular DC current into neurons under current-clamp mode, to elicit action potentials during a 9 second intracellular stimulation protocol. Neurons were held at their resting membrane potential, and the DC current was first injected into the neurons in the absence of ES (“Control” condition), and then same DC current was injected into the neurons while concurrently applying ES at varying frequencies (8–140 Hz) and amplitudes (25 to 200 nA). (DC current amplitude was determined as the current injection necessary to sustain spiking during the protocol) For the spike-phase calculation, i.e. the phase of the extracellular when an action potential is elicited, the trace was first bandpass-filtered with frequency limits from 0.5 Hz to 200 Hz. The trace used was from extracellular electrode closest to the cell soma (15 μm away). We then used the Hilbert transform to calculate the instantaneous phase of the oscillating , closest to the cell soma during spiking. The phase (in degrees) was measured in the range 0° to 360°, with 90° the peak and 270° the trough of the waveform. Traces were analyzed after the first 0.5 s of the protocol to ensure stability of cell spiking for analysis. Spike time for each action potential was measured individually for each spike at the voltage deflection point at which the first-order derivative of the membrane potential (dV/dt) exceeded 20 mV/ms. The spike phase is thus defined as the instantaneous phase at the spike time. Spike times were also used to count and compare the number of spikes during Control vs. ES-application conditions.
We examined the spike phases during the ES via circular statistics (with the ES-modulated as reference) to determine whether a neuron was significantly phase locked. The Rayleigh test was used to assess the uniformity of spike-phase distribution (null hypothesis: distribution is uniform), and population-vector analysis was used to examine whether spikes exhibit a preference to any specific phase of the imposed . The spike distribution during control (i.e. no ES) experiments was examined during alignment to a “virtual” (using the from the subsequent ES experiment) extracellular field and compared to the spike distribution of neurons during ES.
The relationship between spike rate and spike-field entrainment was examined by taking the vector lengths of spike dataset per cell and binning the spikes according to their instantaneous spike frequency (as calculated by the spike ISI). Bootstrap mean values for all cells in each cell class were obtained by shuffling the number of spikes in 10000 trials. The spike-frequency bins were constructed by taking the specific ES frequency inducing entrainment for each cell class (a “center frequency”) and adjusting the bin width by adding/subtracting from the center frequency. The degree of entrainment (as assessed by vector length) for the specific spike-frequency bins, was determined by taking the values of the spike-frequency bins (during ES) and dividing them by the values of the spike-frequency bins during Control conditions.
Statistical analyses
Statistical analysis was performed was performed using Python in SciPy82 and Circstat (https://github.com/circstat/pycircstat). Data was tested for normality (Shapiro-Wilk test), and for normally distributed data, we used the two-sided Welch’s t-test or paired t-test. One-way ANOVAs were used to compare multiple frequencies in Figure 2, and the Cohen’s test and Levene’s test were used to examine effect size and homogeneity of variance, respectively, as indicated in the figures. Significance was determined at p<0.05, as denoted by the following: (* p < 0.05, **: p < 0.01, *** p < 0.001, **** p < 0.0001). Statistical tests performed with multiple comparisons are reported with false discovery rate (FDR)-corrected p-values using the Benjamini-Hochberg method. Values in the text are presented as mean ± standard deviation, or where as noted, also with median and interquartile ranges.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| DAPI (4’,6-diamidino-2-phenylindole, dihydrochloride) | Thermo Fisher | D1306 |
|
| ||
| Biological samples | ||
|
| ||
| Human neurological samples | University of Washington, Swedish, and Harborview Medical Centers | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Kynurenic acid | Sigma Aldrich | K3375 |
| Gabazine | Tocris | SR95531 |
| Cascade Blue | Thermo Fisher | C687 |
| DAB substrate kit | Vector Laboraties | SK-4100 |
|
| ||
| Deposited data | ||
|
| ||
| Subthreshold and Suprathreshold entrainment data | This paper | N/A |
| Subthreshold and Suprathreshold entrainment analysis | This paper | N/A |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Transgenic mice with fluorescent markers for Pvalb, Sst, Sim1, or Tlx3 (see Table S5 for full list, and http://portal.brain-map.org/explore/toolkit/mice for additional details) | Allen Institute | Table S5 |
|
| ||
| Software and algorithms | ||
|
| ||
| Igor Pro | WaveMetrics | https://www.wavemetrics.com/ (RRID: SCR_000325) |
| Python 2.7 and 3.10 | Python Software Foundation | https://www.python.org |
| NEURON 8.2 | Yale University | https://www.neuron.yale.edu/neuron/ |
| BMTK 1.0.7 | Allen Institute | https://alleninstitute.github.io/bmtk/ |
| Pycircstat | Github | https://github.com/circstat/pycircstat |
| Scipy | SciPy community | https://scipy.org/ |
| Custom analysis code (this paper) | Github | https://github.com/anastassiou-team/celltype_brainstim_invitro (https://doi.org/10.5281/zenodo.11111558) https://github.com/anastassiou-team/celltype_brainstim_insilico (DOI: https://doi.org/10.5281/zenodo.11111563) |
| MIES | Allen Institute | https://github.com/AllenInstitute/MIES |
Highlights.
Neurons show robust entrainment to sinusoidal electric fields
Subthreshold entrainment polarizes the cell membrane across cell classes
Spike-field entrainment is cell class specific and depends on stimulation frequency
Field entrainment is seen across neurons in different brain regions and species
ACKNOWLEDGMENTS
We thank the Allen Institute Transgenic Colony Management, Tissue Processing, Histology, Imaging, Morphology, and three-dimensional (3D) reconstruction teams for their assistance and the surgeons at the University of Washington, Swedish, and Harborview Medical Centers for human tissue collection. We thank Ueli Rutishauser for discussions. C.A.A. thanks the board of governors of Cedars-Sinai Medical Center. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award nos. R01NS120300 and R01NS130126. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the Allen Institute founder, Paul G. Allen, for his vision, encouragement, and support.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.neuron.2024.05.009.
DECLARATION OF INTERESTS
S.Y.L. has previously consulted for Starfish Neuroscience, Inc. C.A.A. and S.Y.L. are listed as inventors on a patent application related to this work. C.K. is a board member of and has a financial interest in Intrinsic Powers, Inc.
REFERENCES
- 1.Hitzig E. (1874). Untersuchungen über das Gehirn: Abhandlungen physiologischen und pathologischen Inhalts (A. Hirschwald; ). [Google Scholar]
- 2.Fritsch G. (1870). Uber die elektrische Erregbarkeit des Grosshirns. Arch. Anat. Physiol. Wiss. Med 37, 300–332. [Google Scholar]
- 3.Dumitrascu OM, Kamiński J, Rutishauser U, and Tagliati M. (2016). Subthalamic Nuclei Deep Brain Stimulation Improves Color Vision in Patients with Parkinson’s Disease. Brain Stimul. 9, 948–949. 10.1016/j.brs.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Vitek JL, Jain R, Chen L, Tröster AI, Schrock LE, House PA, Giroux ML, Hebb AO, Farris SM, Whiting DM, et al. (2020). Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 19, 491–501. 10.1016/S1474-4422(20)30108-3. [DOI] [PubMed] [Google Scholar]
- 5.Freund HJ, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, and Sturm V. (2009). COgnitive functions in a patient with parkinson-dementia syndrome undergoing deep brain stimulation. Arch. Neurol 66, 781–785. 10.1001/archneurol.2009.102. [DOI] [PubMed] [Google Scholar]
- 6.Hogg E, During E, E Tan E, Athreya K, Eskenazi J, Wertheimer J, Mamelak AN, Alterman RL, and Tagliati M. (2018). Sustained quality-of-life improvements over 10 years after deep brain stimulation for dystonia. Mov. Disord 33, 1160–1167. 10.1002/mds.27426. [DOI] [PubMed] [Google Scholar]
- 7.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, and Kennedy SH (2005). Deep Brain Stimulation for Treatment-Resistant Depression. Neuron 45, 651–660. 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, and Kennedy SH (2008). Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 64, 461–467. 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, et al. (2012). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 69, 150–158. 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonck K, Boon P, Achten E, De Reuck J, and Caemaert J. (2002). Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann. Neurol 52, 556–565. 10.1002/ana.10323. [DOI] [PubMed] [Google Scholar]
- 11.Hodaie M, Wennberg RA, Dostrovsky JO, and Lozano AM (2002). Chronic Anterior Thalamus Stimulation for Intractable Epilepsy. Epilepsia 43, 603–608. 10.1046/j.1528-1157.2002.26001.x. [DOI] [PubMed] [Google Scholar]
- 12.Kringelbach ML, Jenkinson N, Owen SLF, and Aziz TZ (2007). Translational principles of deep brain stimulation. Nat. Rev. Neurosci 8, 623–635. 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitaki A. (1942). Effects evoked in an axon by the activity of a contiguous one. J. Neurophysiol 5, 89–108. 10.1152/jn.1942.5.2.89. [DOI] [Google Scholar]
- 14.Arvanitaki A, and Chalazonitis N. (1947). De La Nature Des laquoPotentiels D’Activitéraquo II. — Initiation De L’Activité Oscillatoire Neuronique Par Des Anions Aptes A Se Coordonner Avec Les Métaux Des Catalyseurs Respiratoires. Arch. Int. Physiol 54, 423–440. 10.3109/13813454709144832. [DOI] [Google Scholar]
- 15.Holt GR, and Koch C. (1999). Electrical interactions via the extracellular potential near cell bodies. J. Comput. Neurosci 6, 169–184. [DOI] [PubMed] [Google Scholar]
- 16.Anastassiou CA, Montgomery SM, Barahona M, Buzsáki G, and Koch C. (2010). The effect of spatially inhomogeneous extracellular electric fields on neurons. J. Neurosci 30, 1925–1936. 10.1523/JNEUROSCI.3635-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aspart F, Remme MWH, and Obermayer K. (2018). Differential polarization of cortical pyramidal neuron dendrites through weak extracellular fields. PLoS Comp. Biol 14, e1006124. 10.1371/journal.pcbi.1006124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Histed MH, Bonin V, and Reid RC (2009). Direct Activation of Sparse, Distributed Populations of Cortical Neurons by Electrical Microstimulation. Neuron 63, 508–522. 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anastassiou CA, Perin R, Markram H, and Koch C. (2011). Ephaptic coupling of cortical neurons. Nat. Neurosci 14, 217–223. 10.1038/nn.2727. [DOI] [PubMed] [Google Scholar]
- 20.Deans JK, Powell AD, and Jefferys JG (2007). Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J. Physiol 583, 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radman T, Su Y, An JH, Parra LC, and Bikson M. (2007). Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J. Neurosci 27, 3030–3036. 10.1523/JNEUROSCI.0095-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reato D, Rahman A, Bikson M, and Parra LC (2010). Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci 30, 15067–15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang C-C, Shivacharan RS, Wei X, Gonzalez-Reyes LE, and Durand DM (2019). Slow periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling. J. Physiol 597, 249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan CY, and Nicholson C. (1986). Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J. Physiol 371, 89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman DK, Eddington DK, Rizzo JF, and Fried SI (2010). Selective Activation of Neuronal Targets With Sinusoidal Electric Stimulation. J. Neurophysiol 104, 2778–2791. 10.1152/jn.00551.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han K-S, Guo C, Chen CH, Witter L, Osorno T, and Regehr WG (2018). Ephaptic coupling promotes synchronous firing of cerebellar purkinje cells. Neuron 100, 564–578.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freund TF, and Buzsáki G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. . [DOI] [PubMed] [Google Scholar]
- 28.Petilla Interneuron Nomenclature Group, Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, et al. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci 9, 557–568. 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay R, Lee S, and Rudy B. (2016). GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292. 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, and Buzsáki G. (2013). Inhibition-Induced Theta Resonance in Cortical Circuits. Neuron 80, 1263–1276. 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark E, Roux L, Eichler R, Senzai Y, Royer S, and Buzsáki G. (2014). Pyramidal Cell-Interneuron Interactions Underlie Hippocampal Ripple Oscillations. Neuron 83, 467–480. 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radman T, Ramos RL, Brumberg JC, and Bikson M. (2009). Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2, 215–228. 228.e1–228.e3. 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anastassiou CA, Perin R, Buzsáki G, Markram H, and Koch C. (2015). Cell type- and activity-dependent extracellular correlates of intracellular spiking. J. Neurophysiol 114, 608–623. 10.1152/jn.00628.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutishauser U, Ross IB, Mamelak AN, and Schuman EM (2010). Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907. 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Roby KD, and Callaway EM (2010). Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol 518, 389–404. 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouwens NW, Sorensen SA, Berg J, Lee C, Jarsky T, Ting J, Sunkin SM, Feng D, Anastassiou CA, Barkan E, et al. (2019). Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat. Neurosci 22, 1182–1195. 10.1038/s41593-019-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mainen ZF, and Sejnowski TJ (1995). Reliability of spike timing in neocortical neurons. Science 268, 1503–1506. [DOI] [PubMed] [Google Scholar]
- 38.Gimsa J, and Wachner D. (2001). Analytical description of the transmembrane voltage induced on arbitrarily oriented ellipsoidal and cylindrical cells. Biophys. J 81, 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maswiwat K, Wachner D, Warnke R, and Gimsa J. (2007). Simplified equations for the transmembrane potential induced in ellipsoidal cells of rotational symmetry. J. Phys. D 40, 914–923. 10.1088/0022-3727/40/3/033. [DOI] [Google Scholar]
- 40.Nandi A, Chartrand T, Van Geit W, Buchin A, Yao Z, Lee SY, Wei Y, Kalmbach B, Lee B, Lein E, et al. (2022). Single-neuron models linking electrophysiology, morphology, and transcriptomics across cortical cell types. Cell Rep. 40, 111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg J, Sorensen SA, Ting JT, Miller JA, Chartrand T, Buchin A, Bakken TE, Budzillo A, Dee N, Ding S-L, et al. (2021). Human neocortical expansion involves glutamatergic neuron diversification. Nature 598, 151–158. 10.1038/s41586-021-03813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalmbach BE, Buchin A, Long B, Close J, Nandi A, Miller JA, Bakken TE, Hodge RD, Chong P, de Frates R, et al. (2018). h-Channels Contribute to Divergent Intrinsic Membrane Properties of Supragranular Pyramidal Neurons in Human versus Mouse Cerebral Cortex. Neuron 100, 1194–1208.e5. 10.1016/j.neuron.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyal G, Verhoog MB, Testa-Silva G, Deitcher Y, Lodder JC, Benavides-Piccione R, Morales J, DeFelipe J, de Kock CP, Mansvelder HD, and Segev I. (2016). Unique membrane properties and enhanced signal processing in human neocortical neurons. eLife 5, e16553. 10.7554/eLife.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaulieu-Laroche L, Toloza EH, Van der Goes M-S, Lafourcade M, Barnagian D, Williams ZM, Eskandar EN, Frosch MP, Cash SS, and Harnett MT (2018). Enhanced dendritic compartmentalization in human cortical neurons. Cell 175, 643–651.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchin A, de Frates R, Nandi A, Mann R, Chong P, Ng L, Miller J, Hodge R, Kalmbach B, Bose S, et al. (2020). Multi-modal characterization and simulation of human epileptic circuitry. Cell Rep. 41, 111873. 10.1101/2020.04.24.060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch C. (2004). Biophysics of Computation: Information Processing in Single Neurons (Oxford University Press; ). [Google Scholar]
- 47.Gutfreund Y, Yarom Y, and Segev I. (1995). Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. J. Physiol 483, 621–640. 10.1113/jphysiol.1995.sp020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutcheon B, and Yarom Y. (2000). Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 23, 216–222. 10.1016/S0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- 49.Hutcheon B, Miura RM, and Puil E. (1996). Models of subthreshold membrane resonance in neocortical neurons. J. Neurophysiol 76, 698–714. 10.1152/jn.1996.76.2.698. [DOI] [PubMed] [Google Scholar]
- 50.Chagnac-Amitai Y, Luhmann HJ, and Prince DA (1990). Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J. Comp. Neurol 296, 598–613. 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- 51.Buzsáki G, and Kandel A. (1998). Somadendritic backpropagation of action potentials in cortical pyramidal cells of the awake rat. J. Neurophysiol 79, 1587–1591. [DOI] [PubMed] [Google Scholar]
- 52.Williams SR, and Stuart GJ (1999). Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J. Physiol 521, 467–482. 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kandel A, and Buzsáki G. (1997). Cellular–Synaptic Generation of Sleep Spindles, Spike-and-Wave Discharges, and Evoked Thalamocortical Responses in the Neocortex of the Rat. J. Neurosci 17, 6783–6797. 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larkum ME, Nevian T, Sandler M, Polsky A, and Schiller J. (2009). Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325, 756–760. [DOI] [PubMed] [Google Scholar]
- 55.Shai AS, Anastassiou CA, Larkum ME, and Koch C. (2015). Physiology of Layer 5 Pyramidal Neurons in Mouse Primary Visual Cortex: Coincidence Detection through Bursting. PLoS Comput. Biol 11, e1004090. 10.1371/journal.pcbi.1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyal G, Mansvelder HD, de Kock CPJ, and Segev I. (2014). Dendrites Impact the Encoding Capabilities of the Axon. J. Neurosci 34, 8063–8071. 10.1523/JNEUROSCI.5431-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Testa-Silva G, Verhoog MB, Linaro D, de Kock CPJ, Baayen JC, Meredith RM, De Zeeuw CID, Giugliano M, and Mansvelder HD (2014). High Bandwidth Synaptic Communication and Frequency Tracking in Human Neocortex. PLoS Biol. 12, e1002007. 10.1371/journal.pbio.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beaulieu-Laroche L, Brown NJ, Hansen M, Toloza EHS, Sharma J, Williams ZM, Frosch MP, Cosgrove GR, Cash SS, and Harnett MT (2021). Allometric rules for mammalian cortical layer 5 neuron biophysics. Nature 600, 274–278. 10.1038/s41586-021-04072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang J, Huguenard JR, and Prince DA (2000). Voltage-Gated Potassium Channels Activated During Action Potentials in Layer V Neocortical Pyramidal Neurons. J. Neurophysiol 83, 70–80. 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- 60.Bean BP (2007). The action potential in mammalian central neurons. Nat. Rev. Neurosci 8, 451–465. 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 61.Fries P, Reynolds JH, Rorie AE, and Desimone R. (2001). Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science 291, 1560–1563. 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 62.Womelsdorf T, Fries P, Mitra PP, and Desimone R. (2006). Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439, 733–736. 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- 63.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, and Knight RT (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628. 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mosher CP, Mamelak AN, Malekmohammadi M, Pouratian N, and Rutishauser U. (2021). Distinct roles of dorsal and ventral subthalamic neurons in action selection and cancellation. Neuron 109, 869–881.e6. 10.1016/j.neuron.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, and Perret J. (1994). Acute and Long-Term Effects of Subthalamic Nucleus Stimulation in Parkinson’s Disease. Stereotact. Funct. Neurosurg 62, 76–84. 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- 66.Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, and Di Lazzaro V. (2004). Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp. Neurol 188, 480–490. 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, and Robbins TW (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci 6, 115–116. [DOI] [PubMed] [Google Scholar]
- 68.Aron AR, and Poldrack RA (2006). Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci 26, 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk H-J, Cassara AM, Neufeld E, Kuster N, Tsai L-H, et al. (2017). Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169, 1029–1041.e16. 10.1016/j.cell.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buzsáki G, Anastassiou CA, and Koch C. (2012). The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci 13, 407–420. 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, and Buzsáki G. (2008). Entrainment of Neocortical Neurons and Gamma Oscillations by the Hippocampal Theta Rhythm. Neuron 60, 683–697. 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anastassiou CA, and Koch C. (2015). Ephaptic coupling to endogenous electric field activity: why bother? Curr. Opin. Neurobiol 31, 95–103. 10.1016/j.conb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Torres D, Makarova J, Ortuñ o T, Benito N, Makarov VA, and Herreras O. (2019). Local and Volume-Conducted Contributions to Cortical Field Potentials. Cereb. Cortex 29, 5234–5254. 10.1093/cercor/bhz061. [DOI] [PubMed] [Google Scholar]
- 74.Allen Cell Types Database (2017). Electrophysiology. TECHNICAL WHITE PAPER. http://help.brain-map.org/display/celltypes/Documentation. [Google Scholar]
- 75.Campagnola L, Seeman SC, Chartrand T, Kim L, Hoggarth A, Gamlin C, Ito S, Trinh J, Davoudian P, Radaelli C, et al. (2022). Local connectivity and synaptic dynamics in mouse and human neocortex. Science 375, eabj5861. 10.1126/science.abj5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campagnola L, Kratz MB, and Manis PB (2014). ACQ4: an open-source software platform for data acquisition and analysis in neurophysiology research. Front. Neuroinform 8, 3. 10.3389/fninf.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hines ML, and Carnevale NT (1997). The NEURON simulation environment. Neural Comput. 9, 1179–1209. [DOI] [PubMed] [Google Scholar]
- 78.Gratiy SL, Billeh YN, Dai K, Mitelut C, Feng D, Gouwens NW, Cain N, Koch C, Anastassiou CA, and Arkhipov A. (2018). BioNet: A Python interface to NEURON for modeling large-scale networks. PLoS One 13, e0201630. 10.1371/journal.pone.0201630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buchin A, de Frates R, Nandi A, Mann R, Chong P, Ng L, Miller J, Hodge R, Kalmbach B, Bose S, et al. (2022). Multi-modal characterization and simulation of human epileptic circuitry. Cell Rep. 41, 111873. 10.1016/j.celrep.2022.111873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mosher CP, Wei Y, Kamiński J, Nandi A, Mamelak AN, Anastassiou CA, and Rutishauser U. (2020). Cellular Classes in the Human Brain Revealed In Vivo by Heartbeat-Related Modulation of the Extracellular Action Potential Waveform. Cell Rep. 30, 3536–3551.e6. 10.1016/j.celrep.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Geit W, Gevaert M, Chindemi G, Rössert C, Courcol J-D, Muller EB, Schürmann F, Segev I, and Markram H. (2016). BluePyOpt: Leveraging Open Source Software and Cloud Infrastructure to Optimise Model Parameters in Neuroscience. Front. Neuroinform 10, 17. 10.3389/fninf.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, et al. (2020). SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272. 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
The code used to analyze and process the physiology data is available at https://github.com/anastassiou-team/celltype_brainstim_invitro
The code to setup, run and analyze the simulations is available at https://github.com/anastassiou-team/celltype_brainstim_insilico
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.