Abstract
The interconversion of polyamines in the parasite nematode Ascaris suum by a novel type of polyamine oxidase was demonstrated. The nematode enzyme was clearly distinguishable from monoamine and diamine oxidases as well as from the mammalian polyamine oxidase, as shown by the use of the specific inhibitors pargyline, aminoguanidine and MDL 72527 respectively. All three inhibitors had no effect on the parasite polyamine oxidase, and the enzyme did not accept diamines such as putrescine, cadaverine or histamine as substrates. The parasite polyamine oxidase selectively oxidizes spermine and spermidine but not N-acetylated polyamines, whereas the mammalian tissue-type polyamine oxidase shows preference for the N-acetylated polyamines. These results suggest a regulatory function of the nematode polyamine oxidase in the degradation and interconversion of polyamines in parasite nematodes. The enzyme was purified to homogeneity by gel filtration, preparative isoelectric focusing and subsequent affinity chromatography on spermine- and berenil-Sepharose 4B. With respect to reaction type, the prosthetic group FAD, the molecular mass (66 kDa) and the contents of thiol and carbonyl groups, the polyamine oxidase from A. suum is similar to the isofunctional enzyme of mammalian tissue.
Full text
PDF

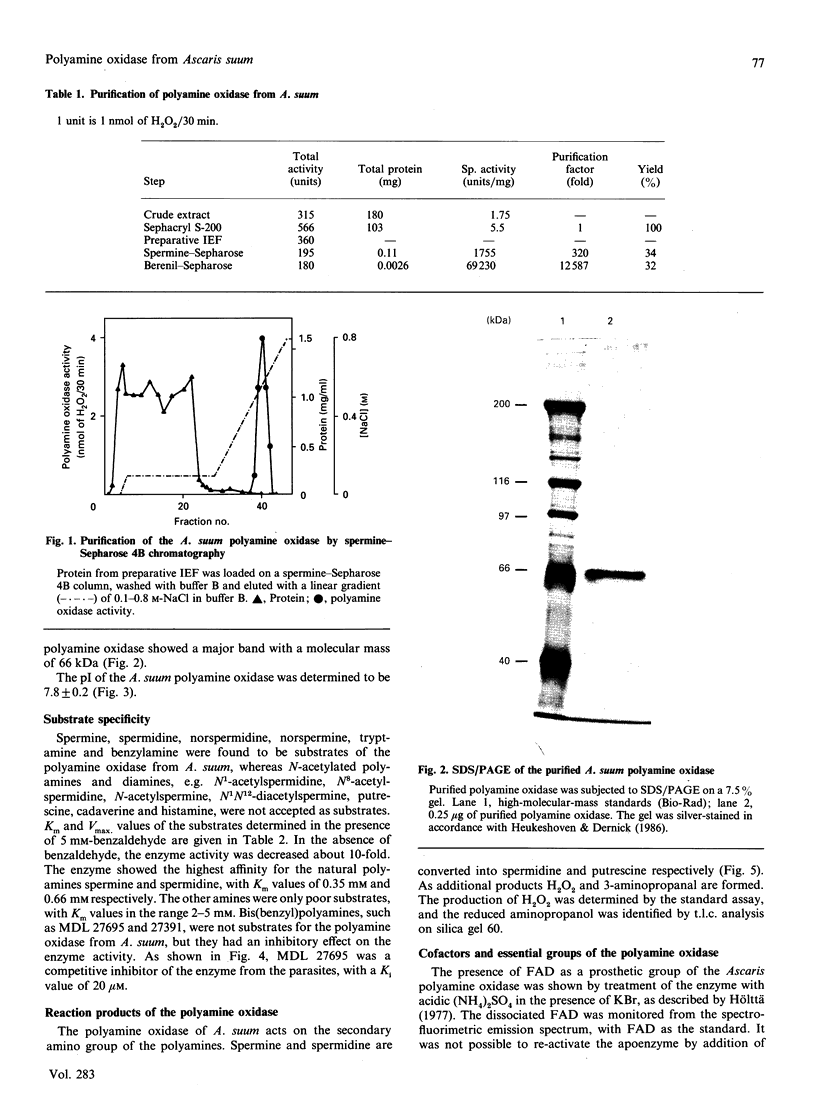
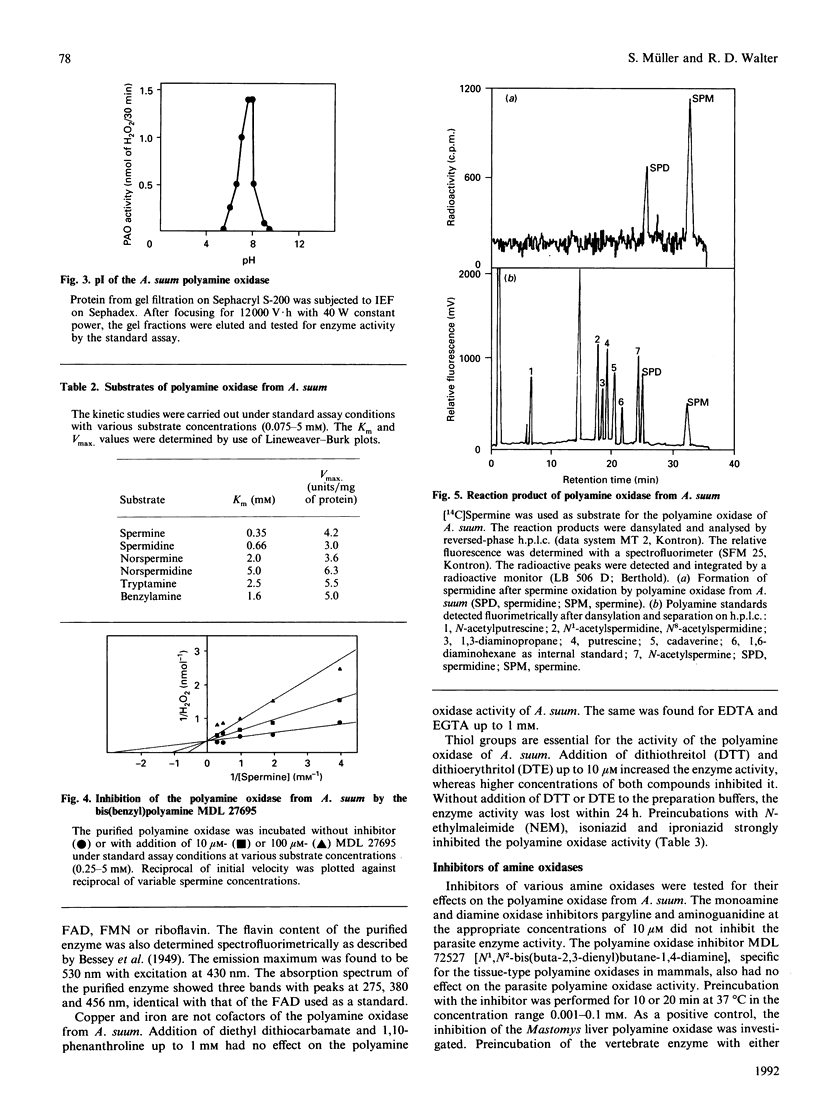


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achee F. M., Chervenka C. H., Smith R. A., Yasunobu K. T. Amine oxidase. XII. The association and dissociation, and number of subunits of beef plasma amine oxidase. Biochemistry. 1968 Dec;7(12):4329–4336. doi: 10.1021/bi00852a027. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey P., Bolkenius F. N., Seiler N., Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985 Jan;28(1):1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., Bush T. L., Stemerick D. M., Edwards M. L., McCann P. P. Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J Biol Chem. 1990 Jan 5;265(1):382–388. [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. The role of polyamine reutilization in depletion of cellular stores of polyamines in non-proliferating tissues. Biochim Biophys Acta. 1987 Jan 20;923(1):125–135. doi: 10.1016/0304-4165(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buffoni F. Histaminase and related amine oxidases. Pharmacol Rev. 1966 Dec;18(4):1163–1199. [PubMed] [Google Scholar]
- Fowler C. J., Mantle T. J., Tipton K. F. The nature of the inhibition of rat liver monoamine oxidase types A and B by the acetylenic inhibitors clorgyline, l-deprenyl and pargyline. Biochem Pharmacol. 1982 Nov 15;31(22):3555–3561. doi: 10.1016/0006-2952(82)90575-5. [DOI] [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Pulkkinen P., Elfving K., Jänne J. Oxidation of polymines by diamine oxidase from human seminal plasma. Biochem J. 1975 Feb;145(2):373–378. doi: 10.1042/bj1450373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. G., Sobota A., Bitonti A. J., McCann P. P., Byers T. J. Polyamine metabolism in Acanthamoeba: polyamine content and synthesis of ornithine, putrescine, and diaminopropane. J Protozool. 1987 Aug;34(3):278–284. doi: 10.1111/j.1550-7408.1987.tb03175.x. [DOI] [PubMed] [Google Scholar]
- Libby P. R., Porter C. W. Separation of two isozymes of polyamine oxidase from murine L1210 leukemia cells. Biochem Biophys Res Commun. 1987 Apr 14;144(1):528–535. doi: 10.1016/s0006-291x(87)80541-7. [DOI] [PubMed] [Google Scholar]
- Mondovì B., Riccio P., Agostinelli E. The biological functions of amine oxidases and their reaction products: an overview. Adv Exp Med Biol. 1988;250:147–161. doi: 10.1007/978-1-4684-5637-0_14. [DOI] [PubMed] [Google Scholar]
- Morgan D. M. Polyamine oxidases. Biochem Soc Trans. 1985 Apr;13(2):322–326. doi: 10.1042/bst0130322. [DOI] [PubMed] [Google Scholar]
- Müller S., Wittich R. M., Walter R. D. The polyamine metabolism of filarial worms as chemotherapeutic target. Adv Exp Med Biol. 1988;250:737–743. doi: 10.1007/978-1-4684-5637-0_65. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Ragione F. D., Pegg A. E. Purification and characterization of spermidine/spermine N1-acetyltransferase from rat liver. Biochemistry. 1982 Nov 23;21(24):6152–6158. doi: 10.1021/bi00267a020. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B., Mamont P. Polyamine oxidase in rat tissues. Biochim Biophys Acta. 1980 Oct;615(2):480–488. doi: 10.1016/0005-2744(80)90514-8. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N. Polyamine reutilization and turnover in brain. Neurochem Res. 1985 Apr;10(4):529–544. doi: 10.1007/BF00964656. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Sharma V., Visen P. K., Katiyar J. C., Wittich R. M., Walter R. D., Ghatak S., Shukla O. P. Polyamine metabolism in Ancylostoma ceylanicum and Nippostrongylus brasiliensis. Int J Parasitol. 1989 Apr;19(2):191–198. doi: 10.1016/0020-7519(89)90007-6. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Saxena J. K., Ghatak S., Shukla O. P., Wittich R. M., Walter R. D. Polyamine metabolism in Setaria cervi, the bovine filarial worm. Parasitol Res. 1989;75(4):311–315. doi: 10.1007/BF00931816. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Hendley E. D. A simple and sensitive fluorescence assay for monoamine oxidase and diamine oxidase. J Pharmacol Exp Ther. 1968 Oct;163(2):386–392. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., BACHRACH U. IDENTIFICATION OF THE AMINOALDEHYDES PRODUCED BY THE OXIDATION OF SPERMINE AND SPERMIDINE WITH PURIFIED PLASMA AMINE OXIDASE. J Biol Chem. 1964 Jul;239:2194–2203. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wittich R. M., Kilian H. D., Walter R. D. Polyamine metabolism in filarial worms. Mol Biochem Parasitol. 1987 Jun;24(2):155–162. doi: 10.1016/0166-6851(87)90102-2. [DOI] [PubMed] [Google Scholar]
- Wittich R. M., Walter R. D. A novel type of putrescine (diamine)-acetylating enzyme from the nematode Ascaris suum. Biochem J. 1989 May 15;260(1):265–269. doi: 10.1042/bj2600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittich R. M., Walter R. D. Putrescine N-acetyltransferase in Onchocerca volvulus and Ascaris suum, an enzyme which is involved in polyamine degradation and release of N-acetylputrescine. Mol Biochem Parasitol. 1990 Jan 1;38(1):13–17. doi: 10.1016/0166-6851(90)90199-v. [DOI] [PubMed] [Google Scholar]