Abstract
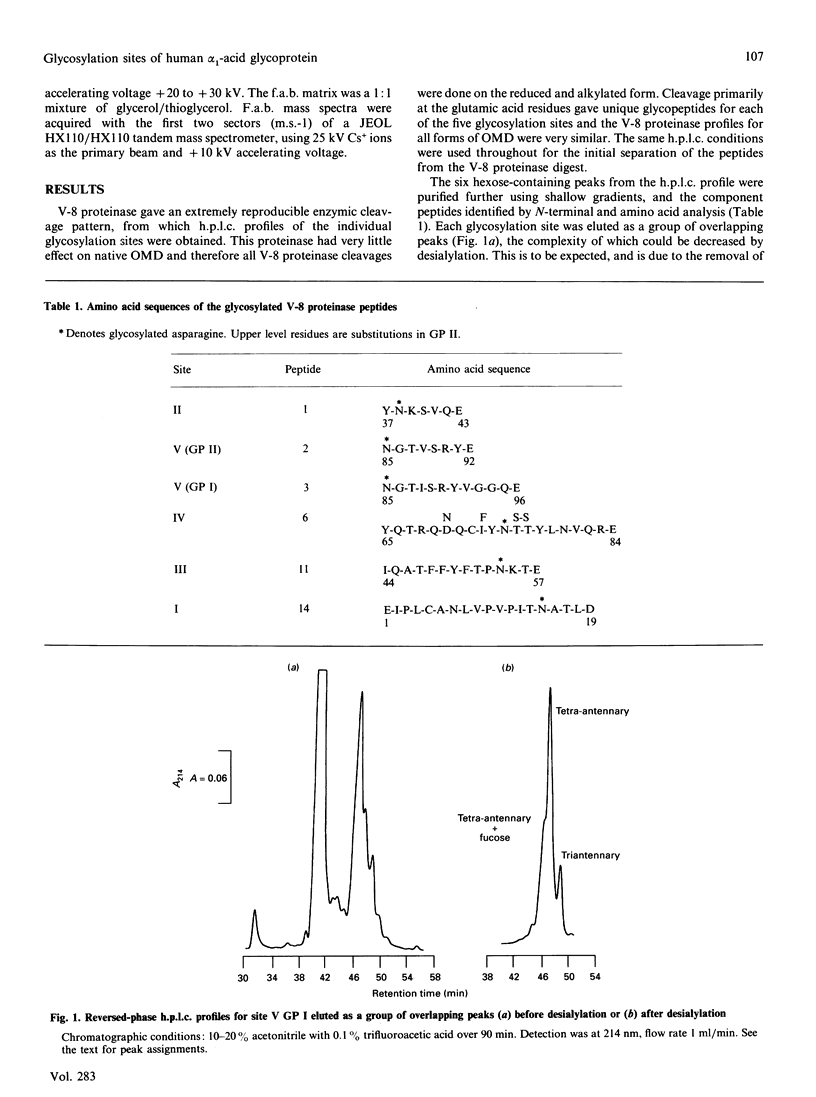
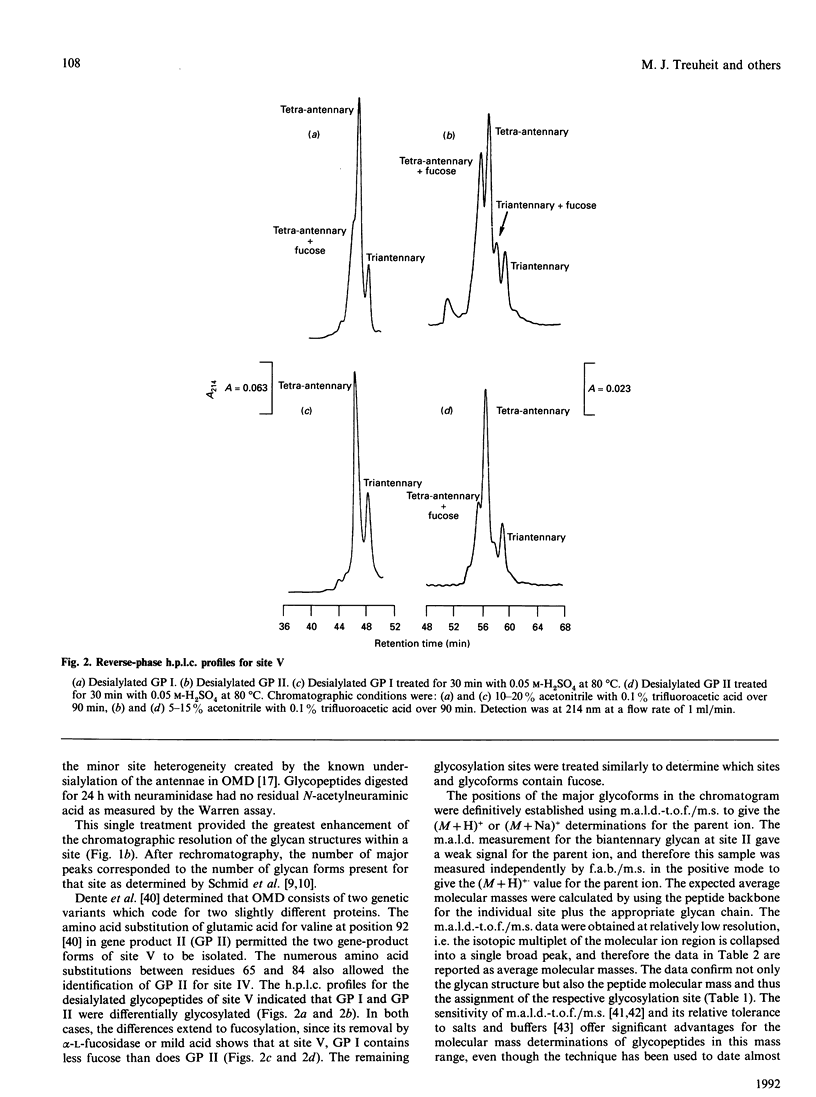
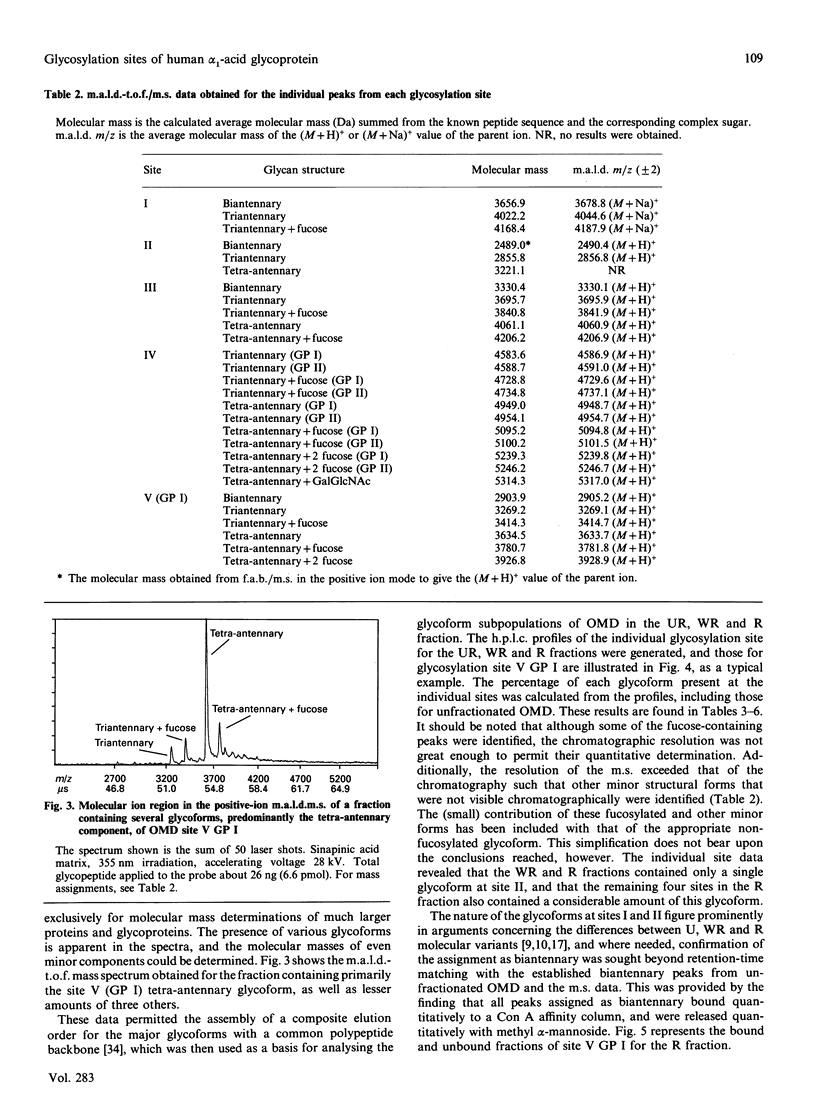
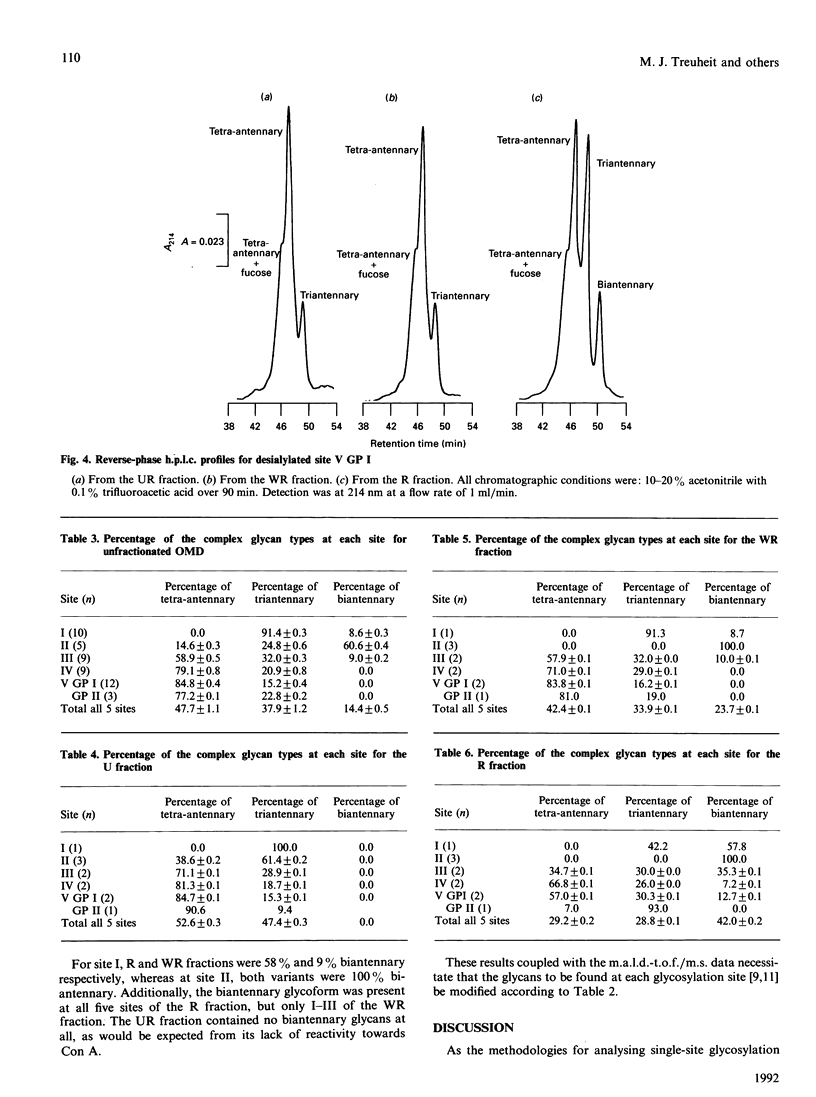
Orosomucoid (OMD) contains complex bi-, tri- and tetra-antennary glycan chains. Subfractionation of OMD into three molecular variants using concanavalin A lectin chromatography is based on variations in these complex structures. Standard h.p.l.c. profiles have been developed to analyse the percentage and distribution of the glycoforms present at each glycosylation site in OMD and its molecular variants. The ability to quantify the glycoforms present at each site allows us to extend the earlier results of others and resolve the remaining questions concerning the glycan structures of these variants. Most significantly, the proportions of bi-, tri- and tetra-antennary chains differ at each site for the three molecular variants. The most strongly retained variant from concanavalin A is uniquely capable of possessing biantennary chains at all five sites, whereas the unretained variant is completely devoid of biantennary chains. Only glycosylation site II of the five present is 100% biantennary in the retained and weakly retained variants. In addition, the two gene products of OMD were differentially glycosylated. Molecular masses of the glycoforms were verified by matrix-assisted u.v. laser desorption mass spectrometry. On the basis of the site distribution of oligosaccharides in the variants, efforts were made to understand the factors that control the processing of the carbohydrate chains in OMD. The results indicate that the 'site-directed' model of processing offers the most consistent explanation for the structures seen at the individual glycosylation sites of OMD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., McKibbin J. M., Hakomori S. The monoclonal antibody directed to difucosylated type 2 chain (Fuc alpha 1 leads to 2Gal beta 1 leads to 4[Fuc alpha 1 leads to 3]GlcNAc; Y Determinant). J Biol Chem. 1983 Oct 10;258(19):11793–11797. [PubMed] [Google Scholar]
- Bacchus H. Serum seromucoid and acid mucopolysaccharide in malignant neoplastic diseases. Cancer. 1965 Oct;18(10):1285–1291. doi: 10.1002/1097-0142(196510)18:10<1285::aid-cncr2820181011>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Bayard B., Kerckaert J. P. Evidence for uniformity of the carbohydrate chains in individual glycoprotein molecular variants. Biochem Biophys Res Commun. 1980 Jul 31;95(2):777–784. doi: 10.1016/0006-291x(80)90854-2. [DOI] [PubMed] [Google Scholar]
- Beavis R. C., Chait B. T. Cinnamic acid derivatives as matrices for ultraviolet laser desorption mass spectrometry of proteins. Rapid Commun Mass Spectrom. 1989 Dec;3(12):432–435. doi: 10.1002/rcm.1290031207. [DOI] [PubMed] [Google Scholar]
- Beavis R. C., Chait B. T. Rapid, sensitive analysis of protein mixtures by mass spectrometry. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6873–6877. doi: 10.1073/pnas.87.17.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhuizen M. F., De Wit M., Govers C. A., Ferwerda W., Koeleman C., Pos O., Van Dijk W. Glycosylation of three molecular forms of human alpha 1-acid glycoprotein having different interactions with concanavalin A. Variations in the occurrence of di-, tri-, and tetraantennary glycans and the degree of sialylation. Eur J Biochem. 1988 Aug 1;175(2):387–394. doi: 10.1111/j.1432-1033.1988.tb14208.x. [DOI] [PubMed] [Google Scholar]
- Bories P. N., Guenounou M., Féger J., Kodari E., Agneray J., Durand G. Human alpha 1-acid glycoprotein-exposed macrophages release interleukin 1 inhibitory activity. Biochem Biophys Res Commun. 1987 Sep 15;147(2):710–715. doi: 10.1016/0006-291x(87)90988-0. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Carver J. P. Solution conformation of asparagine-linked oligosaccharides: alpha(1-6)-linked moiety. Biochemistry. 1983 Jul 19;22(15):3680–3686. doi: 10.1021/bi00284a022. [DOI] [PubMed] [Google Scholar]
- Brockhausen I., Carver J. P., Schachter H. Control of glycoprotein synthesis. The use of oligosaccharide substrates and HPLC to study the sequential pathway for N-acetylglucosaminyltransferases I, II, III, IV, V, and VI in the biosynthesis of highly branched N-glycans by hen oviduct membranes. Biochem Cell Biol. 1988 Oct;66(10):1134–1151. doi: 10.1139/o88-131. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran E. V., Davila M., Nixon D., Mendicino J. Structures of the oligosaccharide chains of two forms of alpha 1-acid glycoprotein purified from liver metastases of lung, colon, and breast tumors. Cancer Res. 1984 Apr;44(4):1557–1567. [PubMed] [Google Scholar]
- Dahms N. M., Hart G. W. Influence of quaternary structure on glycosylation. Differential subunit association affects the site-specific glycosylation of the common beta-chain from Mac-1 and LFA-1. J Biol Chem. 1986 Oct 5;261(28):13186–13196. [PubMed] [Google Scholar]
- Dente L., Pizza M. G., Metspalu A., Cortese R. Structure and expression of the genes coding for human alpha 1-acid glycoprotein. EMBO J. 1987 Aug;6(8):2289–2296. doi: 10.1002/j.1460-2075.1987.tb02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet B., Montreuil J., Strecker G., Dorland L., Haverkamp J., Vliegenthart F. G., Binette J. P., Schmid K. Determination of the primary structures of 16 asialo-carbohydrate units derived from human plasma alpha 1-acid glycoprotein by 360-MHZ 1H NMR spectroscopy and permethylation analysis. Biochemistry. 1978 Nov 28;17(24):5206–5214. doi: 10.1021/bi00617a021. [DOI] [PubMed] [Google Scholar]
- Halsall H. B., Kirley T. L., Friedman M. L. The preparation of orosomucoid from nephrotic urine. Prep Biochem. 1982;12(2):111–120. doi: 10.1080/00327488208065556. [DOI] [PubMed] [Google Scholar]
- Hansen J. E., Larsen V. A., Bøg-Hansen T. C. The microheterogeneity of alpha 1-acid glycoprotein in inflammatory lung disease, cancer of the lung and normal health. Clin Chim Acta. 1984 Mar 27;138(1):41–47. doi: 10.1016/0009-8981(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Ikenaka T., Ishiguro M., Emura J., Kaufmann H., Isemura S., Bauer W., Schmid K. Isolation and partial characterization of the cyanogen bromide fragments of 1 -acid glycoprotein and the elucidation of the amino acid sequence of the carboxyl-terminal cyanogen bromide fragment. Biochemistry. 1972 Sep 26;11(20):3817–3829. doi: 10.1021/bi00770a022. [DOI] [PubMed] [Google Scholar]
- Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988 Oct 15;60(20):2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Whitehead J. S., Curtis K. J. Glycosyltransferases in human blood. II. Study of serum galactosyltransferase and N-acetylgalactosaminyltransferase in patients with liver diseases. J Clin Invest. 1972 Aug;51(8):2033–2039. doi: 10.1172/JCI107009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J. M., Wilting J., Janssen L. H. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol Rev. 1988 Mar;40(1):1–47. [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lainé E., Couderc R., Roch-Arveiller M., Vasson M. P., Giroud J. P., Raichvarg D. Modulation of human polymorphonuclear neutrophil functions by alpha 1-acid glycoprotein. Inflammation. 1990 Feb;14(1):1–9. doi: 10.1007/BF00914025. [DOI] [PubMed] [Google Scholar]
- Lammers G., Jamieson J. C. Studies on the effect of lysosomotropic agents on the release of Gal beta 1-4GlcNAc alpha-2,6-sialytransferase from rat liver slices during the acute-phase response. Biochem J. 1989 Jul 15;261(2):389–393. doi: 10.1042/bj2610389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune P. J., Mallet B., Farnarier C., Kaplanski S. Changes in serum level and affinity for concanavalin A of human alpha 1-proteinase inhibitor in severe burn patients: relationship to natural killer cell activity. Biochim Biophys Acta. 1989 Feb 24;990(2):122–127. doi: 10.1016/s0304-4165(89)80022-4. [DOI] [PubMed] [Google Scholar]
- Martyn J. A., Abernethy D. R., Greenblatt D. J. Plasma protein binding of drugs after severe burn injury. Clin Pharmacol Ther. 1984 Apr;35(4):535–539. doi: 10.1038/clpt.1984.73. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Greene T., Hakomori S. An alpha-N-acetylgalactosaminylation at the threonine residue of a defined peptide sequence creates the oncofetal peptide epitope in human fibronectin. J Biol Chem. 1989 Jun 25;264(18):10472–10476. [PubMed] [Google Scholar]
- Nakaishi H., Sanai Y., Shibuya M., Nagai Y. Analysis of cellular expression of gangliosides by gene transfection. II: Rat 3Y1 cells transformed with several DNAs containing oncogenes (fes, fps, ras & src) invariably express sialosylparagloboside. Biochem Biophys Res Commun. 1988 Jan 29;150(2):766–774. doi: 10.1016/0006-291x(88)90457-3. [DOI] [PubMed] [Google Scholar]
- Nicollet I., Lebreton J. P., Fontaine M., Hiron M. Evidence for alpha-1-acid glycoprotein populations of different pI values after concanavalin A affinity chromatography. Study of their evolution during inflammation in man. Biochim Biophys Acta. 1981 Apr 28;668(2):235–245. doi: 10.1016/0005-2795(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Néel D., Merlu B., Turpin E., Rabourdin-Combe C., Mach B., Goussault Y., Charron D. J. Characterization of N-linked oligosaccharides of an HLA-DR molecule expressed in different cell lines. Biochem J. 1987 Jun 1;244(2):433–442. doi: 10.1042/bj2440433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Rudd P. M., Thomas J. R., Rademacher T. W., Warren T., Wun T. C., Hebert B., Reitz B., Palmier M. N-glycosylation and in vitro enzymatic activity of human recombinant tissue plasminogen activator expressed in Chinese hamster ovary cells and a murine cell line. Biochemistry. 1989 Sep 19;28(19):7670–7679. doi: 10.1021/bi00445a023. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Thomas J. R., Opdenakker G., Rademacher T. W., Wittwer A. J., Howard S. C., Nelson R., Siegel N. R., Jennings M. G. Cell-type-specific and site-specific N-glycosylation of type I and type II human tissue plasminogen activator. Biochemistry. 1989 Sep 19;28(19):7644–7662. doi: 10.1021/bi00445a021. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Tse A. G., Dwek R. A., Williams A. F., Rademacher T. W. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987 May;6(5):1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987 Sep;1(3):209–214. doi: 10.1096/fasebj.1.3.3622999. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Reed R. R., Feinstein P. G., Snyder S. H. Molecular cloning of odorant-binding protein: member of a ligand carrier family. Science. 1988 Jul 15;241(4863):336–339. doi: 10.1126/science.3388043. [DOI] [PubMed] [Google Scholar]
- Pollack L., Atkinson P. H. Correlation of glycosylation forms with position in amino acid sequence. J Cell Biol. 1983 Aug;97(2):293–300. doi: 10.1083/jcb.97.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pos O., Oostendorp R. A., van der Stelt M. E., Scheper R. J., Van Dijk W. Con A-nonreactive human alpha 1-acid glycoprotein (AGP) is more effective in modulation of lymphocyte proliferation than Con A-reactive AGP serum variants. Inflammation. 1990 Apr;14(2):133–141. doi: 10.1007/BF00917452. [DOI] [PubMed] [Google Scholar]
- Pos O., van Dijk W., Ladiges N., Linthorst C., Sala M., van Tiel D., Boers W. Glycosylation of four acute-phase glycoproteins secreted by rat liver cells in vivo and in vitro. Effects of inflammation and dexamethasone. Eur J Cell Biol. 1988 Apr;46(1):121–128. [PubMed] [Google Scholar]
- Rademacher T. W., Dwek R. A. The role of oligosaccharides in modifying protein function. Ciba Found Symp. 1989;145:241–256. doi: 10.1002/9780470513828.ch14. [DOI] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- Schmid K., Binette J. P., Dorland L., Vliegenthart J. F., Fournet B., Montreuil J. The primary structure of the asialo-carbohydrate units of the first glycosylation site of human plasma alpha 1-acid glycoprotein. Biochim Biophys Acta. 1979 Dec 14;581(2):356–359. doi: 10.1016/0005-2795(79)90255-1. [DOI] [PubMed] [Google Scholar]
- Schmid K., Nimerg R. B., Kimura A., Yamaguchi H., Binette J. P. The carbohydrate units of human plasma alpha1-acid glycoprotein. Biochim Biophys Acta. 1977 Jun 24;492(2):291–302. doi: 10.1016/0005-2795(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Swiedler S. J., Freed J. H., Tarentino A. L., Plummer T. H., Jr, Hart G. W. Oligosaccharide microheterogeneity of the murine major histocompatibility antigens. Reproducible site-specific patterns of sialylation and branching in asparagine-linked oligosaccharides. J Biol Chem. 1985 Apr 10;260(7):4046–4054. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wells C., Bøg-Hansen T. C., Cooper E. H., Glass M. R. The use of concanavalin A crossed immuno-affinoelectrophoresis to detect hormone-associated variations in alpha 1-acid glycoprotein. Clin Chim Acta. 1981 Jan 8;109(1):59–67. doi: 10.1016/0009-8981(81)90137-6. [DOI] [PubMed] [Google Scholar]
- Wooten E. W., Bazzo R., Edge C. J., Zamze S., Dwek R. A., Rademacher T. W. Primary sequence dependence of conformation in oligomannose oligosaccharides. Eur Biophys J. 1990;18(3):139–148. doi: 10.1007/BF02427373. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Ohkura T., Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. J Biol Chem. 1985 Apr 10;260(7):3963–3969. [PubMed] [Google Scholar]
- Yet M. G., Shao M. C., Wold F. Effects of the protein matrix on glycan processing in glycoproteins. FASEB J. 1988 Jan;2(1):22–31. doi: 10.1096/fasebj.2.1.3275561. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Matsumoto A., Mizuochi T., Kawasaki T., Kobata A. Comparative study of the carbohydrate moieties of rat and human plasma alpha 1-acid glycoproteins. J Biol Chem. 1981 Aug 25;256(16):8476–8484. [PubMed] [Google Scholar]


