Abstract
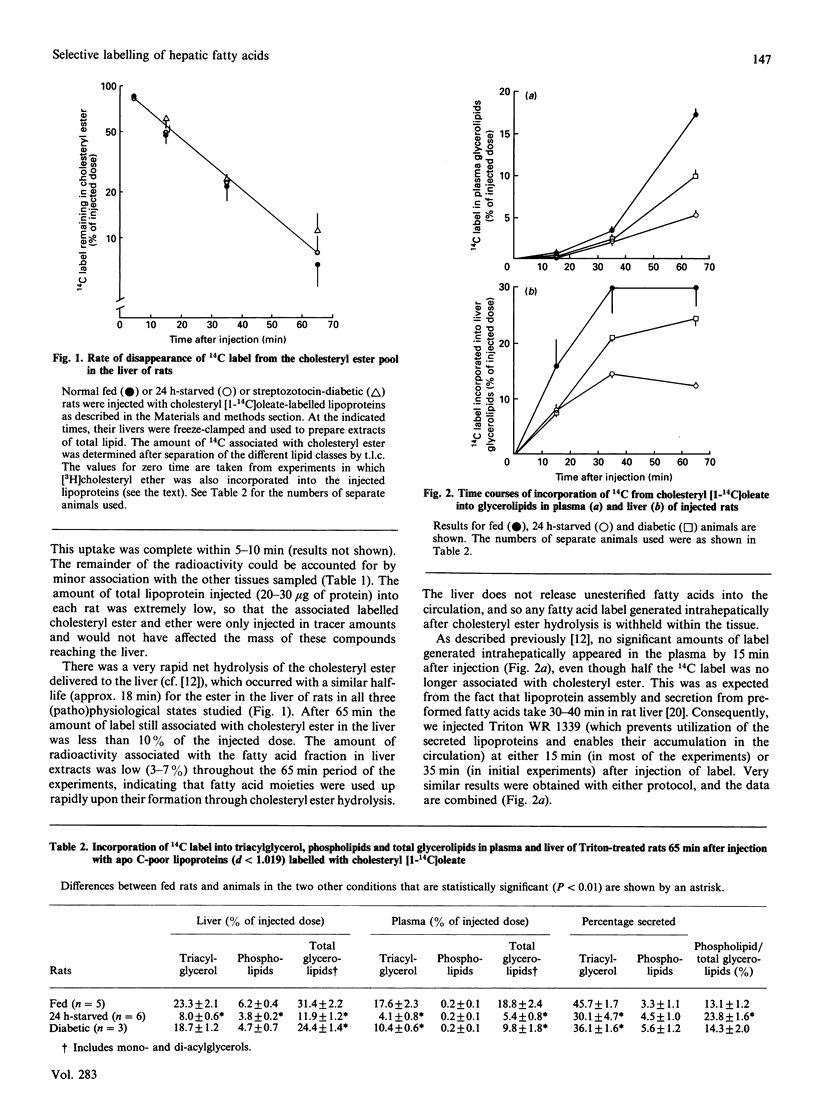
1. We describe a method for the selective labelling of hepatic fatty acids in the rat in vivo. It relies on (i) the rapid and preferential uptake of cholesteryl ester from chylomicron and/or very-low-density-lipoprotein remnants by the liver [Holder, Zammit & Robinson (1990) Biochem. J. 272, 735-741] (without prior exchange of the ester to other lipoproteins in the plasma), and (ii) the very short half-life of the cholesteryl ester in the liver. The 14C-labelled fatty acid moiety generated by cholesteryl ester hydrolysis was shown to be utilized by the liver for glycerolipid synthesis in a very similar pattern to that demonstrated for exogenous fatty acids by isolated cultured hepatocytes in previous studies. 2. Starvation (24 h) was shown to decrease the proportion of fatty acid utilized for glycerolipid synthesis, but to result in a proportionately smaller effect on incorporation into phospholipid. This was accompanied by a decrease in the fraction of synthesized triacylglycerol that was secreted by the liver. 3. Streptozotocin-diabetes did not affect the phospholipid/triacylglycerol ratio, but resulted in a small, but significant, decline in the fraction of triacylglycerol secreted by the liver. 4. In both starved and diabetic animals fatty acid esterification to the glycerol moiety constituted a smaller proportion of the total disposal of label. 5. These findings appear to validate the present method for the selective labelling of liver fatty acids in vivo in a non-invasive manner. Other possible uses for the method are suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbey M., Calvert G. D. Effects of blocking plasma lipid transfer protein activity in the rabbit. Biochim Biophys Acta. 1989 May 15;1003(1):20–29. doi: 10.1016/0005-2760(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Azain M. J., Fukuda N., Chao F. F., Yamamoto M., Ontko J. A. Contributions of fatty acid and sterol synthesis to triglyceride and cholesterol secretion by the perfused rat liver in genetic hyperlipemia and obesity. J Biol Chem. 1985 Jan 10;260(1):174–181. [PubMed] [Google Scholar]
- Bar-On H., Roheim P. S., Stein O., Stein Y. Contribution of floating fat triglyceride and of lecithin towards formation of secretory triglyceride in perfused rat liver. Biochim Biophys Acta. 1971 Oct 5;248(1):1–11. doi: 10.1016/0005-2760(71)90068-3. [DOI] [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Secretion and storage of newly synthesized hepatic triacylglycerol fatty acids in vivo in different nutritional states and in diabetes. Biochem J. 1988 Nov 1;255(3):929–935. doi: 10.1042/bj2550929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Storage, mobilization and secretion of cytosolic triacylglycerol in hepatocyte cultures. The role of insulin. Biochem J. 1990 Dec 15;272(3):583–587. doi: 10.1042/bj2720583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Marsh B., Burnham F. J., Gibbons G. F. Regulation of hepatic synthesis and secretion of cholesterol and glycerolipids in animals maintained in different nutritional states. Biochem J. 1990 Nov 1;271(3):761–766. doi: 10.1042/bj2710761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERETT N. B., SIMMONS B., LASHER E. P. Distribution of blood (Fe 59) and plasma (I 131) volumes of rats determined by liquid nitrogen freezing. Circ Res. 1956 Jul;4(4):419–424. doi: 10.1161/01.res.4.4.419. [DOI] [PubMed] [Google Scholar]
- Faergeman O., Havel R. J. Metabolism of cholesteryl esters of rat very low density lipoproteins. J Clin Invest. 1975 Jun;55(6):1210–1218. doi: 10.1172/JCI108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N., Azain M. J., Ontko J. A. Altered hepatic metabolism of free fatty acids underlying hypersecretion of very low density lipoproteins in the genetically obese Zucker rats. J Biol Chem. 1982 Dec 10;257(23):14066–14072. [PubMed] [Google Scholar]
- Gibbons G. F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem J. 1990 May 15;268(1):1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Burnham F. J. Effect of nutritional state on the utilization of fatty acids for hepatitic triacylglycerol synthesis and secretion as very-low-density lipoprotein. Biochem J. 1991 Apr 1;275(Pt 1):87–92. doi: 10.1042/bj2750087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F. Hyperlipidaemia of diabetes. Clin Sci (Lond) 1986 Nov;71(5):477–486. doi: 10.1042/cs0710477. [DOI] [PubMed] [Google Scholar]
- Groener J. E., van Golde L. M. Effect of fasting and feeding a high-sucrose, fat-free diet on the synthesis of hepatic glycerolipids in vivo and in isolated hepatocytes. Biochim Biophys Acta. 1977 Apr 26;487(1):105–114. doi: 10.1016/0005-2760(77)90047-9. [DOI] [PubMed] [Google Scholar]
- Groener J. E., van Golde L. M. Utilization of exogenously added and endogenously synthesized fatty acids for glycerolipids synthesis in isolated rat hepatocytes. Biochim Biophys Acta. 1978 Apr 28;529(1):88–95. doi: 10.1016/0005-2760(78)90106-6. [DOI] [PubMed] [Google Scholar]
- Holder J. C., Zammit V. A., Robinson D. S. The preferential uptake of very-low-density lipoprotein cholesteryl ester by rat liver in vivo. Biochem J. 1990 Dec 15;272(3):735–741. doi: 10.1042/bj2720735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mortimer B. C., Simmonds W. J., Cockman S. J., Stick R. V., Redgrave T. G. The effect of monostearoylglycerol on the metabolism of chylomicron-like lipid emulsions injected intravenously in rats. Biochim Biophys Acta. 1990 Aug 28;1046(1):46–56. doi: 10.1016/0005-2760(90)90093-d. [DOI] [PubMed] [Google Scholar]
- Ontko J. A., Cheng Q., Yamamoto M. Metabolic factors underlying high serum triglycerides in the normal hamster. J Lipid Res. 1990 Nov;31(11):1983–1992. [PubMed] [Google Scholar]
- Reaven E. P., Reaven G. M. Mechanisms for development of diabetic hypertriglyceridemia in streptozotocin-treated rats. Effect of diet and duration of insulin deficiency. J Clin Invest. 1974 Nov;54(5):1167–1178. doi: 10.1172/JCI107860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser T. R., Reaven G. M., Reaven E. P. Intestinal very low density lipoprotein secretion in insulin-deficient rats. Diabetes. 1978 Sep;27(9):902–908. doi: 10.2337/diab.27.9.902. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Murase T., Yamada N., Iwamoto Y., Akanuma Y., Takaku F. Plasma lipoproteins in streptozotocin-diabetic rats, after Triton WR-1339 treatment. Horm Metab Res. 1984 Dec;16 (Suppl 1):82–84. doi: 10.1055/s-2007-1014904. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Storer G. B., Trimble R. P. Effects of flow rate and insulin on triacylglycerol secretion by perfused rat liver. Am J Physiol. 1988 Sep;255(3 Pt 1):E306–E313. doi: 10.1152/ajpendo.1988.255.3.E306. [DOI] [PubMed] [Google Scholar]
- Van Berkel T. J., Van Tol A. Role of parenchymal and non-parenchymal rat liver cells in the uptake of cholesterolester-labeled serum lipoproteins. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1097–1101. doi: 10.1016/0006-291x(79)92120-x. [DOI] [PubMed] [Google Scholar]
- Van Zuiden P. E., Erickson S. K., Cooper A. D. Effect of removal of lipoproteins of different composition on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and hepatic very low density lipoprotein secretion. J Lipid Res. 1983 Apr;24(4):418–428. [PubMed] [Google Scholar]
- Whitlock M. E., Swenson T. L., Ramakrishnan R., Leonard M. T., Marcel Y. L., Milne R. W., Tall A. R. Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. J Clin Invest. 1989 Jul;84(1):129–137. doi: 10.1172/JCI114132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]
- Windler E., Havel R. J. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985 May;26(5):556–565. [PubMed] [Google Scholar]
- Zammit V. A. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]


