Abstract
Background
Expanding access to clinical trials in community settings is a potential approach to addressing disparities in accrual of historically underrepresented populations. However, little is known about the characteristics of practices that do not participate in research. We investigated differences in patient and practice characteristics of US community oncology practices with high vs low engagement in clinical research.
Methods
We included patients from a real-world, nationwide electronic health record–derived, de-identified database who received active treatment for cancer at community oncology practices between November 1, 2017, and October 31, 2022. We assessed patient and practice characteristics and their associations with high vs low research engagement using descriptive analyses and logistic regression models.
Results
Of the 178 practices, 70 (39.3%) events had high research engagement, treated 57.8% of the overall 568 540 patient cohort, and enrolled 3.25% of their patients on cancer treatment trials during the 5-year observation period (vs 0.27% enrollment among low engagement practices). Practices with low vs high research engagement treated higher proportions of the following patient groups: ages 75 years and older (24.2% vs 21.8%), non-Latinx Black (12.6% vs 10.3%) or Latinx (11.6% vs 6.1%), were within the lowest socioeconomic status quintile (21.9% vs16.5%), and were uninsured or had no documented insurance (22.2% vs 13.6%).
Conclusions
Patient groups historically underrepresented in oncology clinical trials are more likely to be treated at community practices with limited or no access to trials. These results suggest that investments to expand the clinical research footprint among practices with low research engagement could help address persistent inequities in trial representation.
It is well recognized that access to clinical trials is a critical component of high-quality cancer care (1). Furthermore, studying safety and efficacy of cancer therapeutics in representative populations is critical to ensure that results are generalizable and informative for all patients with cancer. Despite these tenets, equitable access to and participation in oncology clinical trials remains suboptimal. Rates of adult oncology clinical trial participation remain low, with estimates varying widely in the literature and ranging between 2% and 8% (2-4). In addition, conduct of cancer clinical research is traditionally skewed toward disease-based specialists at academic medical centers, although by some estimates, more than 80% of cancer patients are diagnosed and treated in community-based oncology clinics (5-8). Last, statistically significant race, ethnicity, sex, and age-based inequities in clinical trial enrollment persist (9-11). Several legislative and regulatory efforts have recently been enacted to promote inclusion of groups whose representation in clinical research is disproportionately low relative to their numbers in the disease populations. These include the Food and Drug Omnibus Reform Act (12), requiring prespecified enrollment goals across age, sex, racial, ethnic, and socioeconomic status (SES) groups from sponsors of clinical trials and the 2022 draft guidance to industry from the US Food and Drug Administration recommending that new agency submissions include race and ethnicity diversity plans (13,14).
Increasingly, research is being conducted to define strategies for research sponsors and sites to optimize recruitment and retention of a diverse pool of trial participants and measure the impact on clinical trial representativeness. Such strategies include broadening of inclusion and exclusion criteria (15,16), facilitated translation of study materials for patients with limited English proficiency (17,18), addressing bias and medical and research mistrust (19), hiring diverse research staff (20), and using technology for enhanced patient screening or site selection (21). Although potentially effective, these strategies are focused on improving representative enrollment at practices and centers, which already offer clinical trials to their patients. To ensure equitable access to research and generalizability of results, clinical trials should be available to all patients, wherever patients receive their care. Little is known about the differences between community oncology practices that do and do not actively participate in clinical trials. Therefore, we aimed to describe and compare the patient demographics and practice characteristics of community oncology practices demonstrating high vs low engagement in clinical research using data from a large de-identified US real-world database. Our goal was to inform which patient groups are more likely to have inequitable access to oncology research studies and how representativeness may be improved by expanding research capabilities in practices that do not currently participate.
Methods
Data source
This retrospective observational study used the nationwide Flatiron Health database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction (22,23). During the study period, the de-identified data originated from approximately 280 US cancer clinics (approximately 800 sites of care). The majority of patients in the database originate from private community oncology practices that operate independently without the ownership or support of health systems or academic medical centers. Institutional review board approval of the study protocol was obtained from WCG institutional review board prior to study conduct and included a waiver of informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.
The study period was between November 1, 2017, and October 31, 2022. Patients were included if they had a cancer diagnosis on or after January 1, 2011; were seen at community practices during our study period; and had active patient status, defined as at least 2 unique-date clinic encounters for a treatment administration. Patients who were seen at academic medical centers–owned community practices or at both academic and community practices were excluded from this analysis.
Variables and endpoints
Cancer treatment clinical trial participation was defined as having at least 1 administration or noncancelled order of an investigational cancer drug during the study period. The index date was defined as the first drug administration date or noncancelled order date of an investigational cancer drug for patients with trial participation; and the index date was defined as the later date of first cancer diagnosis date or November 1, 2017, for patients without trial participation during the study period.
Patient characteristics included age at index date (continuous and categorical), Eastern Cooperative Oncology Group performance status at or closest to the index date (0, 1, ≥2, unknown), sex, race, and ethnicity (non-Latinx Asian [henceforth referred to as Asian], non-Latinx Black [Black], Latinx, non-Latinx White [White], Other [American Indian or Alaska Native, Hawaiian or Pacific Islander, or multiracial], unknown), census region, and insurance status (health insurance a patient had at index date: Medicaid, Medicare, Commercial Health Plan, other, uninsured or unknown). Demographic data (sex, race, and ethnicity) were self-reported by patients to clinical teams. Area-level SES index was calculated using census block group (ie, neighborhood) data from the American Community Survey (2015-2019) per the Yost Index (incorporating income, home values, rental costs, poverty, blue-collar employment, unemployment, and education information) (24,25).
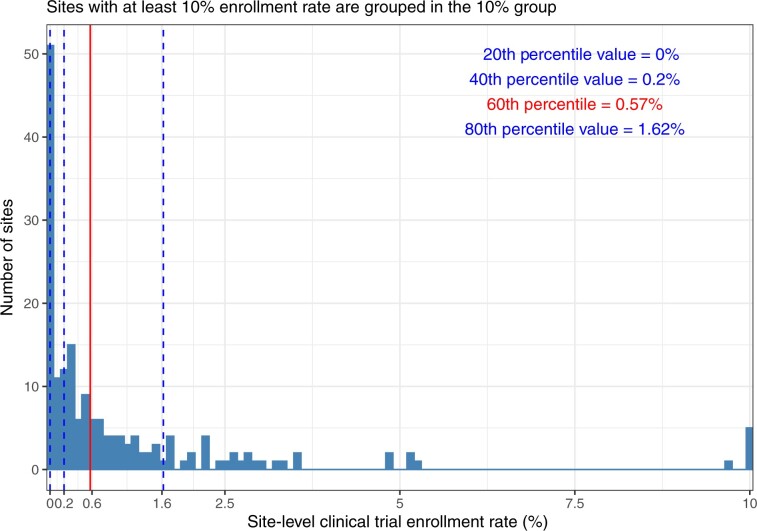
For each practice, the cancer treatment trial enrollment rate was calculated as the number of trial participants divided by the total number of active patients at the practice. Based on the observed quintiles in the right-skewed distribution of the practice-specific enrollment rates, the cutoff value to classify a practice as having high or low research engagement was set for practices within the highest 2 quintiles for enrollment rates (at or above the 60th percentile value of 0.57%).
Practice-level factors, derived during the study period, included numbers of active patients (quintiles); visits per year (quintiles); number of treatment visits; number of physicians (quintiles); patient-to-physician ratio (quintiles); percentages (continuous variable) of active patients with evidence of biomarker testing, patients who identify their race or ethnicity as Black or Latinx, and patients enrolled in Medicaid.
Statistical analyses
Descriptive statistics of patient characteristics were calculated for all patients and stratified by practices with high and low levels of engagement in clinical research. For continuous variables, the descriptive statistics included counts (the number of nonmissing values), medians, interquartile ranges (IQRs), and P values from 2-sample t tests for comparisons between the 2 patient cohorts. For categorical variables, the descriptive statistics included frequencies and percentages (including the missing category if applicable) and P values from χ2 test for group comparisons; P values less than .05 were considered statistically significant.
Because of the high correlation among the practice-level factors, univariate logistic regression models were fitted for each practice-level factor separately, across practices. The models estimated crude odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of a practice having a high research engagement as compared with the highest quintile group of the categorical practice-level factors or a unit increase in the continuous practice-level factors.
Results
Cohort and engagement definition
A total of 568 540 patients from 178 community oncology practices were included in our analysis. Figure 1 depicts the distribution of the observed cancer treatment trial enrollment rates for each of the practices. Based on our definition, 70 practices supported high research engagement, treated 328 694 patients (57.8% of the overall cohort), and enrolled 10 672 (3.25%) of their patients to cancer treatment trials during the 5-year study period. Among the 108 (60.7%) low research engagement practices treating 239 846 (42.2% of overall patients), 643 (0.27%) patients were enrolled in cancer treatment trials, and there were zero enrollments at 51 individual practices.
Figure 1.
Distribution of practice enrollment fractions in cancer treatment trials. Sites with an enrollment rate of at least 10% are categorized at 10% for visual purposes.
Patient characteristics and research engagement
The demographic and clinical characteristics of patients treated at practices with high vs low research engagement are summarized in Table 1. The median age of patients was similar between groups (66 vs 65 years), and the median time from diagnosis to receipt of a clinical study drug (if received) was 26.7 months. The greatest absolute differences among proportions of patients treated within each practice setting were seen in the following groups. Practices with high vs low research engagement had higher percentages of patients who were White (70.5% vs 67.8%), patients who described their race as Other (10.1% vs 6.1%), patients with commercial insurance (31.9% vs 28.5%), patients with Medicare (35.0% vs 31.7%), and patients within the third and fourth highest SES quintiles (22.1% vs 19.4% and 23.4% vs 19.9%, respectively). Conversely, practices with low vs high research engagement had higher percentages of patients who were age 75 years or older (24.2% vs 21.8%), patients who were Black (12.6% vs 10.3%) or Latinx (11.6% vs 6.1%), patients who had uninsured or unknown insurance status (22.2% vs 13.6%), and patients within the lowest socioeconomic quintile (21.9% vs 16.5%).
Table 1.
Patient characteristics by practice research engagement
| Characteristics | All patients diagnosed with cancer | Community practices with high research engagement | Community practices with low research engagement | P |
|---|---|---|---|---|
| (n = 568 540) | (n = 328 694) | (n = 239 846) | ||
| Time from diagnosis to first clinical study drug use or November 1, 2017, 2017, mo | <.001 | |||
| Median (IQR) | 26.7 (12.4-44.0) | 26.6 (12.2-43.6) | 27.0 (12.7-44.3) | |
| No. of cancer treatment trial participants during study period, No. (%) | 11 315 (1.99) | 10 672 (3.25) | 643 (0.27) | <.001 |
| Age at November 1, 2017, y | <.001 | |||
| Median (IQR) | 66.0 (57.0-74.0) | 65.0 (56.0-73.0) | 66.0 (57.0-74.0) | |
| Age category, y, No. (%) | <.001 | |||
| 49 and younger | 75 972 (13.4) | 46 060 (14.0) | 29 912 (12.5) | |
| 50-64 | 187 932 (33.1) | 110 845 (33.7) | 77 087 (32.1) | |
| 65-74 | 174 954 (30.8) | 100 085 (30.4) | 74 869 (31.2) | |
| 75 and older | 129 682 (22.8) | 71 704 (21.8) | 57 978 (24.2) | |
| Sex, No. (%) | .526 | |||
| Male | 248 518 (43.7) | 143 795 (43.7) | 104 723 (43.7) | |
| Female | 320 022 (56.3) | 184 899 (56.3) | 135 123 (56.3) | |
| Race and ethnicity, No. (%) | <.001 | |||
| Asian | 12 677 (2.6) | 9014 (3.0) | 3663 (1.9) | |
| Black | 55 506 (11.2) | 30 532 (10.3) | 24 974 (12.6) | |
| Latinx | 41 217 (8.3) | 18 255 (6.1) | 22 962 (11.6) | |
| Not documented | 73 190 | 30 984 | 42 206 | |
| Othera | 42 046 (8.5) | 30 023 (10.1) | 12 023 (6.1) | |
| White | 343 904 (69.4) | 209 886 (70.5) | 134 018 (67.8) | |
| Eastern Cooperative Oncology Group performance status at index, No. (%) | <.001 | |||
| 0 | 267 255 (53.3) | 147 867 (50.2) | 119 388 (57.7) | |
| 1 | 174 241 (34.7) | 109 096 (37.0) | 65 145 (31.5) | |
| ≥2 | 60 185 (12.0) | 37 709 (12.8) | 22 476 (10.9) | |
| Unknown or missing | 66 859 | 34 022 | 32 837 | |
| Insurance type, No. (%) | <.001 | |||
| Commercial | 173 151 (30.5) | 104 830 (31.9) | 68 321 (28.5) | |
| Medicaid | 60 900 (10.7) | 35 745 (10.9) | 25 155 (10.5) | |
| Medicare | 191 065 (33.6) | 115 061 (35.0) | 76 004 (31.7) | |
| Other | 45 341 (8.0) | 28 337 (8.6) | 17 004 (7.1) | |
| Uninsured or unknown | 98 083 (17.3) | 44 721 (13.6) | 53 362 (22.2) | |
| Socioeconomic status index (quintile), No. (%) | <.001 | |||
| 5 - Highest | 96 074 (18.5) | 53 319 (17.7) | 42 755 (19.6) | |
| 4 | 113 603 (21.9) | 70 259 (23.4) | 43 344 (19.9) | |
| 3 | 108 502 (20.9) | 66 339 (22.1) | 42 163 (19.4) | |
| 2 | 103 510 (19.9) | 61 277 (20.4) | 42 233 (19.4) | |
| 1 - Lowest | 97 276 (18.7) | 49 541 (16.5) | 47 735 (21.9) | |
| Missing or unknown | 49 575 | 27 959 | 21 616 | |
| Area, No. (%) | <.001 | |||
| Urban | 374 456 (72.2) | 214 968 (71.5) | 159 488 (73.1) | |
| Suburban | 69 188 (13.3) | 41 272 (13.7) | 27 916 (12.8) | |
| Rural | 75 322 (14.5) | 44 495 (14.8) | 30 827 (14.1) | |
| Missing or unknown | 49 574 | 27 959 | 21 615 |
Other includes American Indian or Alaska Native, Hawaiian or Pacific Islander, and/or multiracial. IQR = interquartile range.
Practice characteristics and research engagement
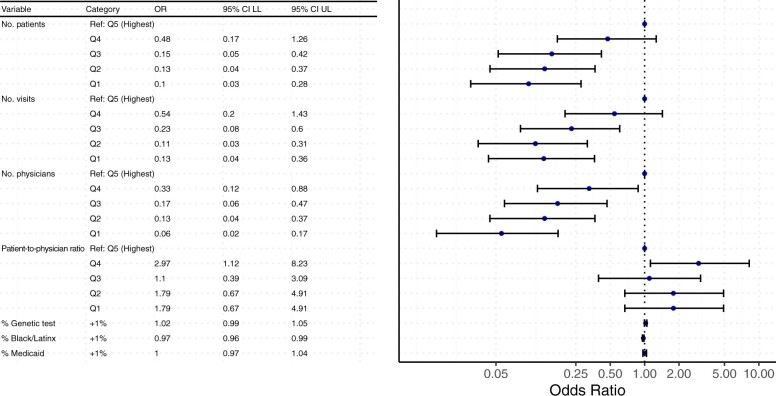
The practice-level characteristics are summarized in Table 2, overall and by research engagement. The 178 community oncology practices had a median of 6 physicians (IQR = 2-12 physicians), 1406 active patients (IQR = 634-3287 active patients), 9537 annual visits (IQR = 3999-23 840 annual visits), and 5629 treatment visits annually (IQR = 2456-12 069 treatment visits annually). Practices with high research engagement had statistically significantly higher median numbers of physicians and active patients than practices with low engagement (11.5 vs 4 and 2750 vs 1007, respectively; P < .001), though the patient-to-physician ratio was similar between groups. In univariate logistic regression analyses, there was a statistically significant inverse association between high research engagement and the lowest three quintiles for number of patients (quintile 3: OR = 0.15, 95% CI = 0.05 to 0.42; quintile 2: OR = 0.13, 95% CI = 0.04 to 0.37; quintile 1: OR = 0.1, 95% CI = 0.03 to 0.28) and number of visits per year (quintile 3: OR = 0.23, 95% CI = 0.08 to 0.60; quintile 2: OR = 0.11, 95% CI = 0.03 to 0.31; quintile 1: OR = 0.13, 95% CI = 0.04 to 0.36) compared with the highest quintile for each factor. Likelihood of research engagement statistically significantly declined as the median number of physicians in the practice decreased. The percentage of active patients with evidence of a tumor genomic test and percentage of active patients on Medicaid were not statistically significantly associated with a practice’s research engagement level (Figure 2).
Table 2.
Practice-level characteristics by site research engagementa
| Characteristics | All community practices-median (IQR) | Community practices with high research engagement-median (IQR) | Community practices with low research engagement-median (IQR) | P |
|---|---|---|---|---|
| (n = 178) | (n = 70) | (n = 108) | ||
| No. of active patients | 1406 (634-3287) | 2750 (1211-6076) | 1007 (565-2067) | <.001 |
| No. of visits | 9537 (3999-23840) | 20405 (9030-44951) | 7061 (3287-13424) | <.001 |
| No. of treatment visits | 5629 (2456-12069) | 9621 (5050-21577) | 3752 (1848-7357) | <.001 |
| No. of physicians | 6 (2-12) | 11.5 (5-21) | 4 (2-8) | <.001 |
| Patient-to-physician ratio | 307 (182-407) | 310 (192-391) | 302 (181-430) | .586 |
| % of active patients with evidence of a tumor genetic test | 22.3 (13.0-32.3) | 23.8 (15.4-33.1) | 20.6 (11.5-32.2) | .127 |
| % of active patients who are Black or Latinx | 14.0 (4.9-26.9) | 9.7 (4.1-18.1) | 18.1 (5.8-37.5) | .002 |
| % of active patients who have Medicaid | 8.8 (4.2-14.1) | 8.7 (4.8-13.2) | 8.8 (4.1-14.3) | .845 |
IQR = interquartile range.
Figure 2.
Odds ratios (ORs) of a community practice having high research engagement obtained from univariate logistic regression adjusting for practice-level factors. CI = confidence interval; LL = lower limit; Q = quintile; Ref. = referent; UL = upper limit.
Discussion
In this comparison between US community oncology practices with high and low research engagement, important findings regarding differences among practice and patient-level characteristics emerged. Though more numerous, low research engagement practices served a slightly lower but still substantial percentage (42%) of the total active patient population in this analysis, and 51 practices (29% of practices in our sample) had no patients at all with a recorded clinical study drug administration or order during our observation period spanning the past 5 years. Practices with high research engagement were on average larger, with higher numbers of patients, annual visits, and physicians. However, practices with low research engagement had higher proportions of Black and Latinx patients among their active patient population. Importantly, we found no statistically significant associations between research engagement and the percentage of patients with evidence of structured biomarker testing. Although this is only a single performance indicator, this suggests similar quality of clinical care delivery as defined by genomic testing across both practice settings.
We found several interesting trends among key demographic groups and practice settings with respect to research engagement. Sex distributions did not statistically significantly differ, and although the geographic differences (site of residence in urban, suburban, or rural areas) did achieve statistical significance, the point estimates were within 2% and therefore not likely clinically meaningful. Historically, patients who reside in rural areas have been recognized to have reduced access to and participation in oncology trials (26,27). Notably, practices with high and low research engagement in our cohort served nearly identical proportions of patients residing in rural areas, underscoring the ability of community oncology practices to extend clinical trial options for patients living in remote areas. However, we did identify some clinically meaningful differences among the patients seen at practices with and without robust research programs. Practices with high research engagement served higher proportions of patients who are White, insured, and at higher SES levels, whereas practices with low research engagement served higher proportions of patients who are Black, Latinx, without insurance or unknown status, and at the lowest SES levels. The finding that Black and Latinx patients were more likely to receive care at practices without robust research programs was consistent between our patient and practice characteristic analyses.
Notably, the demographic groups more likely to be treated at low- or nonresearch practices in our analysis are the same groups historically underrepresented in cancer clinical trials. Multiple studies have consistently reported the lack of cancer clinical trial representation among Black and Latinx patients (9,25,28-30), and a study evaluating recent trials supporting US Food and Drug Administration oncology drug approvals showed that not only are Black and Latinx patients underrepresented in trials compared with their expected cancer incidence (22% and 44%, respectively) but these rates have changed minimally over a 10-year period (31). A study among older patients with breast cancer, using Surveillance, Epidemiology, and End Results data, found an inverse association with low SES and trial participation (32), and a recent retrospective analysis at the University of Alabama at Birmingham found patients living in more disadvantaged areas (based on Area of Deprivation Index score) were less than half as likely to enroll in breast or ovarian treatment trials (33). More recently, a prospective survey study of barriers to trial participation found patients with low annual household income had 32% lower odds of trial participation than those with higher income and that trial participation further decreased as annual income decreased from less than $50 000 to less than $20 000 (34). However, a recent meta-analysis shows that when trials are offered to patients, rates of enrollment are similar across races and ethnicities, highlighting the role unequal access to clinical research plays in the perpetuation of inequities in trial participation (3).
To our knowledge, this is the first national study to report that patient groups underrepresented in cancer treatment trials are those more likely to be seen at community practices that lack engagement with (and possibly infrastructure and/or resources to offer) clinical research studies. These findings suggest that an underlying contributor to low accrual among historically underrepresented racial, ethnic, and sociodemographic groups is access to the facilities that offer clinical trials and support the hypothesis that trial representativeness may be improved if inequities in research access are addressed.
Several public and private initiatives are focused on improving equitable access to and participation in clinical research for patients with cancer. Some of these initiatives are specifically focused on expanding support for sites and investigators not currently engaged in research. For example, the American Association of Cancer Research co-sponsored Robert A. Winn Diversity in Clinical Trials award program offers a training program for new community investigators and community-based clinical research externships for medical students (35). The Association of Cancer Care Centers established the Community Oncology Research Institute in 2021, and with support from the National Quality Minority Forum, aims to recruit, train, and set up new research programs in communities serving rural and historically underrepresented minorities (36). Recommendations from a cancer trial accrual symposium co-sponsored by the National Cancer Institute and the American Society of Clinical Oncology specifically call for community practitioner engagement, incentives for clinicians to participate in research, and implementation of performance standards to qualify clinical investigators (37). Community–academic partnerships have been successful to establish conduits for investigator-initiated studies to expand to community settings (38), and several private companies offer paid end-to-end services for staff training, study coordination, and financial management for new research sites. Last, there is an emerging role for technology to facilitate screening, data capture, and automated study data transfer to reduce staff burden of research conduct for new and established community research programs to remain viable and sustainable (39-41). The growing availability and capabilities of electronic health record–embedded research tools promise to reduce the investments needed to onboard research-naive sites, which, as shown here, tend to be smaller and treat fewer patients. Our study helps shed light on the potential impact of these and other efforts to expand the footprint of oncology community research.
This study has several limitations inherent to retrospective analyses using observational data. The use of clinical study drug administration as a surrogate for clinical trial participation captured only cancer drug treatment trial accrual and was unable to measure surgical and radiation oncology trial participation or participation in research for which there was not an investigational study drug. We therefore may have underestimated overall clinical trial accrual. Though covering a broad range of types, sizes, and geographic locations of sites, it is unclear if the trends observed among community oncology practices in this cohort are generalizable to all of community oncology in the United States at large. Lastly, the results of this analysis are dependent on the cutoff set to define high vs low research engagement. As there was no precedent in the literature to inform our methodology, we used a data-driven approach, choosing practices with the highest 2 quintiles of clinical study drug administrations (as opposed to the median) because of the relatively high number of practices with no trial participants. However, the overall enrollment rate of research-enabled practices by our definition (3.25%) is similar to contemporary estimates of patient enrollment fractions in community oncology cancer clinics (4%), according to a recent analysis from data reported to the Commission of Cancer (4,42).
Despite wide recognition and myriad attempted interventions to improve poor representativeness in clinical trials, inequities in study participation for patients with cancer persist. Our work found that the patient groups historically underrepresented in clinical trials are the same populations that are more likely to be treated at community practices that have limited or no active research programs. These results suggest that investments to expand and promote research infrastructure and engagement among practices that do not participate in research may help move the needle on equitable access, trial representativeness, and generalizability of oncology study results.
Acknowledgements
The authors, who currently are or once were employed by the sponsors, were responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors would like to thank Anjali Ramoutar from Flatiron Health, Inc, for editorial support.
Contributor Information
Ivy Altomare, Flatiron Health, Inc, New York, NY, USA.
Xiaoliang Wang, Flatiron Health, Inc, New York, NY, USA.
Maneet Kaur, Flatiron Health, Inc, New York, NY, USA.
Jenny S Guadamuz, Flatiron Health, Inc, New York, NY, USA; School of Public Health, Division of Health Policy and Management, University of California, Berkeley, Berkeley, CA, USA.
Sam Falk, Flatiron Health, Inc, New York, NY, USA.
Forrest Xiao, Flatiron Health, Inc, New York, NY, USA.
Neal J Meropol, Flatiron Health, Inc, New York, NY, USA; Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, US.
Yihua Zhao, Flatiron Health, Inc, New York, NY, USA.
Data availability
The data that support the findings of this study were originated by and are the property of Flatiron Health, Inc, which has restrictions prohibiting the authors from making the dataset publicly available. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to publicationsdataaccess@flatiron.com.
Author contributions
Ivy Altomare, MD (Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—Original draft; Writing—Review & editing), Xiaoliang Wang, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing—Review & editing), Maneet Kaur, PhD, MPH (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing—Original draft; Writing—Review & editing), Jenny S. Guadamuz, PhD (Conceptualization; Investigation; Methodology; Software; Supervision; Validation; Visualization; Writing—Review & editing), Sam Falk, MS (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing—Review & editing), Forrest Xiao, PhD (Conceptualization; Data curation; Formal analysis; Methodology; Software; Visualization; Writing—Review & editing), Neal J. Meropol, MD (Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—Review & editing), and Yihua Zhao, PhD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—Original draft).
Funding
This work was supported by Flatiron Health, Inc, an independent member of the Roche Group.
Conflicts of interest
During the study period, all authors reported employment with Flatiron Health, Inc, and stock ownership in Roche. JSG, SF, FX, and YZ no longer report employment with Flatiron Health; SF reports employment with Thyme Care, Data Science, New York, NY, USA; FX reports employment with Turquoise Health (Quantitative Research, San Diego, CA, USA); YZ reports employment with Jiangsu Hengrui Pharmaceuticals (Biometrics Department, Shanghai, P.R. China).
References
- 1. National Comprehensive Cancer Network. National Comprehensive Cancer Network. https://www.nccn.org/home. Accessed July 3, 2023.
- 2. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728-1733. doi: 10.1200/jco.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 3. Unger JM, Hershman DL, Till C, et al. “When offered to participate:” a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J Natl Cancer Inst. 2020;113(3):244-257. doi: 10.1093/jnci/djaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Unger JM, Shulman LN, Facktor MA, Nelson H, Fleury ME.. National estimates of the participation of patients with cancer in clinical research studies based on commission on cancer accreditation data. JCO. 2024;42(18):2139-2148. doi: 10.1200/jco.23.01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Copur MS, Ramaekers R, Gönen M, et al. ReCAP: impact of the national cancer institute community cancer centers program on clinical trial and related activities at a community cancer center in rural Nebraska. JOP. 2015;12(1):67-68. doi: 10.1200/jop.2015.005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Copur MS. Inadequate awareness of and participation in cancer clinical trials in the community oncology setting. Oncol (Williston Park, NY). 2019;33(2):54-57. [PubMed] [Google Scholar]
- 7. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME.. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245-255. doi: 10.1093/jnci/djy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg AK. Community-based cancer care quality and expertise in a COVID-19 era and beyond. Am J Clin Oncol. 2020;43(8):537-538. doi: 10.1097/coc.0000000000000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 10. Javier‐DesLoges J, Nelson TJ, Murphy JD, et al. Disparities and trends in the participation of minorities, women, and the elderly in breast, colorectal, lung, and prostate cancer clinical trials. Cancer. 2022;128(4):770-777. doi: 10.1002/cncr.33991 [DOI] [PubMed] [Google Scholar]
- 11. American Association for Cancer Research. AACR Cancer Disparities Progress Report.2022. https://cancerprogressreport.aacr.org/wp-content/uploads/sites/2/2022/06/AACR_CDPR_2022.pdf. Accessed July 3, 2023. [DOI] [PubMed]
- 12. Rep. Connolly GE. Making Consolidated Appropriations for the Fiscal Year Ending September 30, 2023, and for Providing Emergency Assistance for the Situation in Ukraine, and for Other Purposes. https://www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf. Accessed January 15, 2024.
- 13. US Food and Drug Administration. Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry; Availability. 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations. Accessed June 9, 2023.
- 14. Varma T, Gross CP, Miller JE.. Clinical trial diversity—will we know it when we see it? JAMA Oncol. 2023;9(6):765-767. doi: 10.1001/jamaoncol.2023.0143 [DOI] [PubMed] [Google Scholar]
- 15. Kaur M, Frahm F, Ascha M, et al. Trial eligibility criteria (EC) and diversity among patients with advanced non small cell lung cancer (advNSCLC). J Clin Oncol 2023;41(suppl 16):1566. 10.1200/jco.2023.41.16_suppl.1566 [DOI] [Google Scholar]
- 16. Liu R, Rizzo S, Whipple S, et al. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592(7855):629-633. doi: 10.1038/s41586-021-03430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahn JM, Gray DM, Oliveri JM, Washington CM, DeGraffinreid CR, Paskett ED.. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128(2):216-221. doi: 10.1002/cncr.33905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JS, Pérez-Stable EJ, Gregorich SE, et al. Increased access to professional interpreters in the hospital improves informed consent for patients with limited English proficiency. J Gen Intern Med. 2017;32(8):863-870. doi: 10.1007/s11606-017-3983-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: Perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126(9):1958-1968. doi: 10.1002/cncr.32755 [DOI] [PubMed] [Google Scholar]
- 20. Bains A, Osathanugrah P, Sanjiv N, et al. Diverse research teams and underrepresented groups in clinical studies. JAMA Ophthalmol. 2023;141(11):1037-1044. doi: 10.1001/jamaophthalmol.2023.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St. Germain DC, McCaskill‐Stevens W.. Use of a clinical trial screening tool to enhance patient accrual. Cancer. 2021;127(10):1630-1637. doi: 10.1002/cncr.33399 [DOI] [PubMed] [Google Scholar]
- 22. Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health, SEER, and NPCR. medRxiv. 2023;2020:03.16.20037143. doi: 10.1101/2020.03.16.20037143 [DOI] [Google Scholar]
- 23. Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. 2020. doi: 10.48550/arxiv.2001.09765 [DOI]
- 24. Yost K, Perkins C, Cohen R, Morris C, Wright W.. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 25. Guadamuz J, Mamtani R, Wang X, et al. Abstract A049: Racial/ethnic and socioeconomic inequities in clinical trial participation among US community oncology patients, 2011-2021. Cancer Epidemiology, Biomark Prev. 2023;32(suppl 1):A049-A049. doi: 10.1158/1538-7755.disp22-a049 [DOI] [Google Scholar]
- 26. Virani S, Burke L, Remick SC, Abraham J.. Barriers to recruitment of rural patients in cancer clinical trials. JOP. 2011;7(3):172-177. doi: 10.1200/jop.2010.000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mudaranthakam DP, Gajewski B, Krebill H, et al. Barriers to clinical trial participation: comparative study between rural and urban participants. JMIR Cancer. 2022;8(2):e33240. doi: 10.2196/33240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Unger JM, Hershman DL, Osarogiagbon RU, et al. Representativeness of black patients in cancer clinical trials sponsored by the National Cancer Institute compared to pharmaceutical companies. JNCI Cancer Spectr. 2020;4(4):pkaa034. doi: 10.1093/jncics/pkaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guadamuz J, Wang X, Altomare I, Calip GS.. Mediators of racial/ethnic inequities in clinical trial participation among US patients with cancer, 2011-2022. J Clin Oncol. 2023;41(suppl 16):6511-6511. doi: 10.1200/jco.2023.41.16_suppl.6511 [DOI] [Google Scholar]
- 30. Pittell H, Calip GS, Pierre A, et al. Racial and ethnic inequities in US oncology clinical trial participation from 2017 to 2022. JAMA Netw Open. 2023;6(7):e2322515. doi: 10.1001/jamanetworkopen.2023.22515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870. doi: 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gross CP, Filardo G, Mayne ST, Krumholz HM.. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491. doi: 10.1002/cncr.20792 [DOI] [PubMed] [Google Scholar]
- 33. Caston NE, Lalor F, Wall J, et al. Ineligible, unaware, or uninterested? Associations between underrepresented patient populations and retention in the pathway to cancer clinical trial enrollment. J Clin Oncol Oncol Pr. 2022;18(11):e1854-e1865. doi: 10.1200/op.22.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL.. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2015;2(1):137-133. doi: 10.1001/jamaoncol.2015.3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robert A. Winn Diversity in Clinical Trials Award Program. https://diversityinclinicaltrials.org/. Accessed June 9, 2023.
- 36. Association of Community Cancer Centers. ACORI: ACCC Community Oncology Research Institute. https://www.accc-cancer.org/home/learn/community-oncology-research/accc-community-oncology-research-institute. Accessed July 31, 2023.
- 37. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American society of clinical oncology cancer trial accrual symposium: summary and recommendations. JOP. 2013;9(6):267-276. doi: 10.1200/jop.2013.001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vicioso NL, Lin D, Gomez DR, et al. Implementation strategies to increase clinical trial enrollment in a community-academic partnership and impact on Hispanic representation: an interrupted time series analysis. J Clin Oncol Oncol Pr. 2022;18(5):e780-e785. doi: 10.1200/op.22.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baer A, Bechar N, Cohen G, Devine S.. Basic steps to building a research program. JOP. 2010;6(1):45-47. doi: 10.1200/jop.091070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parikh RB, Basen-Enquist KM, Bradley C, et al. Digital health applications in oncology: an opportunity to seize. J Natl Cancer Inst. 2022;114(10):1338-1339. doi: 10.1093/jnci/djac108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Royce TJ, Falk S, Xiao F, et al. Technology-enabled clinical trials: intentional capture of source data (IDC) in the electronic-health record (EHR) and direct transfer to trial database (electronic data capture [EDC]) in a phase II multicenter trial. JCO. 41(16):1568-1568. doi: 10.1200/jco.2023.41.16_suppl.1568 [DOI]
- 42. Unger JM, Fleury M. Nationally representative estimates of the participation of cancer patients in clinical research studies according to the commission on cancer. JCO. 39(28):74-74. doi: 10.1200/jco.2020.39.28_suppl.74. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study were originated by and are the property of Flatiron Health, Inc, which has restrictions prohibiting the authors from making the dataset publicly available. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to publicationsdataaccess@flatiron.com.