Abstract
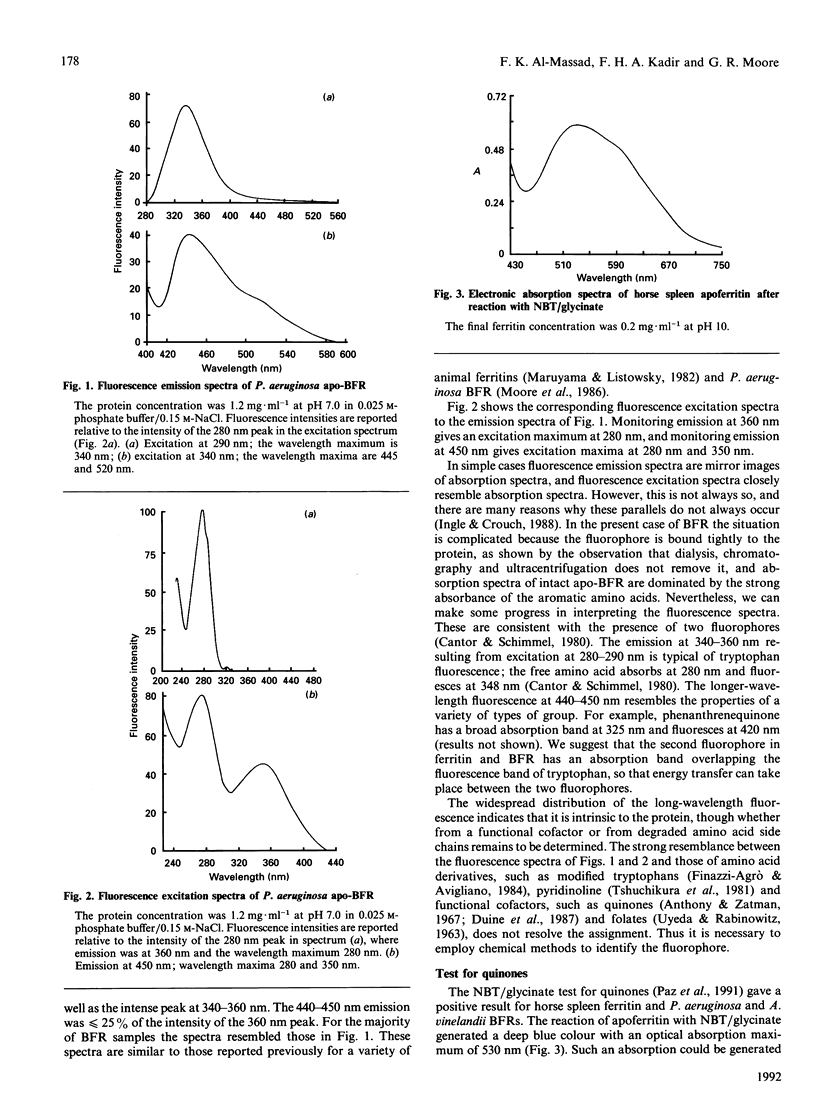
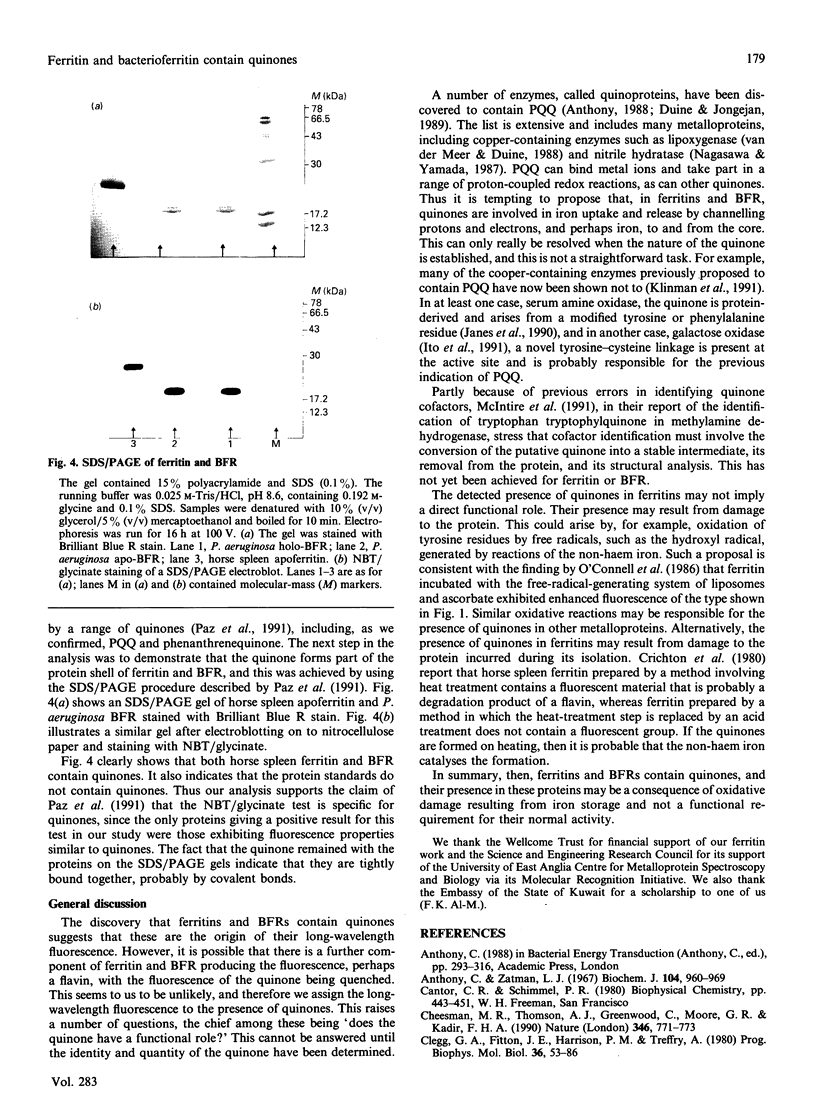
The origin of the 440 nm fluorescence of horse spleen ferritin and of Pseudomonas aeruginosa and Azotobacter vinelandii bacterioferritin has been investigated using a Nitro Blue Tetrazolium/glycinate colorimetric test specific for quiones [Paz, Flückiger, Boak, Kagan & Gallop (1991) J. Biol. Chem. 266, 689-692]. The results of the analysis indicate that ferritin and bacterioferritins contain quinones. A possible functional role of these quinones in iron uptake and release is described, as is the possibility that the presence of quinones in these proteins results from oxidative damage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J. 1967 Sep;104(3):960–969. doi: 10.1042/bj1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman M. R., Thomson A. J., Greenwood C., Moore G. R., Kadir F. Bis-methionine axial ligation of haem in bacterioferritin from Pseudomonas aeruginosa. Nature. 1990 Aug 23;346(6286):771–773. doi: 10.1038/346771a0. [DOI] [PubMed] [Google Scholar]
- Clegg G. A., Fitton J. E., Harrison P. M., Treffry A. Ferritin: molecular structure and iron-storage mechanisms. Prog Biophys Mol Biol. 1980;36(2-3):56–86. [PubMed] [Google Scholar]
- Crichton R. R., Roman F., Roland F. Iron mobilization from ferritin by chelating agents. J Inorg Biochem. 1980 Dec;13(4):305–316. doi: 10.1016/s0162-0134(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jongejan J. A. Enzymology of quinoproteins. Adv Enzymol Relat Areas Mol Biol. 1987;59:169–212. doi: 10.1002/9780470123058.ch4. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Jongejan J. A. Quinoproteins, enzymes with pyrrolo-quinoline quinone as cofactor. Annu Rev Biochem. 1989;58:403–426. doi: 10.1146/annurev.bi.58.070189.002155. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Ito N., Phillips S. E., Stevens C., Ogel Z. B., McPherson M. J., Keen J. N., Yadav K. D., Knowles P. F. Novel thioether bond revealed by a 1.7 A crystal structure of galactose oxidase. Nature. 1991 Mar 7;350(6313):87–90. doi: 10.1038/350087a0. [DOI] [PubMed] [Google Scholar]
- Janes S. M., Mu D., Wemmer D., Smith A. J., Kaur S., Maltby D., Burlingame A. L., Klinman J. P. A new redox cofactor in eukaryotic enzymes: 6-hydroxydopa at the active site of bovine serum amine oxidase. Science. 1990 May 25;248(4958):981–987. doi: 10.1126/science.2111581. [DOI] [PubMed] [Google Scholar]
- Kadir F. H., Moore G. R. Bacterial ferritin contains 24 haem groups. FEBS Lett. 1990 Oct 1;271(1-2):141–143. doi: 10.1016/0014-5793(90)80391-u. [DOI] [PubMed] [Google Scholar]
- Klinman J. P., Dooley D. M., Duine J. A., Knowles P. F., Mondovi B., Villafranca J. J. Status of the cofactor identity in copper oxidative enzymes. FEBS Lett. 1991 Apr 22;282(1):1–4. doi: 10.1016/0014-5793(91)80431-2. [DOI] [PubMed] [Google Scholar]
- McIntire W. S., Wemmer D. E., Chistoserdov A., Lidstrom M. E. A new cofactor in a prokaryotic enzyme: tryptophan tryptophylquinone as the redox prosthetic group in methylamine dehydrogenase. Science. 1991 May 10;252(5007):817–824. doi: 10.1126/science.2028257. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Mann S., Bannister J. V. Isolation and properties of the complex nonheme-iron-containing cytochrome b557 (bacterioferritin) from Pseudomonas aeruginosa. J Inorg Biochem. 1986 Oct-Nov;28(2-3):329–336. doi: 10.1016/0162-0134(86)80097-6. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Yamada H. Nitrile hydratase is a quinoprotein. A possible new function of pyrroloquinoline quinone: activation of H2O in an enzymatic hydration reaction. Biochem Biophys Res Commun. 1987 Sep 15;147(2):701–709. doi: 10.1016/0006-291x(87)90987-9. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Baum H., Peters T. J. Haemosiderin-like properties of free-radical-modified ferritin. Biochem J. 1986 Nov 15;240(1):297–300. doi: 10.1042/bj2400297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz M. A., Flückiger R., Boak A., Kagan H. M., Gallop P. M. Specific detection of quinoproteins by redox-cycling staining. J Biol Chem. 1991 Jan 15;266(2):689–692. [PubMed] [Google Scholar]
- Smith J. M., Quirk A. V., Plank R. W., Diffin F. M., Ford G. C., Harrison P. M. The identity of Escherichia coli bacterioferritin and cytochrome b1. Biochem J. 1988 Oct 15;255(2):737–740. [PMC free article] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Tsuchikura O., Gotoh Y., Saito S. Pyridinoline fluorescence in cyanogen bromide peptides of collagen. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1203–1208. doi: 10.1016/s0006-291x(81)80139-8. [DOI] [PubMed] [Google Scholar]
- UYEDA K., RABINOWITZ J. C. Fluorescence properties of tetrahydrofolate and related compounds. Anal Biochem. 1963 Jul;6:100–108. doi: 10.1016/0003-2697(63)90012-5. [DOI] [PubMed] [Google Scholar]



