Abstract
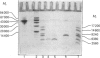
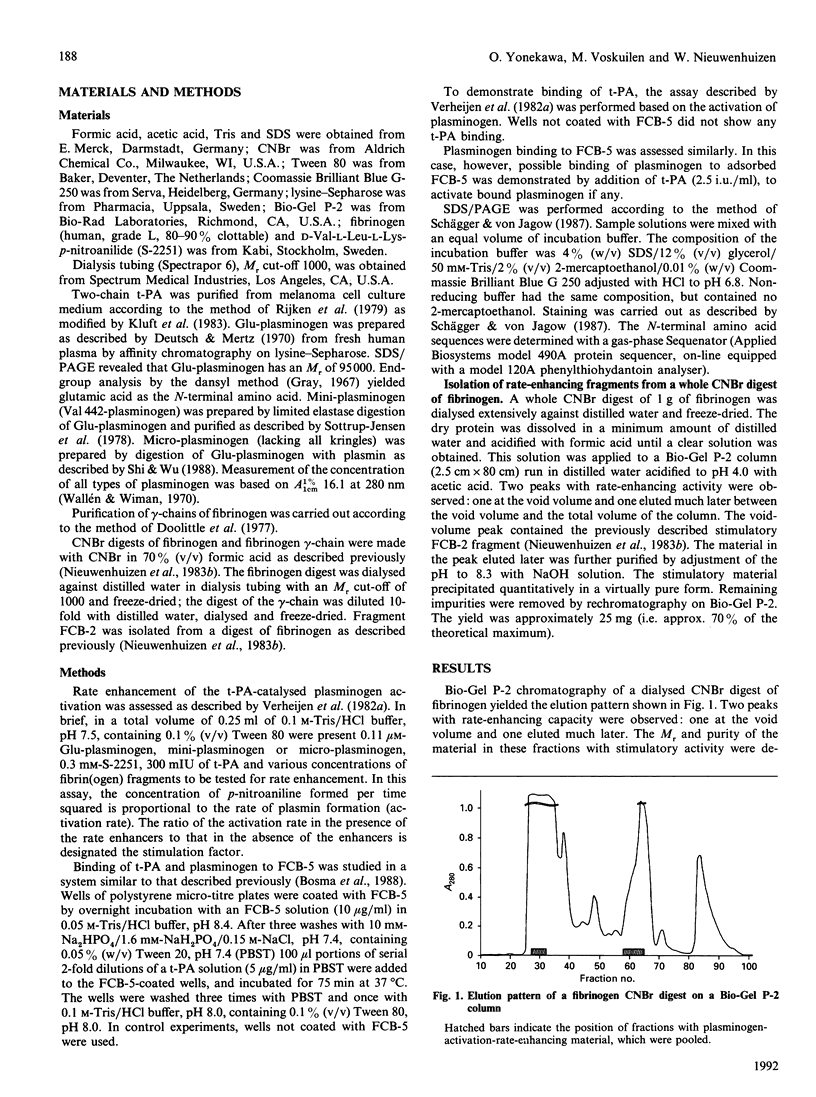
In previous publications [e.g. Voskuilen, Vermond, Veeneman, Van Boom, Klasen, Zegers & Nieuwenhuizen (1987) J. Biol. Chem. 262, 5944-5946] we have shown that fibrin(ogen) chain fragment A alpha-(148-160) contains a site that contributes to the acceleration of Glu-plasminogen activation by tissue-type plasminogen activator (t-PA). In contrast with fibrin, this peptide, however, does not enhance the rate of mini-plasminogen activation. Therefore, possibly more stimulatory sites than A alpha-(148-160) are present in fibrin. In the present investigation we have localized a possible second type of stimulatory site in the fibrin(ogen) molecule. A whole CNBr digest of fibrinogen was applied to a Bio-Gel P-2 column run in water, pH 4. Two peaks with stimulatory activity were observed, one at the void volume and one between the void volume and the total volume. The former contained the previously described stimulating fragment FCB-2 [which comprises A alpha-(148-160)]; the latter had not been observed before and was characterized further. The stimulating material in the low-M(r) fraction of the Bio-Gel P-2 column was precipitated at pH 8.3 in a virtually pure form. It has a high tryptophan content, and an M(r) of 6500 as assessed by SDS/PAGE. On reduction, a main band of M(r) 2500 is seen, plus a weakly staining band of M(r) 4000. These properties plus the amino acid sequence data identify the fragment as FCB-5. FCB-5 consists of two chains, i.e. gamma-(311-336) and gamma-(337-379), linked by a single disulphide bond between Cys-gamma-326 and Cys-gamma-339. Both these chains and the disulphide bond appear to be essential for rate enhancement. FCB-5 enhances the activation rates of Glu-, mini- and micro-plasminogen, with all five kringles, only kringle V and without kringles respectively. FCB-5 binds t-PA, but none of the plasminogen forms binds to FCB-5. This indicates that the rate enhancements induced by FCB-5 are due to an effect on t-PA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma P. J., Rijken D. C., Nieuwenhuizen W. Binding of tissue-type plasminogen activator to fibrinogen fragments. Eur J Biochem. 1988 Mar 1;172(2):399–404. doi: 10.1111/j.1432-1033.1988.tb13900.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Cassman K. G., Cottrell B. A., Friezner S. J., Hucko J. T., Takagi T. Amino acid sequence studies on the alpha chain of human fibrinogen. Characterization of 11 cyanogen bromide fragments. Biochemistry. 1977 Apr 19;16(8):1703–1709. doi: 10.1021/bi00627a028. [DOI] [PubMed] [Google Scholar]
- Gårdlund B., Hessel B., Marguerie G., Murano G., Blombäck B. Primary structure of human fibrinogen. Characterization of disulfide-containing cyanogen-bromide fragments. Eur J Biochem. 1977 Aug 1;77(3):595–610. doi: 10.1111/j.1432-1033.1977.tb11704.x. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Nieuwenhuizen W., Verheijen J. H., Vermond A., Chang G. T. Plasminogen activation by tissue activator is accelerated in the presence of fibrin(ogen) cyanogen bromide fragment FCB-2. Biochim Biophys Acta. 1983 Feb 22;755(3):531–533. doi: 10.1016/0304-4165(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Vermond A., Voskuilen M., Traas D. W., Verheijen J. H. Identification of a site in fibrin(ogen) which is involved in the acceleration of plasminogen activation by tissue-type plasminogen activator. Biochim Biophys Acta. 1983 Oct 17;748(1):86–92. doi: 10.1016/0167-4838(83)90030-4. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Voskuilen M., Vermond A., Hoegee-de Nobel B., Traas D. W. The influence of fibrin(ogen) fragments on the kinetic parameters of the tissue-type plasminogen-activator-mediated activation of different forms of plasminogen. Eur J Biochem. 1988 May 16;174(1):163–169. doi: 10.1111/j.1432-1033.1988.tb14077.x. [DOI] [PubMed] [Google Scholar]
- Radcliffe R. A critical role of lysine residues in the stimulation of tissue plasminogen activator by denatured proteins and fibrin clots. Biochim Biophys Acta. 1983 Mar 30;743(3):422–430. doi: 10.1016/0167-4838(83)90401-6. [DOI] [PubMed] [Google Scholar]
- Radcliffe R., Heinze T. Stimulation of tissue plasminogen activator by denatured proteins and fibrin clots: a possible additional role for plasminogen activator? Arch Biochem Biophys. 1981 Oct 15;211(2):750–761. doi: 10.1016/0003-9861(81)90512-9. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Zaal-de Jong M., Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim Biophys Acta. 1979 Sep 29;580(1):140–153. doi: 10.1016/0005-2795(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Schielen W. J., Adams H. P., van Leuven K., Voskuilen M., Tesser G. I., Nieuwenhuizen W. The sequence gamma-(312-324) is a fibrin-specific epitope. Blood. 1991 May 15;77(10):2169–2173. [PubMed] [Google Scholar]
- Schielen W. J., Voskuilen M., Tesser G. I., Nieuwenhuizen W. The sequence A alpha-(148-160) in fibrin, but not in fibrinogen, is accessible to monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8951–8954. doi: 10.1073/pnas.86.22.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shi G. Y., Wu H. L. Isolation and characterization of microplasminogen. A low molecular weight form of plasminogen. J Biol Chem. 1988 Nov 15;263(32):17071–17075. [PubMed] [Google Scholar]
- Verheijen J. H., Mullaart E., Chang G. T., Kluft C., Wijngaards G. A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemost. 1982 Dec 27;48(3):266–269. [PubMed] [Google Scholar]
- Verheijen J. H., Nieuwenhuizen W., Traas D. W., Chang G. T., Hoegee E. Differences in effects of fibrin(ogen) fragments on the activation of 1-glu-plasminogen and 442-val-plasminogen by tissue-type plasminogen activator. Thromb Res. 1983 Oct 1;32(1):87–92. doi: 10.1016/0049-3848(83)90157-3. [DOI] [PubMed] [Google Scholar]
- Verheijen J. H., Nieuwenhuizen W., Wijngaards G. Activation of plasminogen by tissue activator is increased specifically in the presence of certain soluble fibrin(ogen) fragments. Thromb Res. 1982 Aug 15;27(4):377–385. doi: 10.1016/0049-3848(82)90055-x. [DOI] [PubMed] [Google Scholar]
- Voskuilen M., Vermond A., Veeneman G. H., van Boom J. H., Klasen E. A., Zegers N. D., Nieuwenhuizen W. Fibrinogen lysine residue A alpha 157 plays a crucial role in the fibrin-induced acceleration of plasminogen activation, catalyzed by tissue-type plasminogen activator. J Biol Chem. 1987 May 5;262(13):5944–5946. [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. I. On the relationship between different molecular forms of plasminogen demonstrated in plasma and found in purified preparations. Biochim Biophys Acta. 1970 Oct 20;221(1):20–30. [PubMed] [Google Scholar]