Abstract
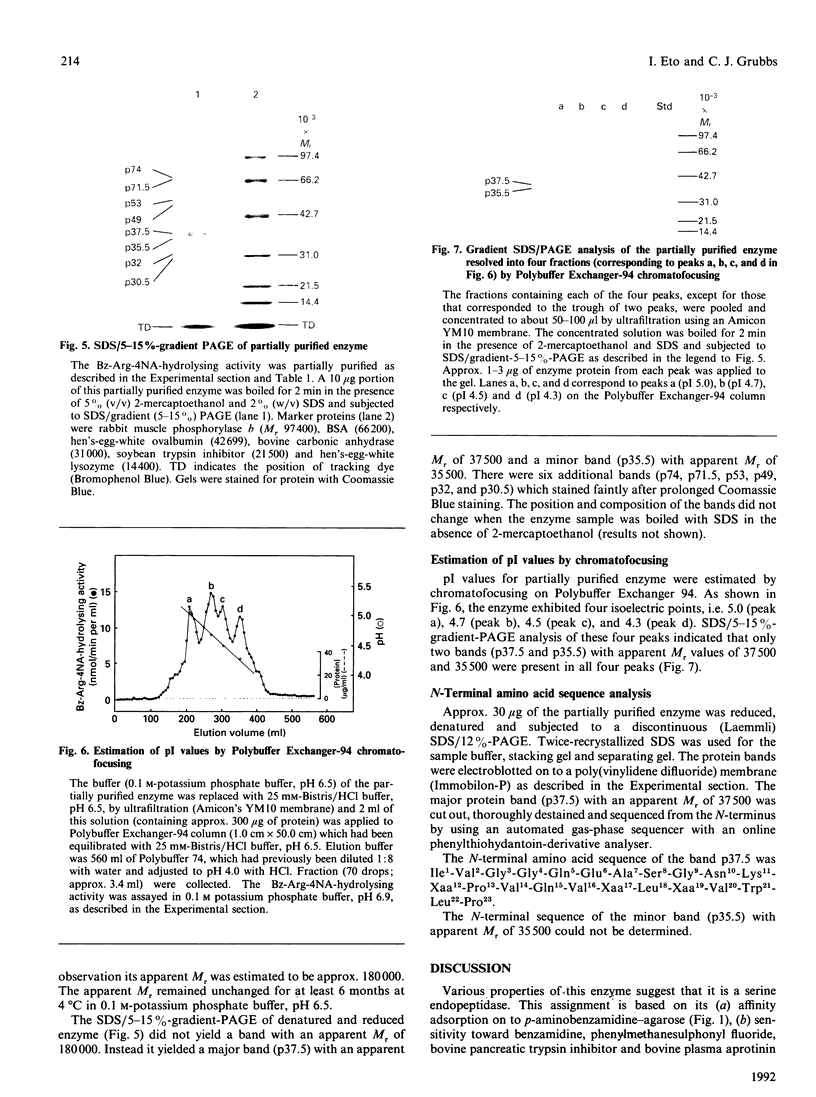
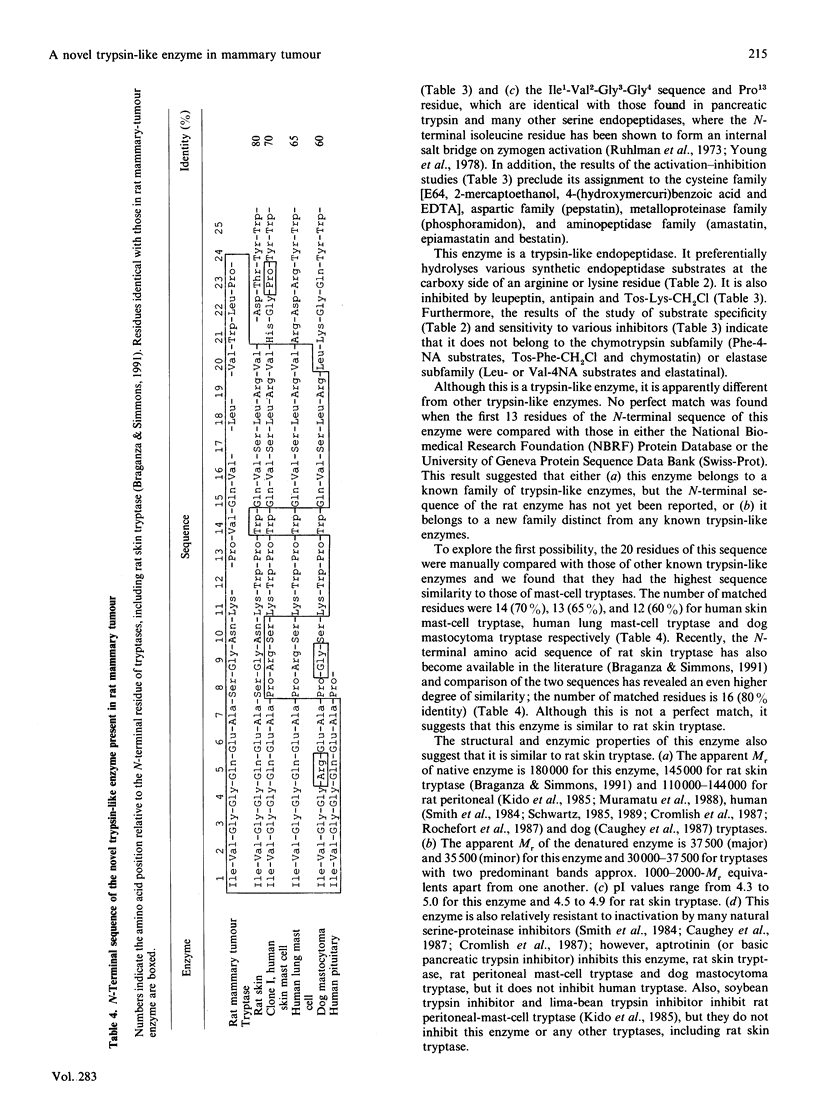
Leupeptin is a small peptide microbially derived inhibitor of certain proteolytic enzymes. Using N-alpha-benzoyl-DL-arginine 4-nitroanilide as substrate, we found a novel leupeptin-sensitive proteolytic enzyme in N-methyl-N-nitrosourea(MNU)-induced rat mammary adenocarcinoma. This enzyme was apparently different from urokinase-type plasminogen activator or cathepsin B and was present in mammary tumour at levels at least 20 times higher than those in normal mammary tissue. This enzyme was separated and purified from crude extracts of MNU-induced mammary adenocarcinoma approx. 1900-fold with 34% yield. It was a trypsin-like serine endopeptidase and had a pH optimum at 7.0. The native enzyme had an apparent M(r) of 180,000 and exhibited four isoelectric points ranging from 4.3 to 5.0. Electrophoresis of denatured enzyme, however, yielded, with reduction, a major band with an apparent M(r) of 37,500 and a minor band with an apparent M(r) of 35,500. The N-terminal 23 residues of the major band were Ile1-Val2-Gly3-Gly4-Gln5-Glu6-Ala7-+ ++Ser8-Gly9-Asn10-Lys11-Xaa12-Pro13- Val14- Gln15-Val16-Xaa17-Leu18-Xaa19-Val20- Trp21-Leu22-Pro23. These and other properties of this enzyme suggested that it most closely resembles rat skin tryptase, followed by rat peritoneal mast-cell tryptase and then by tryptases from other species. The rat, like human and mouse, may carry multiple tryptase genes, and this mammary-tumour enzyme may be an additional form of rat tryptase within a new serine-proteinase family.
Full text
PDF

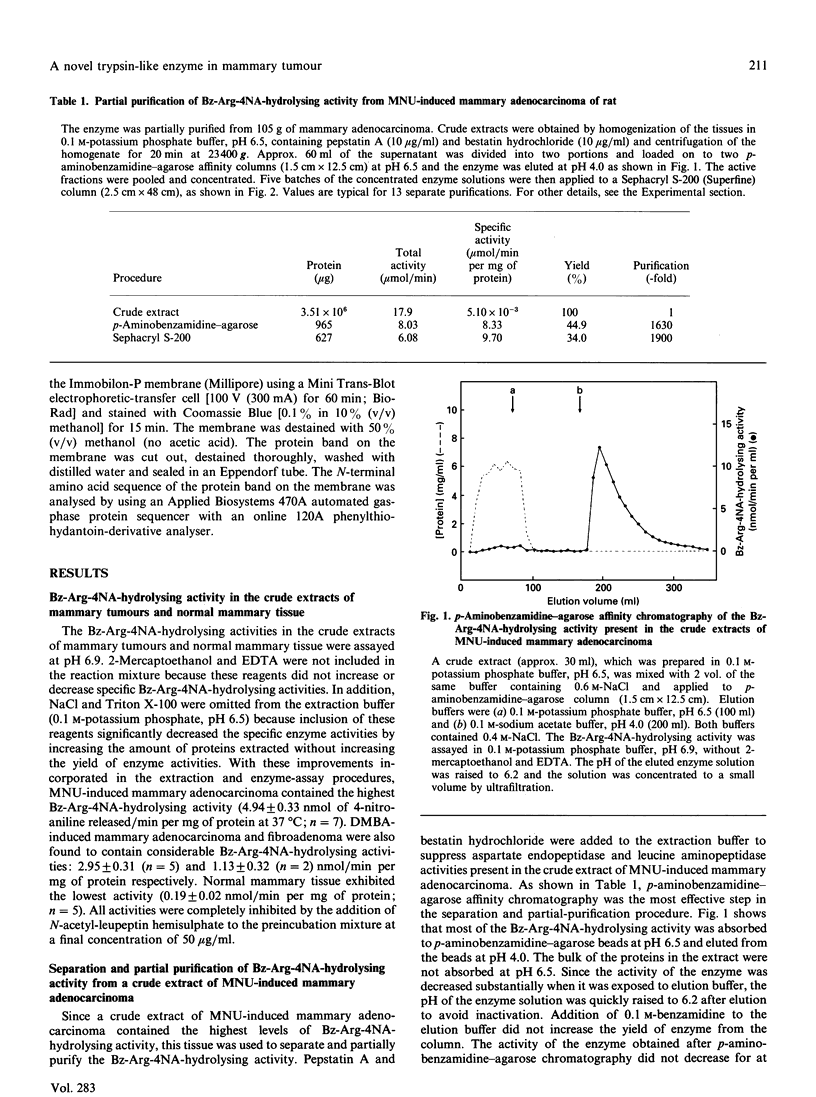
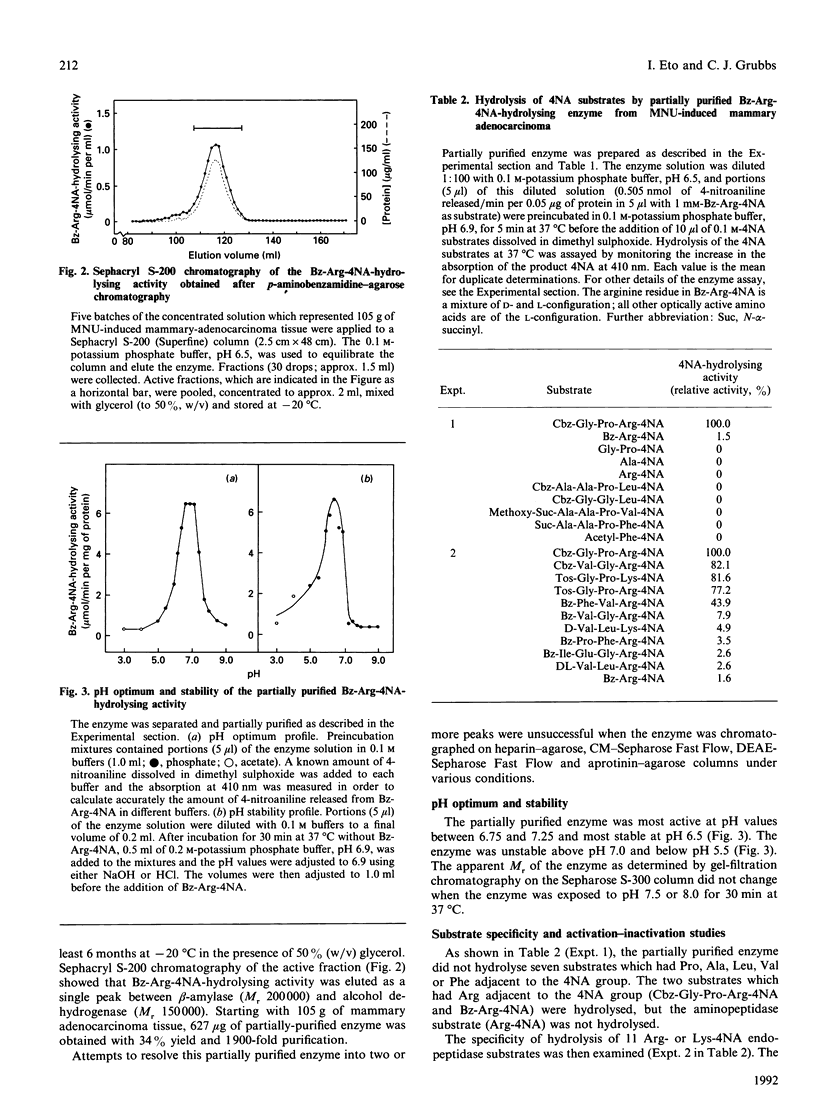
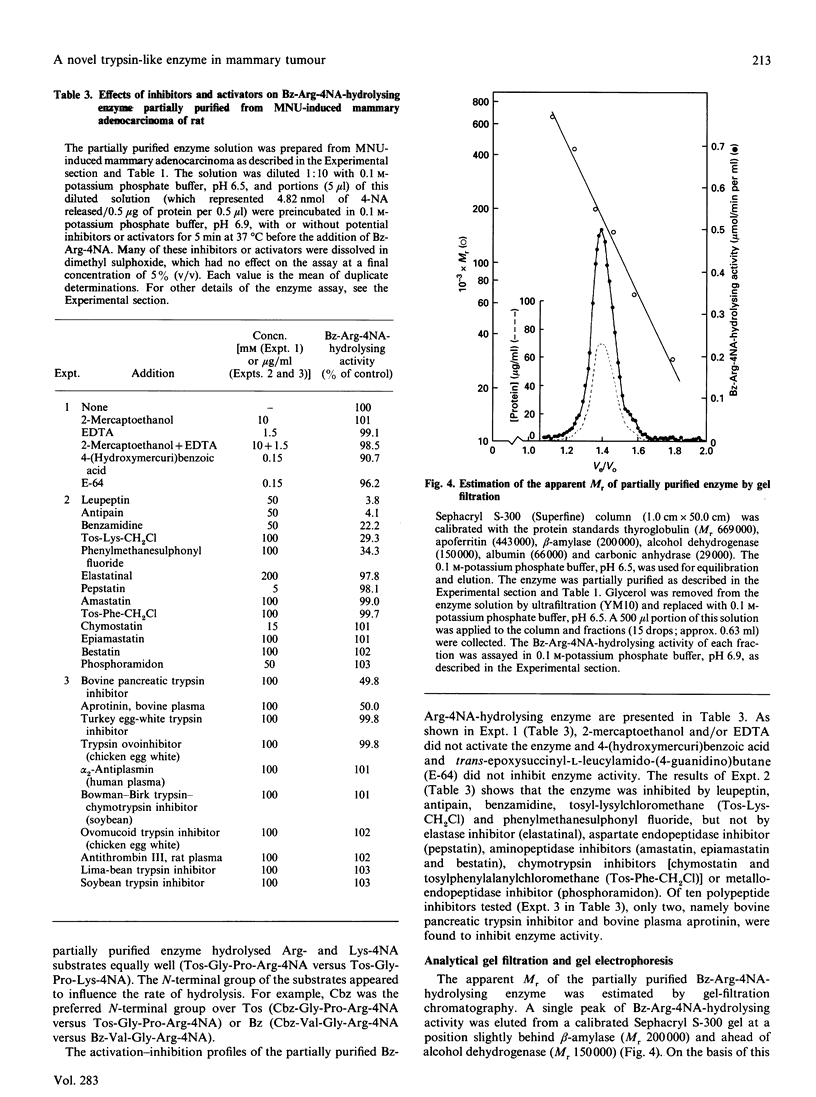

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter S. C., Metcalfe D. D., Bradford T. R., Schwartz L. B. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem J. 1987 Dec 15;248(3):821–827. doi: 10.1042/bj2480821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T., Miyata S., Nanbo M., Kojima F., Matsuzaki M. Biological activities of leupeptins. J Antibiot (Tokyo) 1969 Nov;22(11):558–568. doi: 10.7164/antibiotics.22.558. [DOI] [PubMed] [Google Scholar]
- Aoyagi T., Takeuchi T., Matsuzaki A., Kawamura K., Kondo S. Leupeptins, new protease inhibitors from Actinomycetes. J Antibiot (Tokyo) 1969 Jun;22(6):283–286. doi: 10.7164/antibiotics.22.283. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braganza V. J., Simmons W. H. Tryptase from rat skin: purification and properties. Biochemistry. 1991 May 21;30(20):4997–5007. doi: 10.1021/bi00234a023. [DOI] [PubMed] [Google Scholar]
- Brown S., Miller W. G. Determination of magnesium in serum by the technicon SMAC with a calmagite method with blank correction. Clin Chem. 1990 Nov;36(11):1990–1991. [PubMed] [Google Scholar]
- Caughey G. H., Viro N. F., Ramachandran J., Lazarus S. C., Borson D. B., Nadel J. A. Dog mastocytoma tryptase: affinity purification, characterization, and amino-terminal sequence. Arch Biochem Biophys. 1987 Nov 1;258(2):555–563. doi: 10.1016/0003-9861(87)90377-8. [DOI] [PubMed] [Google Scholar]
- Clavel C., Birembaut P. Protéases et carcinomes mammaires. Ann Pathol. 1988;8(1):20–24. [PubMed] [Google Scholar]
- Cromlish J. A., Seidah N. G., Marcinkiewicz M., Hamelin J., Johnson D. A., Chrétien M. Human pituitary tryptase: molecular forms, NH2-terminal sequence, immunocytochemical localization, and specificity with prohormone and fluorogenic substrates. J Biol Chem. 1987 Jan 25;262(3):1363–1373. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Duffy M. J., O'Grady P., Devaney D., O'Siorain L., Fennelly J. J., Lijnen H. J. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report. Cancer. 1988 Aug 1;62(3):531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Eto I., Bandy M. D. A novel leupeptin-sensitive serine endopeptidase present in normal and malignant rat mammary tissues. Mol Cell Biochem. 1990 Apr 18;94(1):19–36. doi: 10.1007/BF00223559. [DOI] [PubMed] [Google Scholar]
- Evers J. L., Patel J., Madeja J. M., Schneider S. L., Hobika G. H., Camiolo S. M., Markus G. Plasminogen activator activity and composition in human breast cancer. Cancer Res. 1982 Jan;42(1):219–226. [PubMed] [Google Scholar]
- Goldfarb R. H., Liotta L. A. Proteolytic enzymes in cancer invasion and metastasis. Semin Thromb Hemost. 1986 Oct;12(4):294–307. doi: 10.1055/s-2007-1003570. [DOI] [PubMed] [Google Scholar]
- Grubbs C. J., Farnell D. R., Hill D. L., McDonough K. C. Chemoprevention of N-nitroso-N-methylurea-induced mammary cancers by pretreatment with 17 beta-estradiol and progesterone. J Natl Cancer Inst. 1985 Apr;74(4):927–931. [PubMed] [Google Scholar]
- Grubbs C. J., Peckham J. C., McDonough K. D. Effect of ovarian hormones on the induction of 1-methyl-1-nitrosourea-induced mammary cancer. Carcinogenesis. 1983;4(4):495–497. doi: 10.1093/carcin/4.4.495. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Rehemtulla A. Plasminogen activators and their inhibitors: regulators of extracellular proteolysis and cell function. Comp Biochem Physiol B. 1988;90(4):691–708. doi: 10.1016/0305-0491(88)90323-9. [DOI] [PubMed] [Google Scholar]
- Hozumi M., Ogawa M., Sugimura T., Takeuchi T., Umezawa H. Inhibition of tumorigenesis in mouse skin by leupeptin, a protease inhibitor from Actinomycetes. Cancer Res. 1972 Aug;32(8):1725–1728. [PubMed] [Google Scholar]
- Kido H., Fukusen N., Katunuma N. Chymotrypsin- and trypsin-type serine proteases in rat mast cells: properties and functions. Arch Biochem Biophys. 1985 Jun;239(2):436–443. doi: 10.1016/0003-9861(85)90709-x. [DOI] [PubMed] [Google Scholar]
- Kondo S. I., Kawamura K., Iwanaga J., Hamada M., Aoyagi T. Isolation and characterization of leupeptins produced by Actinomycetes. Chem Pharm Bull (Tokyo) 1969 Sep;17(9):1896–1901. doi: 10.1248/cpb.17.1896. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Moxley G., Schwartz L. B. Cloning and characterization of a second complementary DNA for human tryptase. J Clin Invest. 1990 Sep;86(3):864–870. doi: 10.1172/JCI114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira-y-Lopez R., Reich E., Ossowski L. Modulation of plasminogen activator in rodent mammary tumors by hormones and other effectors. Cancer Res. 1983 Nov;43(11):5467–5477. [PubMed] [Google Scholar]
- Moon R. C., Grubbs C. J., Sporn M. B. Inhibition of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis by retinyl acetate. Cancer Res. 1976 Jul;36(7 Pt 2):2626–2630. [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D. Interrelationship of active and latent secreted human cathepsin B precursors. Biochem J. 1986 Jan 1;233(1):57–63. doi: 10.1042/bj2330057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Characterization of a thiol proteinase secreted by malignant human breast tumours. Biochim Biophys Acta. 1980 Jul 10;614(1):134–143. doi: 10.1016/0005-2744(80)90174-6. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Muramatu M., Itoh T., Takei M., Endo K. Tryptase in rat mast cells: properties and inhibition by various inhibitors in comparison with chymase. Biol Chem Hoppe Seyler. 1988 Jul;369(7):617–625. doi: 10.1515/bchm3.1988.369.2.617. [DOI] [PubMed] [Google Scholar]
- Nagy B., Ban J., Brdar B. Fibrinolysis associated with human neoplasia: production of plasminogen activator by human tumours. Int J Cancer. 1977 May 15;19(5):614–620. doi: 10.1002/ijc.2910190504. [DOI] [PubMed] [Google Scholar]
- Needham G. K., Nicholson S., Angus B., Farndon J. R., Harris A. L. Relationship of membrane-bound tissue type and urokinase type plasminogen activators in human breast cancers to estrogen and epidermal growth factor receptors. Cancer Res. 1988 Nov 15;48(22):6603–6607. [PubMed] [Google Scholar]
- O'Grady R. L., Upfold L. I., Stephens R. W. Rat mammary carcinoma cells secrete active collagenase and activate latent enzyme in the stroma via plasminogen activator. Int J Cancer. 1981 Oct 15;28(4):509–515. doi: 10.1002/ijc.2910280418. [DOI] [PubMed] [Google Scholar]
- Pacheco M. M., Brentani M. M., Franco E. L., Fontelles J. A., Chamone D. F., Marques L. A. Plasminogen activator expression and steroid hormone receptors in female breast cancer: a multifactorial study. Int J Cancer. 1988 Jun 15;41(6):798–804. doi: 10.1002/ijc.2910410604. [DOI] [PubMed] [Google Scholar]
- Pereyra-Alfonso S., Bal de Kier Joffe E. Enhancement of urokinase-type plasminogen activator activity during the growth of a murine mammary adenocarcinoma. Int J Cancer. 1989 Feb 15;43(2):356–357. doi: 10.1002/ijc.2910430232. [DOI] [PubMed] [Google Scholar]
- Pereyra-Alfonso S., Haedo A., Bal de Kier Joffé E. Correlation between urokinase-type plasminogen activator production and the metastasizing ability of two murine mammary adenocarcinomas. Int J Cancer. 1988 Jul 15;42(1):59–63. doi: 10.1002/ijc.2910420112. [DOI] [PubMed] [Google Scholar]
- Peterson H. I., Petrusson B., Korsan-Bengtsen K. Fibrinolytic activity of human carcinomas. A comparative methodological study. Thromb Diath Haemorrh. 1973 Sep 15;30(1):133–137. [PubMed] [Google Scholar]
- Poole A. R., Tiltman K. J., Recklies A. D., Stoker T. A. Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature. 1978 Jun 15;273(5663):545–547. doi: 10.1038/273545a0. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Stephens R., Salonen E. M., Vaheri A. Proteolytic mechanisms operating at the surface of invasive cells. Adv Exp Med Biol. 1988;233:187–199. doi: 10.1007/978-1-4899-5037-6_21. [DOI] [PubMed] [Google Scholar]
- Recklies A. D., Mort J. S., Poole A. R. Secretion of a thiol proteinase from mouse mammary carcinomas and its characterization. Cancer Res. 1982 Mar;42(3):1026–1032. [PubMed] [Google Scholar]
- Recklies A. D., Poole A. R., Mort J. S. A cysteine proteinase secreted from human breast tumours is immunologically related to cathepsin B. Biochem J. 1982 Dec 1;207(3):633–636. doi: 10.1042/bj2070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recklies A. D., Tiltman K. J., Stoker T. A., Poole A. R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 1980 Mar;40(3):550–556. [PubMed] [Google Scholar]
- Rochefort H., Capony F., Garcia M., Cavaillès V., Freiss G., Chambon M., Morisset M., Vignon F. Estrogen-induced lysosomal proteases secreted by breast cancer cells: a role in carcinogenesis? J Cell Biochem. 1987 Sep;35(1):17–29. doi: 10.1002/jcb.240350103. [DOI] [PubMed] [Google Scholar]
- Rühlmann A., Kukla D., Schwager P., Bartels K., Huber R. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. Crystal structure determination and stereochemistry of the contact region. J Mol Biol. 1973 Jul 5;77(3):417–436. doi: 10.1016/0022-2836(73)90448-8. [DOI] [PubMed] [Google Scholar]
- Saito D., Sawamura M., Umezawa K., Kanai Y., Furihata C., Matsushima T., Sugimura T. Inhibition of experimental blood-borne lung metastasis by protease inhibitors. Cancer Res. 1980 Jul;40(7):2539–2542. [PubMed] [Google Scholar]
- Schwartz L. B. Monoclonal antibodies against human mast cell tryptase demonstrate shared antigenic sites on subunits of tryptase and selective localization of the enzyme to mast cells. J Immunol. 1985 Jan;134(1):526–531. [PubMed] [Google Scholar]
- Sherman M. R., Tuazon F. B., Miller L. K. Estrogen receptor cleavage and plasminogen activation by enzymes in human breast tumor cytosol. Endocrinology. 1980 Jun;106(6):1715–1727. doi: 10.1210/endo-106-6-1715. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Hougland M. W., Johnson D. A. Human lung tryptase. Purification and characterization. J Biol Chem. 1984 Sep 10;259(17):11046–11051. [PubMed] [Google Scholar]
- Sutherland D. J. Plasminogen-activating activity: association with steroid binding by cytosols of human breast cancers. J Natl Cancer Inst. 1980 Jan;64(1):3–7. [PubMed] [Google Scholar]
- Thorsen T. Association of plasminogen activator activity and steroid receptors in human breast cancer. Eur J Cancer Clin Oncol. 1982 Feb;18(2):129–132. doi: 10.1016/0277-5379(82)90055-4. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- Vanderslice P., Ballinger S. M., Tam E. K., Goldstein S. M., Craik C. S., Caughey G. H. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci U S A. 1990 May;87(10):3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]