Abstract
We have explored the role of mitochondrial 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase in regulating ketogenesis. We had previously cloned the cDNA for mitochondrial HMG-CoA synthase and have now studied the regulation in vivo of the expression of this gene in rat liver. The amount of processed mitochondrial HMG-CoA synthase mRNA is rapidly changed in response to cyclic AMP, insulin, dexamethasone and refeeding, and is greatly increased by starvation, fat feeding and diabetes. We conclude that one point of ketogenic control is exercised at the level of genetic expression of mitochondrial HMG-CoA synthase.
Full text
PDF
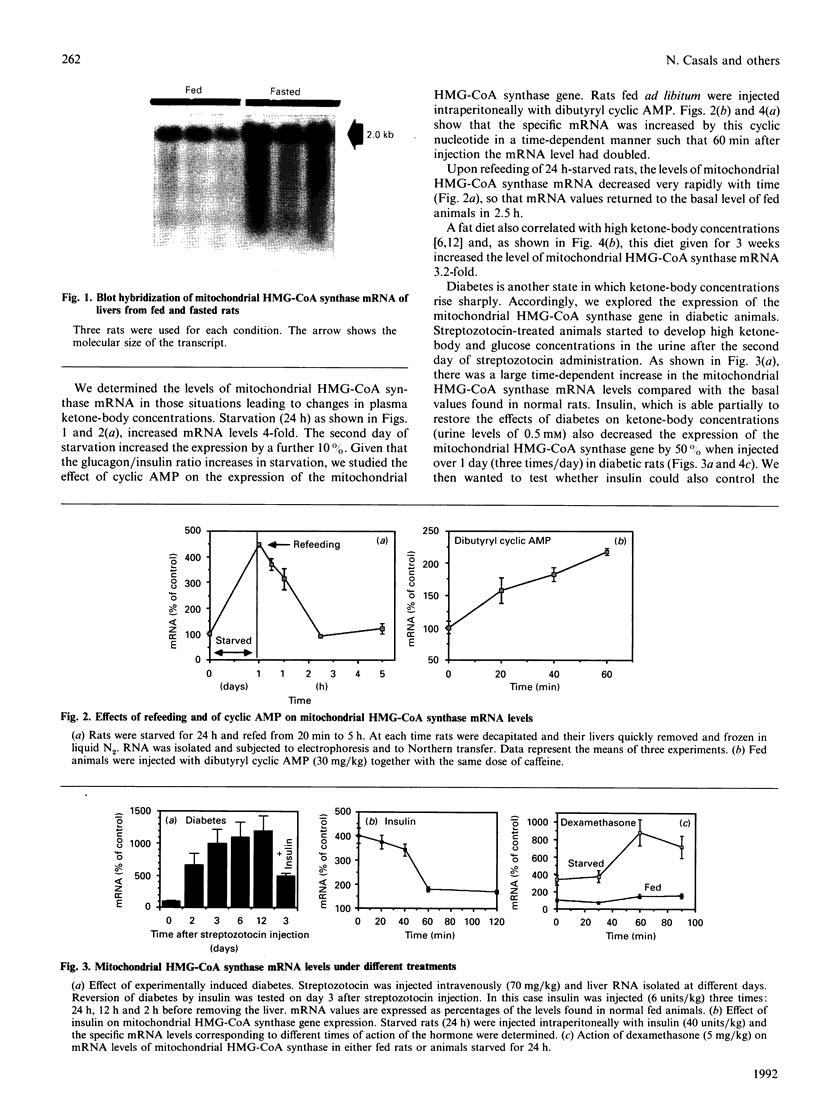


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benito M., Whitelaw E., Williamson D. H. Regulation of ketogenesis during the suckling-weanling transition in the rat. Studies with isolated hepatocytes. Biochem J. 1979 Apr 15;180(1):137–144. doi: 10.1042/bj1800137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciudad C. J., Vila J., Mor M. A., Guinovart J. J. Effects of glucagon and insulin on the cyclic AMP binding capacity of hepatocyte cyclic AMP-dependent protein kinase. Mol Cell Biochem. 1987 Jan;73(1):37–44. doi: 10.1007/BF00229374. [DOI] [PubMed] [Google Scholar]
- Dashti N., Ontko J. A. Rate-limiting function of 3-hydroxy-3-methylglutaryl-coenzyme A synthase in ketogenesis. Biochem Med. 1979 Dec;22(3):365–374. doi: 10.1016/0006-2944(79)90024-3. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Robin D., Robin P., Ferré P., Girard J. Intramitochondrial factors controlling hepatic fatty acid oxidation at weaning in the rat. FEBS Lett. 1988 May 9;232(1):156–158. doi: 10.1016/0014-5793(88)80407-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foster D. W. Banting lecture 1984. From glycogen to ketones--and back. Diabetes. 1984 Dec;33(12):1188–1199. doi: 10.2337/diab.33.12.1188. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Quant P. A., Robin D., Robin P., Ferre P., Brand M. D., Girard J. Control of hepatic mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase during the foetal/neonatal transition, suckling and weaning in the rat. Eur J Biochem. 1991 Jan 30;195(2):449–454. doi: 10.1111/j.1432-1033.1991.tb15724.x. [DOI] [PubMed] [Google Scholar]
- Quant P. A., Tubbs P. K., Brand M. D. Glucagon activates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in vivo by decreasing the extent of succinylation of the enzyme. Eur J Biochem. 1990 Jan 12;187(1):169–174. doi: 10.1111/j.1432-1033.1990.tb15291.x. [DOI] [PubMed] [Google Scholar]
- Quant P. A., Tubbs P. K., Brand M. D. Treatment of rats with glucagon or mannoheptulose increases mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase activity and decreases succinyl-CoA content in liver. Biochem J. 1989 Aug 15;262(1):159–164. doi: 10.1042/bj2620159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. D., Clinkenbeard D., Lane M. D. Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem. 1975 Apr 25;250(8):3117–3123. [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Royo T., Ayté J., Albericio F., Giralt E., Haro D., Hegardt F. G. Diurnal rhythm of rat liver cytosolic 3-hydroxy-3-methylglutaryl-CoA synthase. Biochem J. 1991 Nov 15;280(Pt 1):61–64. doi: 10.1042/bj2800061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satabin P., Bois-Joyeux B., Chanez M., Guezennec C. Y., Peret J. Effects of long-term feeding of high-protein or high-fat diets on the response to exercise in the rat. Eur J Appl Physiol Occup Physiol. 1989;58(6):583–590. doi: 10.1007/BF00418503. [DOI] [PubMed] [Google Scholar]
- Schofield P. S., French T. J., Sugden M. C. Ketone-body metabolism after surgical stress or partial hepatectomy. Evidence for decreased ketogenesis and a site of control distal to carnitine palmitoyltransferase I. Biochem J. 1987 Jan 15;241(2):475–481. doi: 10.1042/bj2410475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Krebs H. A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J. 1968 Jul;108(3):353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Cox W. F., Schroeder J. G., Liao S. T., Foster D. W., McGarry J. D. Inter-tissue and inter-species characteristics of the mitochondrial carnitine palmitoyltransferase enzyme system. J Biol Chem. 1990 Jun 25;265(18):10714–10719. [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Sen A., Cox W. F., McPhaul M. J., Slaughter C. A., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver mitochondrial carnitine palmitoyltransferase II. J Biol Chem. 1990 Jun 25;265(18):10720–10725. [PubMed] [Google Scholar]