Abstract
Introduction
HIV self‐testing (HIVST) has been shown to increase the uptake of HIV testing and help achieve the UNAIDS 95‐95‐95 targets. This study assessed the acceptability, usability (ease of use and result interpretation) and the willingness to pay for HIVST kits distributed through three distribution models, namely the community‐based, PLHIV network‐led and private practitioners models, in India.
Methods
This cross‐sectional study was implemented across 14 states in India between September 2021 and June 2022. All participants could choose between blood‐based or oral‐fluid‐based test kits. Participants were shown a test‐kit usage demonstration video, and pre‐ and post‐test counselling was provided for all. Participants were followed‐up after testing, and if reported reactive, were further supported for linkage to confirmatory testing and antiretroviral therapy (ART) initiation.
Results
Among the 90,605 participants found eligible, 88,080 (97%) accepted an HIVST kit. Among the 87,976 who reported using an HIVST kit, 45,207 (51%) preferred a blood‐based kit, and 42,120 (48%) reported testing for the first time. For future testing, 77,064 (88%) reported preferring HIVST over other HIV testing methods. Among those who used the kit, 83,308 (95%) found the kit easy to use, and 83,237 (95%) reported that the test results were easy to interpret. Among those who preferred HIVST for future use, 52,136 (69%) were willing to pay for the kit, with 35,854 (69%) of those willing to pay less than US$ 1.20. Only one instance of social harm was reported, with a participant reporting suicidal tendencies due to discord with their partner.
Out of 328 participants (0.4%) who tested reactive with HIVST, 291 (89%) were linked to confirmatory testing; of these, 254 were confirmed HIV positive, and 216 (85%) successfully initiated ART.
Conclusions
Overall, we report that nearly all participants were willing to accept HIVST, found the test kits easy to use and interpret, and about two‐thirds were willing to pay for HIVST. Given the high levels of acceptance and the ability to reach a large proportion of first‐time testers, HIVST in India could contribute to achieving the UNAIDS first 95 and ending the HIV epidemic.
Keywords: HIV/AIDS, HIV testing, HIV self‐testing, operational research, key population, India
1. INTRODUCTION
Substantial progress with access to HIV testing has been made, with 85% of people living with HIV (PLHIV) globally and 79% of estimated PLHIV in India knowing their HIV status in 2023 [1, 2]. However, a wider range of HIV testing options are needed to achieve the UNAIDS 95‐95‐95 targets. HIV self‐testing (HIVST) is an important tool to reach those who otherwise would not seek testing at healthcare facilities or community‐based testing sites [3].
Given the evidence available on the benefits of HIVST, the World Health Organization incorporated HIVST into its 2021 Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring [4]. These guidelines recommend the distribution of HIVST kits through various community and facility‐based models. It also recommends private practitioners, pharmacies, workplaces and online delivery of HIVST kits. HIVST kit delivery through innovative distribution mechanisms has been shown to be feasible and acceptable [5, 6].
Considering these benefits associated with the introduction of HIVST kits, India is also taking steps towards adapting its HIV‐testing guidelines to include HIVST. Currently, HIVST kits are not available for purchase in the country, and there is no established HIVST policy. The National AIDS and STD Control Programme (NACP) Phase‐V (2021−2026) calls for the generation of evidence regarding implementation modalities for introducing HIVST in the country [7]. Studies from India report that HIVST is acceptable among key populations, such as men who have sex with men (MSM), transgender people (TG), female sex workers (FSWs) and truckers [8, 9, 10, 11, 12, 13, 14].
However, most of these studies were qualitative, and there is limited evidence available on models for the distribution of HIVST. To fill this evidence gap, we implemented a demonstration study to understand the acceptability, usability and willingness to pay for HIVST distributed through various distribution models and among diverse population groups in India. Here, we present results from the distribution of HIVST through three different models.
2. METHODS
2.1. Study design and setting
This cross‐sectional study was conducted across 50 districts in 14 states in India with a high prevalence of HIV and/or a high number of PLHIV [2]. In 2020, these states contributed over 55% of the new HIV cases in the country [2]. The study included states from all regions of the country and included both urban and rural areas. The complete list of models implemented by states and districts is provided in Table S1.
2.2. HIVST distribution models
HIVST kits were distributed in the study using five models. Three models focused on in‐person distribution and reaching key populations, their partners, clients and partners of PLHIV are presented here. These include the community‐based, PLHIV network‐led and private practitioners models. The other models implemented were workplace and virtual models, the results of which are yet to be published.
2.3. Community‐based model
The community‐based model was implemented in all 14 states. In this model, HIVST kits were distributed by study staff who were supported by community‐based organisations (CBOs) providing HIV testing services through targeted intervention or other community‐based testing methods. This model was designed to reach key populations (FSWs, MSM, TG and people who inject drugs [PWID]), their sexual and injecting partners, and clients, and partners of PLHIV. Bridge populations such as migrant workers and truckers were also reached. HIVST distribution in this model took place from September 2021 to June 2022.
Study staff were recruited mainly from key population communities. All staff received two days in‐person training from the study investigators and master trainers in their local language. The training covered the basics of HIV transmission and treatment, HIV testing approaches and study procedures which included conducting the eligibility assessment, consent‐taking procedures, study definitions, pre‐and post‐test counselling, HIVST kit demonstrations, follow‐up protocol, social harms assessment and responding to any reported social harms, and data capture. The study process is shown in Figure 1.
Figure 1.
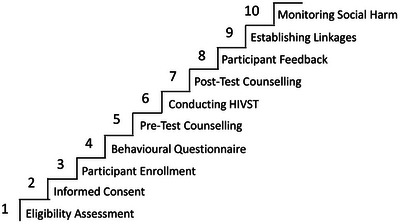
Study process followed in the STAR III study in India.
The study staff conducted demand‐generation activities such as conducting camps and community information sessions at hotspots, CBO offices and other community‐based testing sites. Participants who met the eligibility criteria and provided written informed consent were enrolled in the study and offered to select between blood‐based (Mylan HIV self‐test, Atomo Diagnostics Pvt. Ltd, Australia or Insti HIV self‐test, bioLytical Lab., Canada) or oral‐fluid‐based (OraQuick HIV self‐test, OraSure Technologies, USA) HIVST kits and to test in an assisted or unassisted approach. Assisted testing was defined as testing in the physical presence of study staff. All participants were shown kit demonstration videos (available at https://www.youtube.com/@starhivselftesting3897) before selecting a kit. Pre‐ and post‐test counselling was conducted. Participants who opted for the unassisted approach could test at the distribution site or take the test kit to test later. Participants who took the test kits with them were followed up on days 3, 5 and 7 before being declared lost to follow‐up. Participants were followed‐up by phone or in‐person. Any participant who requested additional time for testing was further followed‐up.
All participants with an HIV reactive self‐test were offered accompanied referral to the nearest public Integrated Counselling and Testing Centre (ICTC) for confirmatory testing and to an antiretroviral therapy (ART) centre for ART initiation for those who were confirmed HIV positive.
All participants were followed up by phone or in person after 7 days of testing to check for social harm and those who reported it were offered appropriate support.
2.4. PLHIV network‐led model
This model aimed to reach the partners of PLHIV in six districts of one state (Table S1). It was implemented by a CBO formed by PLHIV that runs a peer‐led integrated health centre and community pharmacy offering positive prevention counselling, HIV testing for spouses/partners and children of PLHIV, subsidised ART, pre‐exposure prophylaxis (PrEP) and post‐exposure prophylaxis (PEP). The distribution of kits through this model occurred between January and June 2022.
The staff, who were PLHIV, received standard training and followed study procedures as described in the community‐based model. Demand generation was conducted through physical and virtual modes with the registered PLHIV, their partners and any client seeking HIV testing services at one of the service delivery sites.
2.5. Private practitioners model
The private practitioners model was implemented in nine districts from five states, selected based on administrative approvals and operational feasibility (Table S1). Private practitioners who provided sexually transmitted infection (STI) services, tuberculosis clinics, dermatologists and family physicians/general practitioners (including allopathic, ayurvedic and homoeopathic doctors) providing HIV and STI services were mapped. A total of 700 private practitioners and labs were mapped and approached. Among these, 7.1% (50) practitioners agreed to participate in the study. The availability of in‐house laboratory services and the perceived lengthiness of the study procedures were the main reasons reported for low participation. A two‐day training on HIVST and the study procedures were conducted for the private practitioner or their support staff (nurses and lab technicians). A study staff was assigned to support study activities at the clinics. Study staff would be present at the clinic on days and times agreed with the practitioner. For high‐burden clinics, monetary incentives were given to clinic staff to act as the study focal person. Follow‐up visits were conducted by the study staff every two weeks to discuss challenges and to arrive at appropriate solutions.
Posters and takeaway leaflets on HIVST were kept inside the clinics. Clients willing to receive HIVST were asked to approach the designated clinic staff. The practitioners also referred their clients to the study staff. The study staff guided all potential participants through the study processes as described in the community‐based model. Kit distribution through this model occurred between December 2021 and June 2022.
2.6. Study participants
The study included participants who were 18 years of age or above and identified with one of the following groups—member of a key population group (FSW, MSM, TG, PWID), partner or client of key population, partner of PLHIV, referred from identified private practitioners or individuals self‐identified at high‐risk of HIV. HIV‐positive persons, those on PrEP or PEP and pregnant women were not included in the study. While both the blood and oral‐fluid‐based HIVST kits were offered to all the participants, those who self‐reported ever testing positive for hepatitis B or C were offered only the blood‐based HIVST kit.
2.7. Data collection and analysis
A structured questionnaire was used to collect data. Data were collected directly into a mobile phone‐based web application. Staff were trained to collect data in real‐time while completing the study procedures. In locations with a poor network, data were collected using hard‐copy forms and then entered into the online tool once the network was available.
The primary outcomes measured were the acceptability of HIVST for current and future testing, usability of the HIVST kits (including ease of use and result interpretation) and willingness to pay (Table 1). To understand participants’ willingness to pay, after the testing process, they were asked for a range within which they would be willing to pay for an HIVST kit. To determine the factors associated with the primary outcomes, we first calculated the unadjusted odds ratios and 95% confidence intervals. Variables with p‐value<0.20 were selected for multiple regression. The variables population group and gender were found to be collinear, hence, only population group was used for further analysis. Using these variables, directed acyclic graphs were constructed (Figures S1−S3) to identify potential confounders for each outcome‐factor association. Confounders to be adjusted using the multiple logistic regression were identified by comparing the −2 log‐likelihood ratios of the models with and without the confounding variables, separately for each exposure variable [15]. Data were analysed using SPSS (Version 20, 2011; IBM Inc., Chicago, IL, USA) and Stata (Version 16, StataCorp LLC, Texas, USA).
Table 1.
Outcome definitions of primary outcomes for the STAR III study in India
| Outcome | Definition |
|---|---|
| Acceptance of HIVST | The proportion of participants who accepted to receive HIVST among those eligible |
| Acceptance of HIVST for future testing | Among those who used the HIVST kit, the proportion of participants who indicated willingness to use HIVST for future testing |
| Ease of using the HIVST kit | Among those who used the HIVST kit, the proportion of participants who reported finding the test kit easy or very easy to use |
| Ease of result interpretation | Among those who used the HIVST kit, the proportion of participants who reported finding the test kit easy or very easy to interpret |
| Willingness to pay | Among those who preferred HIVST for future testing, the proportion of those who were willing to pay |
2.8. Ethics
The study was approved by the ethics committees of The Humsafar Trust, Mumbai, India (HST‐IRB‐49‐10/2020), and WCG IRB, USA (20212973). The study also received a research determination from the Scientific Integrity Branch of the Division of Global HIV and TB, CDC, Atlanta. Written informed consent was obtained from all participants.
3. RESULTS
A total of 90,771 people agreed to check their eligibility, among whom 90,605 (99.8%) were found eligible (Figure 2). Among those eligible, 88,080 (97%) consented to accept an HIVST kit, while 87,976 (99.9%) received an HIVST kit. Thus, 97% (87,976/90,605) of those eligible accepted an HIVST kit. Most of those who received an HIVST kit were recruited through the community‐based model (79,324/87,976; 90%), followed by the private practitioners model (6984; 8%) and the PLHIV network‐led model (1668; 2%) (Table 2).
Figure 2.
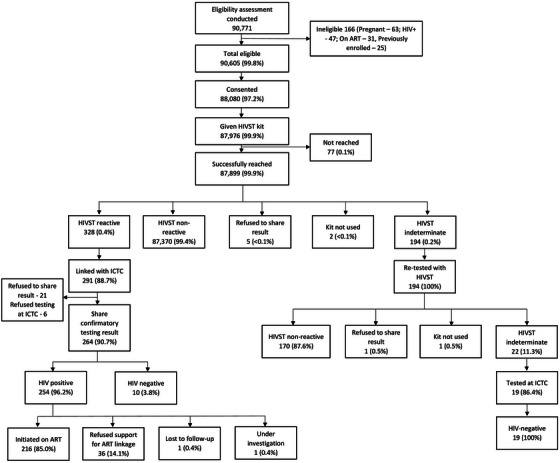
Study cascade for the STAR III study in India.
Table 2.
Socio‐demographic characteristics of the population enrolled in the STAR III study in India
| Community‐based model | PLHIV network‐led model | Private practitioners model | Total | |
|---|---|---|---|---|
| Total | 79,324 (100) | 1668 (100) | 6984 (100) | 87,976 (100) |
| Place of recruitment | ||||
| CBO/NGO/other community‐based testing sites | 47,161 (59) | 170 (10) | 436 (6) | 47,767 (54) |
| Home visit | 21,889 (28) | 992 (59) | 34 (0.5) | 22,915 (26) |
| Private clinic/pharmacy | 233 (0.3) | 482 (30) | 6510 (93) | 7225 (8) |
| Virtual outreach | 560 (0.7) | 0 (0) | 0 (0) | 560 (0.6) |
| Other | 9481 (12) | 24 (1) | 4 (0.1) | 9509 (11) |
| Median age (IQR) | 30 (25−35) | 32 (24−42) | 32 (28−38) | 30 (26−35) |
| Age | ||||
| 18−24 years | 16,151 (20) | 425 (25) | 761 (11) | 17,337 (20) |
| 25−34 years | 41,078 (52) | 493 (30) | 3607 (51) | 45,178 (51) |
| 35−44 years | 18,231 (23) | 393 (24) | 2196 (31) | 20,820 (24) |
| >44 years | 3864 (5) | 357 (21) | 420 (6) | 4641 (5) |
| Gender | ||||
| Male | 51,479 (65) | 815 (49) | 5633 (81) | 57,927 (66) |
| Female | 23,008 (29) | 850 (51) | 1349 (19) | 25,207 (29) |
| Transgender | 4765 (6) | 3 (0.2) | 2 (<0.1) | 4770 (5) |
| Other | 72 (0.1) | 0 | 0 | 72 (0.1) |
| Population group | ||||
| FSW | 19,513 (25) | 24 (1) | 295 (4) | 19,832 (22) |
| MSM | 15,180 (19) | 26 (2) | 36 (0.5) | 15,242 (17) |
| TG | 4765 (6) | 3 (0.2) | 2 (<0.1) | 4770 (5) |
| PWID | 5528 (7) | 3 (0.2) | 2 (<0.1) | 5533 (6) |
| Partner/client of key population | 10,934 (14) | 24 (1) | 41 (0.6) | 10,999 (12) |
| Partner of PLHIV | 1855 (2) | 537 (32) | 3 (<0.1) | 2395 (3) |
| Family member of PLHIV | 0 | 340 (20) | 0 | 340 (0.4) |
| Private practitioner/other referrals | 353 (0.4) | 91 (5) | 6111 (87) | 6555 (7) |
| Self‐identified high‐risk individuals | 21,199 (27) | 620 (37) | 494 (7) | 22,313 (25) |
| Education (n = 87,493) | ||||
| No formal education | 5382 (7) | 123 (7) | 136 (2) | 5641 (6) |
| Primary | 30,128 (38) | 447 (27) | 2469 (35) | 33,044 (38) |
| High school | 36,451 (46) | 987 (59) | 3605 (52) | 41,043 (47) |
| Higher education | 6888 (9) | 103 (6) | 774 (11) | 7765 (9) |
| HIV risk perception (n = 86,599) | ||||
| Low risk | 22,218 (28) | 833 (51) | 1687 (24) | 24,738 (29) |
| Medium risk | 33,634 (43) | 413 (25) | 2953 (43) | 37,000 (43) |
| High risk | 11,953 (15) | 294 (18) | 839 (12) | 13,086 (15) |
| Don't know | 10,271 (13) | 97 (6) | 1407 (20) | 11,775 (14) |
| Last HIV test (n = 87,974) | ||||
| Never tested | 35,740 (45) | 1144 (69) | 5236 (75) | 42,120 (48) |
| 0−12 months | 26,874 (34) | 176 (11) | 706 (10) | 27,756 (32) |
| More than 12 months | 11,681 (15) | 290 (17) | 493 (7) | 12,464 (14) |
| Time since last test not known | 5027 (6) | 58 (3) | 549 (8) | 5634 (6) |
| Kit preference | ||||
| Blood‐based | 39,967 (50) | 713 (43) | 4526 (65) | 45,206 (51) |
| Oral‐fluid based | 39,357 (50) | 955 (57) | 2458 (35) | 42,770 (49) |
Abbreviations: CBO, community‐based organization; FSW, female sex worker; IQR, inter quartile range; MSM, men who have sex with men; NGO, non‐governmental organisation; PWID, people who inject drugs; TG, transgender people.
3.1. Population characteristics
Participants median age was 30 years (IQR: 26−35 years) (Table 2). The median age was higher for participants recruited through the PLHIV network‐led (32 years; IQR: 24−42) and private practitioners (32 years; IQR: 28−38) models. Overall, 65.8% of participants were men, the proportion of women in the PLHIV network‐led model was relatively higher (51%). TG were mainly recruited through the community‐based model. Based on the recruitment strategies, the community‐based model enrolled mostly FSW, MSM and other self‐identified high‐risk individuals, while the PLHIV network‐based model reached partners and family members of PLHIV.
3.2. First‐time testers
The study reached 42,120 (48%) first‐time testers (Table 3). The proportion of first‐time testers was 75% in the private practitioners model and 69% in the PLHIV network‐led model. A higher proportion of men (55%) and younger individuals (54% among those between 18 and 24 years) were first‐time HIV testers. Almost all family members of PLHIV (98%) reported testing for the first time. The proportion of first‐time testers was also high among private practitioner referrals (76%), partners/clients of key population groups (68%) and MSM (43%).
Table 3.
Time since last HIV test and HIVST kit type preference for participants who accepted an HIVST kit in the STAR III study in India
| Time since last HIV test | HIVST kit type preference | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Never tested | 0−12 months | 12+ months | Unknown time of last HIV test | Total | Oral fluid‐based | Blood‐based | |
| Total | 87,974 (100) | 42,120 (48) | 27,756 (32) | 12,464 (14) | 5634 (6) | 87,976 (100) | 42,769 (49) | 45,207 (51) |
| Model | ||||||||
| Community‐based model | 79,322 (100) | 35,740 (45) | 26,874 (34) | 11,681 (15) | 5027 (6) | 79,324 (100) | 39,341 (50) | 39,983 (50) |
| PLHIV network‐led model | 1668 (100) | 1144 (69) | 176 (11) | 290 (17) | 58 (3) | 1668 (100) | 959 (57) | 709 (42) |
| Private practitioners’ model | 6984 (100) | 5236 (75) | 706 (10) | 493 (7) | 549 (8) | 6984 (100) | 2469 (35) | 4515 (65) |
| Age | ||||||||
| 18−24 years | 17,337 (100) | 9440 (54) | 4700 (27) | 1927 (11) | 1270 (7) | 17,337 (100) | 8606 (50) | 8731 (50) |
| 25−34 years | 45,117 (100) | 21,103 (47) | 14,846 (33) | 6903 (15) | 2325 (5) | 45,178 (100) | 22,151 (49) | 23,027 (51) |
| 35−44 years | 20,819 (100) | 9293 (45) | 6903 (33) | 2979 (14) | 1644 (8) | 20,820 (100) | 9935 (48) | 10,885 (52) |
| >44 years | 4641 (100) | 2284 (49) | 1307 (28) | 655 (14) | 395 (8) | 4641 (100) | 2077 (45) | 2564 (55) |
| Gender | ||||||||
| Male | 57,926 (100) | 32,153 (55) | 13,853 (24) | 7207 (12) | 4713 (8) | 57,927 (100) | 27,023 (47) | 30,904 (53) |
| Female | 25,206 (100) | 8293 (33) | 12,125 (48) | 4032 (16) | 756 (3) | 25,207 (100) | 13,112 (52) | 12,095 (48) |
| Transgender | 4770 (100) | 1635 (34) | 1775 (37) | 1223 (26) | 137 (3) | 4770 (100) | 2583 (54) | 2187 (46) |
| Other | 72 (100) | 39 (54) | 3 (4) | 2 (3) | 28 (39) | 72 (100) | 51 (71) | 21 (29) |
| Population group | ||||||||
| FSW | 19,831 (100) | 5168 (26) | 11,261 (57) | 2921 (15) | 481 (2) | 19,832 (100) | 10,419 (52) | 9413 (47) |
| MSM | 15,241 (100) | 6558 (43) | 5409 (35) | 2280 (15) | 994 (6) | 15,242 (100) | 8551 (56) | 6691 (44) |
| TG | 4764 (100) | 1633 (34) | 1773 (6) | 1221 (26) | 137 (3) | 4770 (100) | 2583 (54) | 2187 (46) |
| PWID | 5534 (100) | 1366 (25) | 3044 (55) | 805 (14) | 319 (6) | 5533 (100) | 1875 (34) | 3658 (66) |
| Partner/client of key population | 11,001 (100) | 7471 (68) | 1410 (13) | 1173 (11) | 947 (9) | 10,999 (100) | 4644 (42) | 6355 (58) |
| Partner of PLHIV | 2392 (100) | 800 (33) | 769 (32) | 727 (30) | 96 (4) | 2392 (100) | 1273 (53) | 1119 (47) |
| Family member of PLHIV | 340 (100) | 334 (98) | 1 (0.3) | 0 (0) | 5 (1) | 340 (100) | 161 (47) | 179 (53) |
| Private practitioner/other referrals | 6555 (100) | 4992 (76) | 527 (8) | 464 (7) | 572 (9) | 6555 (100) | 2524 (38) | 4031 (61) |
| Self‐identified high‐risk individuals | 22,313 (100) | 13,798 (62) | 3561 (16) | 2871 (13) | 2083 (9) | 22,313 (100) | 10,739 (48) | 11,574 (52) |
Abbreviations: FSW, female sex worker; MSM, men who have sex with men; PWID, people who inject drugs; TG, transgender people.
3.3. HIVST testing cascade
Among the 87,976 participants who received an HIVST kit, 87,899 (99.9%) were reached for follow‐up (Figure 2). Five participants (<0.01%) declined to share their test results. One hundred ninety‐four (0.2%) tested indeterminate on the first HIVST kit, and were retested; 22 (11%) tested indeterminate the second time and were offered linkage to the nearest ICTC for retesting. All 22 participants had used a blood‐based kit and used the assisted testing approach. Of these, 19 (86%) participants were tested at ICTC and all tested HIV negative.
A total of 328 (0.4%) participants received a reactive HIVST result. Among these, 291 (89%) were linked for confirmatory testing at a public ICTC. Among 254 who were confirmed HIV positive, 216 (85%) were linked to ART. A total of 10 (4%) tested negative in confirmatory testing. Of the 328 who tested HIVST reactive, 175 (53%) reported testing for the first time.
3.4. Test kit type preference
Among the 87,976 participants, a slightly higher preference was seen for blood‐based kits with 45,207 (51%) participants opting for them (Table 3). However, females (12,112; 52%), TG (2583; 54%) and MSM (8551; 56%) selected the oral‐fluid‐based test kit. A higher proportion of people referred by private practitioners preferred the blood‐based kit (4031; 61%).
3.5. Acceptance of HIVST for future testing
Among the 87,976 participants who tested with an HIVST kit, 77,064 (88%) reported willingness to use HIVST over other HIV testing services in the future (Table 4). Partner/family members of PLHIV (OR: 3.668; 95 CI: 2.995−4.492) and TG (OR: 1.564; 95 CI: 1.400−1.747) had higher odds of preferring HIVST over other testing methods. People who considered themselves at high risk of HIV (aOR: 0.600; 95 CI: 0.561−0.643) and those referred by private practitioners (OR: 0.582; 95 CI: 0.541−0.626) had lower odds for using HIVST in the future compared to those who were unaware of their HIV risk and those who self‐identified themselves at high risk, respectively.
Table 4.
Acceptance of HIVST for future testing, ease of using an HIVST kit and ease of interpreting the result among participants of the STAR III study in India
| Acceptance for future use | Ease of using the kit | Ease of interpretation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Yes n (%) | OR | 95 CI | aOR | 95 CI | Easy n (%) | OR | 95 CI | aOR | 95 CI | Easy n (%) | OR | 95 CI | aOR | 95 CI | |
| Total | 87,976 | 77,064 (88) | 83,308 (95) | 83,273 (95) | ||||||||||||
| Age | ||||||||||||||||
| 18−24 years | 17,337 | 15,639 (90) | Ref | 16,172 (93) | Ref | 16,136 (93) | Ref | |||||||||
| 25−34 years | 45,178 | 39,400 (87) | 0.74 | 0.699−0.784 | 42,768 (95) | 1.278 | 1.189−1.374 | 42,787 (95) | 1.332 | 1.240−1.431 | ||||||
| 35−44 years | 20,820 | 17,834 (86) | 0.648 | 0.609−0.691 | 19,870 (95) | 1.507 | 1.380−1.646 | 19,877 (95) | 1.569 | 1.437−1.713 | ||||||
| >44 years | 4641 | 4191 (90) | 1.011 | 0.906−1.128 | 4498 (97) | 2.266 | 1.899−2.704 | 4473 (96) | 1.982 | 1.681−2.337 | ||||||
| Gender | ||||||||||||||||
| Male | 57,927 | 51,418 (89) | Ref | 54,352 (94) | Ref | 54,243 (94) | Ref | |||||||||
| Female | 25,207 | 21,228 (84) | 0.675 | 0.647−0.705 | 24,358 (97) | 1.887 | 1.748−2.037 | 24,521 (97) | 2.428 | 2.235−2.637 | ||||||
| Transgender | 4770 | 4376 (92) | 1.406 | 1.264−1.564 | 4536 (95) | 1.275 | 1.113−1.460 | 4452 (93) | 0.951 | 0.845−1.071 | ||||||
| Prefer not to disclose | 72 | 42 (58) | 0.177 | 0.111−0.283 | 62 (86) | 0.408 | 0.209−0.796 | 57 (79) | 0.258 | 0.146−0.456 | ||||||
| Population group | ||||||||||||||||
| Self‐identified high‐risk individuals | 22,313 | 19,562 (88) | Ref | 20,904 (94) | Ref | 20,885 (94) | Ref | |||||||||
| FSW | 19,832 | 16,546 (83) | 0.708 | 0.670−0.748 | 19,193 (97) | 2.025 | 1.840−2.227 | 19,289 (97) | 2.429 | 2.196−2.686 | ||||||
| MSM | 15,242 | 13,669 (90) | 1.222 | 1.144−1.305 | 14,281 (94) | 1.002 | 0.920−1.090 | 14,277 (94) | 1.012 | 0.930−1.101 | ||||||
| TG | 4764 | 4371 (92) | 1.564 | 1.400−1.747 | 4531 (95) | 1.311 | 1.137−1.511 | 4450 (93) | 0.969 | 0.854−1.100 | ||||||
| PWID | 5534 | 4814 (87) | 0.94 | 0.861−1.027 | 4959 (90) | 0.581 | 0.525−0.644 | 4922 (89) | 0.55 | 0.498−0.608 | ||||||
| Partner/client of key population | 11,001 | 10,188 (93) | 1.762 | 1.624−1.913 | 10,406 (95) | 1.179 | 1.068−1.301 | 10,394 (94) | 1.171 | 1.062−1.291 | ||||||
| Partner/family member of PLHIV | 2735 | 2634 (96) | 3.668 | 2.995−4.492 | 2658 (97) | 2.327 | 1.843−2.937 | 2678 (98) | 3.212 | 2.458−4.199 | ||||||
| Private practitioner/other referrals | 6555 | 5280 (80) | 0.582 | 0.541−0.626 | 6376 (97) | 2.401 | 2.050−2.812 | 6378 (97) | 2.464 | 2.102−2.887 | ||||||
| Education (n = 87,493) | ||||||||||||||||
| No formal education | 5641 | 4945 (88) | Ref | 5497 (97) | Ref | Ref a | 5457 (97) | Ref | Ref | |||||||
| Primary | 33,044 | 28,927 (87) | 0.989 | 0.908−1.078 | 31,747 (96) | 0.641 | 0.539−0.763 | 0.653 | 0.548−0.778 | 31,694 (96) | 0.792 | 0.677−0.926 | 0.802 | 0.686−0.939 | ||
| High school | 41,043 | 36,025 (88) | 1.01 | 0.928−1.100 | 38,435 (94) | 0.386 | 0.326−0.458 | 0.408 | 0.344−0.484 | 38,546 (94) | 0.521 | 0.447−0.606 | 0.548 | 0.471−0.639 | ||
| Higher education | 7765 | 6825 (88) | 1.022 | 0.920−1.135 | 7262 (93) | 0.378 | 0.313−0.457 | 0.405 | 0.335−0.490 | 7250 (93) | 0.475 | 0.400−0.564 | 0.506 | 0.426−0.602 | ||
| HIV risk perception (n = 86,599) | ||||||||||||||||
| Don't know | 11,775 | 10,036 (85) | Ref | Ref b | 10,856 (92) | Ref | Ref c | 10,778 (91) | Ref | Ref c | ||||||
| Low risk | 24,738 | 23,089 (93) | 2.426 | 2.259−2.606 | 2.321 | 2.159−2.495 | 23,576 (95) | 1.718 | 1.571−1.878 | 1.764 | 1.609−1.933 | 23,521 (95) | 1.788 | 1.639−1.905 | 1.87 | 1.710−2.044 |
| Medium risk | 37,000 | 32,602 (88) | 1.284 | 1.210−1.364 | 1.247 | 1.173−1.325 | 35,050 (95) | 1.522 | 1.403−1.651 | 1.483 | 1.364−1.612 | 35,117 (95) | 1.725 | 1.593−1.868 | 1.744 | 1.606−1.894 |
| High risk | 13,086 | 10,253 (78) | 0.627 | 0.587−0.670 | 0.6 | 0.561−0.643 | 12,591 (96) | 2.153 | 1.925−2.409 | 2.028 | 1.805−2.279 | 12,641 (97) | 2.628 | 2.343−2.947 | 2.469 | 2.192−2.781 |
| Last HIV test (n = 87,974) | ||||||||||||||||
| Never tested | 42,120 | 37,427 (89) | Ref | Ref d | 39,704 (94) | Ref | Ref e | 39,681 (94) | Ref | Ref e | ||||||
| 0−12 months | 27,756 | 23,460 (84) | 0.685 | 0.655−0.716 | 0.717 | 0.680−0.755 | 26,584 (96) | 1.38 | 1.285−1.483 | 1.392 | 1.284−1.509 | 26,622 (96) | 1.443 | 1.343−1.551 | 1.429 | 1.317−1.551 |
| More than 12 months | 12,464 | 11,202 (90) | 1.113 | 1.042−1.189 | 1.137 | 1.061−1.218 | 11,829 (95) | 1.134 | 1.036−1.240 | 1.183 | 1.076−1.302 | 11,894 (95) | 1.283 | 1.168−1.408 | 1.349 | 1.220−1.490 |
| Time since last test not known | 5634 | 4974 (88) | 0.945 | 0.867−1.031 | 1.01 | 0.919−1.109 | 5190 (92) | 0.711 | 0.640−0.790 | 0.798 | 0.713−0.892 | 5075 (90) | 0.558 | 0.507−0.615 | 0.627 | 0.565−0.695 |
| Kit used | ||||||||||||||||
| Oral‐fluid | 42,769 | 37,563 (88) | Ref | Ref f | 41,106 (96) | Ref | Ref g | 40,831 (95) | Ref | Ref g | ||||||
| Blood‐based | 45,207 | 39,501 (87) | 0.959 | 0.922−0.999 | 0.946 | 0.907−0.986 | 42,202 (93) | 0.568 | 0.534−0.604 | 0.555 | 0.521−0.591 | 42,442 (94) | 0.729 | 0.686−0.773 | 0.725 | 0.682−0.772 |
Adjusted for age.
Adjusted for age and population group.
Adjusted for age, education and population group.
Adjusted for age, population group and HIV risk perception.
Adjusted for age, education, HIV risk perception and population group.
Adjusted for age, HIV risk perception and last HIV test.
Adjusted for age, population group, HIV risk perception and last HIV test.
The major reasons that participants reported for selecting HIVST over other testing methods were privacy (84%; 65,011/77,064), getting quick results with HIVST (81%, 62,371/77,064), convenience (44%; 34,157/77,064) and ease of administration (40%, 31,077/77,064). Participants reported that the possibility of interpreting the result incorrectly (8%; 6172/77,064), getting a false negative or positive result (8%; 5959/77,064) and the loss of opportunity to interact with a healthcare provider and counselling (6%; 4635/77,064) were the concerns with selecting HIVST over healthcare provider delivered HIV testing.
3.6. Ease of using the kit and interpreting the result
Overall, 83,380 (95%) and 83,237 (95%) participants reported that they found the test kits easy to use and to interpret the result, respectively (Table 4). Participants with higher education had lower odds of finding the kits easy to use (aOR: 0.405; 95 CI: 0.335−0.490) and interpret (aOR: 0.506; 95 CI: 0.426−0.602) than those with no formal education. Also, participants using the blood‐based kits had lower odds of finding the kits as easy to use (aOR: 0.555; 95 CI: 0.521−0.591) and interpret (aOR: 0.725; 95 CI: 0.682−0.772) compared to those using oral‐fluid based test kits. Higher risk perception levels, recent HIV testing and use of oral fluid kit were associated with greater ease of interpretation, while low and medium HIV risk perceptions and use of oral‐fluid kit increased the likelihood of future use.
3.7. Willingness to pay
Among the 75,206 participants who preferred HIVST for future testing, 52,136 (69%) reported willingness to pay for the test kit in the future (Table 5). A large proportion of TG (88%) and MSM (72%) reported willingness to pay for the kit. A total of 35,854 (48%) participants were willing to pay less than US$ 1.2 (INR 100) to purchase the kit, 13,857 (18%) reported a willingness to pay between US$ 1.2 and 3 (INR 101−250), and only 2425 (3%) reported a willingness to pay more than US$ 3 (INR 250). Just over one‐third of TG (40%) were willing to pay between US$ 1.2 and 3 (INR 101−250) for the HIV self‐test kits.
Table 5.
Willingness to pay for HIV self‐testing among participants of the STAR III study in India
| Not willing to pay | Less than USD 1.2 (INR 100) | Between USD 1.2 and USD 3 (INR 101−250) | More than USD 3 (INR 250) | Total | |
|---|---|---|---|---|---|
| Total | 23,070 (1) | 35,854 (48) | 13,857 (18) | 2425 (3) | 75,206 |
| Model | |||||
| Community‐based model | 20,252 (29) | 33,003 (48) | 13,023 (19) | 2238 (3) | 68,561 |
| PLHIV network‐led model | 420 (26) | 905 (56) | 255 (16) | 31 (2) | 1611 |
| Private practitioners model | 2398 (48) | 1946 (39) | 579 (11) | 111 (2) | 5034 |
| Age | |||||
| 18−24 years | 4665 (31) | 7206 (48) | 2759 (18) | 472 (3) | 15,092 |
| 25−34 years | 11,845 (31) | 18,041 (47) | 7206 (19) | 1320 (3) | 38,412 |
| 35−44 years | 5314 (30) | 8677 (49) | 3121 (18) | 449 (2) | 17,561 |
| >44 years | 1256 (30) | 1930 (46) | 771 (17) | 184 (4) | 4141 |
| Gender | |||||
| Male | 15,314 (30) | 25,383 (51) | 8197 (16) | 1300 (3) | 50,194 |
| Female | 7217 (35) | 8566 (41) | 3909 (19) | 939 (4) | 20,631 |
| Transgender | 537 (12) | 1887 (43) | 1733 (40) | 182 (4) | 4339 |
| Other | 2 (5) | 18 (25) | 18 (43) | 4 (9) | 42 |
| Population group | |||||
| FSW | 5486 (34) | 6513 (41) | 3220 (20) | 786 (5) | 16,005 |
| MSM | 3671 (28) | 6812 (51) | 2256 (17) | 507 (4) | 13,246 |
| TG | 537 (12) | 1887 (43) | 1733 (40) | 182 (4) | 4339 |
| PWID | 1516 (33) | 2033 (44) | 1009 (22) | 57 (1) | 4615 |
| Partner/client of key population | 3896 (40) | 3895 (40) | 1813 (18) | 200 (2) | 9804 |
| Partner of PLHIV | 547 (24) | 1180 (52) | 461 (20) | 91 (4) | 2279 |
| Family member of PLHIV | 257 (78) | 72 (22) | 0 | 0 | 329 |
| Private practitioner/other referrals | 2472 (47) | 2049 (39) | 628 (12) | 115 (2) | 5264 |
| Self‐identified high‐risk individuals | 4688 (24) | 11,413 (59) | 2737 (14) | 487 (2) | 19,325 |
| Preferred HIVST kit for future use | |||||
| Blood‐based | 11,650 (33) | 16,642 (48) | 5654 (16) | 949 (3) | 34,895 |
| Oral fluid‐based | 11,298 (28) | 19,164 (48) | 8182 (20) | 1469 (4) | 40,113 |
Abbreviations: FSW, female sex worker; MSM, men who have sex with men; PWID, people who inject drugs; TG, transgender people.
3.8. Social harms
One participant identifying as MSM, with a history of depression and suicidal thoughts, who tested HIV positive reported having conflict with his partner and suicidal thoughts. That participant was linked with psychological counselling.
4. DISCUSSION
HIVST kits distributed through various community‐based organisations and private providers was seen to be highly acceptable among a wide range of key populations and other high‐risk groups in India. Over 97% of those eligible accepted HIVST kits and almost 9 out of 10 participants reported preferring to receive HIVST over provider‐delivered testing methods in the future. Several studies from India have previously reported that HIVST was acceptable among key population groups in the country [8–12, 14]. While most of these studies were either qualitative or had a limited sample size, this study, for the first time in the country, demonstrated the high acceptability of HIVST empirically through different distribution models. HIVST distribution in private clinics/hospitals could be encouraged by offering training to clinic staff to help integrate HIVST distribution into the clinic workflows and displaying educational materials on HIVST in the clinics.
As of 2021−22, the NACP reports coverage of over 90% of the estimated number of people in the key population groups [2]. However, many study participants from these groups reported testing for the first time. We also found a large number of first‐time testers among the partners and clients of key populations. This proportion was as high as reported from Vietnam and China and higher than seen in other settings such as Malawi, Zambia and Zimbabwe, underscoring its value in expanding reach and improving HIV diagnosis as India advances towards epidemic control goals [16, 17, 18].
ART initiation in this study at 85% was only slightly lower than the national linkage of 92% from public ICTCs to ART centres, however, importantly diagnosed people who never before tested [19]. Previous studies among FSWs, pregnant women and truckers in India reported a preference for oral‐fluid test kits over blood‐based [9, 10, 11, 12]. However, in the present study, we found that an almost equal proportion of participants preferred oral‐fluid and blood‐based test kits.
Along with high acceptance, this study also found high ease of use and ease of interpretation for both, oral‐fluid and blood‐based test kits, irrespective of the education level of the participants. This finding is in concurrence with other studies around the world [5, 6]. Given the high acceptability and usability of both oral‐fluid and blood‐based HIVST kits, it will be imperative to have both kit types made available in the country.
Seven out of 10 participants who preferred HIVST kits for future testing reported willingness to pay at least some amount for them. This proportion is similar to studies from low‐ and middle‐income countries that report willingness to pay between 65% and 92% [20, 21, 22]. Among those who were willing to pay, 69% were willing to pay up to US$ 1.2 or lower, and 26.6% were willing to pay up to US$ 3. This amount is similar to that reported by other studies from low‐ and middle‐income countries [20, 21, 22, 23, 24]. Given the mismatch between the current costs of the test kits (US$ 7−US$ 12 in the private sector) and the price most participants are willing to pay, it will be imperative to continue with market interventions to reduce the costs of test kits for end users [25]. Given the ability to reach a large proportion of first‐time testers and high levels of acceptance and usability of HIVST, there is value in exploring making HIVST available from the public sector.
We found extremely low levels of reported social harms in our study, with only one participant reporting suicidal tendencies after testing HIV positive. Further investigation revealed that the suicidal tendency was due to extraneous factors and, therefore, was not associated with the use of HIVST. These findings are in line with the international literature that report a very low level of social harms associated with the use of HIVST [6].
Based on overall outcomes, TG reported a high level of acceptance, usability and willingness to pay for the use of HIVST kits. This might be due to the inherent confidentiality and privacy that HIVST has to offer, thus sparing them from the discrimination they might face at healthcare facilities [26].
This study is among the first that provides evidence on the distribution of HIVST through different service delivery models among a wide range of population groups across 14 states and 50 districts in India. This study was conducted at existing HIV service sites and thus represents real‐world scenarios in the country. The COVID‐19 pandemic, despite leading to delays in obtaining regulatory approvals for importing HIVST kits, may not have significantly impacted the outcome. However, the study had some limitations. The study sampling frame was not designed to be nationally representative of key populations and their sexual/injecting partners or clients. It did not include pregnant women or private sector pharmacies nor allowed for secondary distribution. Future studies can look at the feasibility of using secondary distribution to reach population groups such as partners of MSM and FSWs that might be even more difficult to reach. As data were collected face‐to‐face, the possibility of desirability bias cannot be ruled out.
5. CONCLUSIONS
Overall, we found that nearly all participants were willing to accept HIVST, found the test kits easy to use and interpret, and about two‐thirds were willing to pay for HIVST distributed through the community‐based, PLHIV network‐led and private practitioners models in India, with good linkage to confirmatory testing and ART initiation. Given the high levels of acceptance and the ability to reach a large proportion of first‐time testers combined with the provision of comprehensive prevention solutions, HIVST in India could contribute to achieving the UNAIDS first 95 and ending the HIV epidemic.
COMPETING INTERESTS
The authors declared no competing interests.
AUTHORS’ CONTRIBUTIONS
CL, KM, AD, AH, VC, VV, CD, JVRPR, DCSR and KG have made contributions to the conception. CL, KM, AD, AH, MS, MP, VC, SG, TB and DCSR designed the work. CL, KM, SM, MS, MD, MM, GSS, MP, VJ, VRP, MP, CU, SC, SU and MN led the acquisition of data. CL, KM, AD, AH, VR, YB, JC, VC, SG, TB and DCSR conducted the analysis and interpretation of data. CL has drafted the work. KM, AD, AH, MS, SM, MS, MD, MM, GSS, MP, VJ, VRP, VR, YB, JC, MP, CU, SC, SU, MN, VC, SG, TB, VV, CD, JVRPR, DCSR and KG substantively reviewed and revised it. All authors have approved the submitted version.
FUNDING
This study was conducted under the STAR III Initiative grant from Unitaid and led by Population Services International (PSI). The HIVST kits used in the study were supplied to the Indian investigators under the UNITAID STAR‐III grant, with necessary licenses obtained from the Central Drugs Standard Control Organisation (CDSCO) under the Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India to ensure compliance with Indian regulations.
DISCLAIMER
The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the funding agencies.
Supporting information
Table S1: State and district‐wise HIVST distribution models implemented under the STAR III study in India
Table S2: Questions, responses and dichotomization of the primary outcomes
Figure S1: Directed acyclic graph (DAG) of ease of interpreting the HIV self‐test result.
Figure S2: Directed acyclic graph (DAG) of ease of use of HIV self‐testing kit.
Figure S3: Directed acyclic graph (DAG) of acceptance of HIV self‐testing for next use.
ACKNOWLEDGEMENTS
The authors would like to thank the National AIDS Control Organisation and all States AIDS Control Societies for their support in implementing this study. The authors would further like to acknowledge Dr. Rachel Baggaley (WHO, Geneva), Dr. Cheryl Johnson (WHO, Geneva), Dr. Mohammad Jamil (WHO, Geneva), Dr Karin Hatzold (PSI), Dr. B.B. Rewari (WHO SEARO), Dr. Po‐Lin Chan and Dr. Rajatashuvra Adhikary (WHO India), Dr. Salil Panakadan (UNAIDS), Ms. Purvi Shah (UNAIDS and WHO), Ms. Nandini Kapoor Dhingra and Dr. David Bridger (UNAIDS, India), Mr Aayush Solanki (PSI), Mr. Rohit Sarkar and Mr. Pawan Kumar (India HIV/AIDS Alliance), and Dr. Vikas Oswal (Sai hospital, Mumbai). The authors would also like to thank the members of the PATH India HIV self‐testing project advisory group (PISPAG) and the co‐chair Dr. Ashok Kumar, community advisory board (CAB) chaired by Ms. Selvi Shanmugam and co‐chaired by Mx. Yashwinder Singh, and all the state oversight committees (SOC) and community monitoring boards (CMB) for their support during the entire project duration. The authors would also like to acknowledge the technical support of the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). Additionally, the authors would like to thank Mr. Anupam Hazra (SAATHII) and Ms. Vaishnavi Jondhale, Mr. Haresh Patel, Dr. Satyabrata Routray, Mr. Kanduri Ananth Balaji, Dr. Shibu Vijayan, Ms. Pranati Jha, Ms. Isha Jain, Mr. K. G. Venkateswaran, Mr. Rishabh Chopra, Mr. Kushal Mazumdar, Mr. Neeraj Jain, Ms. Davina Canagasabey, Ms. Leecreesha Hicks, Ms. Lisa Mueller Scott, Ms. Krista Granger, Dr. Kerry Thomson, Mr. Purusottam Sahu, Ms. Varsha Nagwekar and Ms. Sandrine Fimbi of PATH for their support. Lastly, we would like to acknowledge the hard work and support of all our staff, partners, community leaders and communities for making this study possible.
The STAR III initiative was implemented through a collaboration between PSI, PATH, Jhpiego and International Labor Organization (ILO) with technical oversight from WHO. In India, the study was led by PATH and implemented by The Humsafar Trust, Solidarity and Action Against The HIV Infection (SAATHII), PEPFAR/CDC implementing partners (International Training and Education Centre for Health [I‐TECH India], Society for Health Allied Research & Education India [SHARE India], Voluntary Health Services [VHS]), the National Coalition of People Living with HIV in India (NCPI+) and Network of Maharashtra By People Living with HIV/AIDS (NMP+).
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are available from the corresponding author on reasonable request and after approval from the ethics committee.
REFERENCES
- 1. UNAIDS . Factsheet 2022. Accessed 12 Feb 2023. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 2. National AIDS Control Organisation . Sankalak: Status of National AIDS Response (5th edition). New Delhi; 2023. [Google Scholar]
- 3. Hatzold K, Gudukeya S, Mutseta MN, Chilongosi R, Nalubamba M, Nkhoma C, et al. HIV self‐testing: breaking the barriers to uptake of testing among men and adolescents in sub‐Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. 2019;22(Suppl 1):e25244. 10.1002/jia2.25244/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva; 2021. [PubMed] [Google Scholar]
- 5. Witzel TC, Eshun‐Wilson I, Jamil MS, Tilouche N, Figueroa C, Johnson CC, et al. Comparing the effects of HIV self‐testing to standard HIV testing for key populations: a systematic review and meta‐analysis. BMC Med. 2020;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson CC, Kennedy C, Fonner V, Siegfried N, Figueroa C, Dalal S, et al. Examining the effects of HIV self‐testing compared to standard HIV testing services: a systematic review and meta‐analysis. J Int AIDS Soc. 2017;20(1):21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National AIDS Control Organization . National AIDS and STD Control Programme Phase‐V (2021–2026). Anchoring the national response towards ending the AIDS epidemic. 2022.
- 8. Rawat S, Solomon SS, Dange A, Srikrishnan AK, Chakrapani V, Mohan B, et al. Community perspectives on making HIV self‐testing accessible as a comprehensive prevention package. New York: Springer; 2018. [Google Scholar]
- 9. Rao A, Patil S, Aheibam S, Kshirsagar P, Hemade P, Panda S. Acceptability of HIV oral self‐test among men having sex with men and transgender population: a qualitative investigation from Pune, India. Infect Dis (Auckl). 2020;13:1178633720962809. doi: 10.1177/1178633720962809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao A, Patil S, Kulkarni PP, Devi AS, Borade SS, Ujagare DD, et al. Acceptability of HIV oral self‐test among truck drivers and youths: a qualitative investigation from Pune, Maharashtra. BMC Public Health. 2021;21:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao A, Patil S, Nirmalkar A, Bagul R, Ghule U, Panchal N, et al. HIV oral self‐screening test among HIV/STD/TB clinic attendees: a mixed‐method pilot investigation examining merit for larger evaluation. Indian J Med Res. 2022;155:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang YM, Sevekari T, Duerr A, Molina Y, Gilada T. HIV self‐testing in Pune, India: perspectives and recommendations of female sex workers and peer educators. AIDS Care. 2020;32:182–185. [DOI] [PubMed] [Google Scholar]
- 13. Vashisht S, Rai S, Kant S, Halder P, Misra P, Goswami K, et al. Oral HIV self‐testing among men who have sex with men in New Delhi, India: perceptions & apprehensions: a qualitative study. Indian J Med Res. 2022;156(6):764–770. 10.4103/ijmr.ijmr_718_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarkar A, Mburu G, Shivkumar PV, Sharma P, Campbell F, Behera J, et al. Feasibility of supervised self‐testing using an oral fluid‐based HIV rapid testing method: a cross‐sectional, mixed method study among pregnant women in rural India. J Int AIDS Soc. 2016;19:20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin Y, Tang W, Nowacki A, Mollan K, Reifeis SA, Hudgens MG, et al. Benefits and potential harms of human immunodeficiency virus self‐testing among men who have sex with men in China: an implementation perspective. Sex Transm Dis. 2017;44:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatzold K, Gudukeya S, Mutseta MN, Chilongosi R, Nalubamba M, Nkhoma C, et al. HIV self‐testing: breaking the barriers to uptake of testing among men and adolescents in sub‐Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J Int AIDS Soc. 2019;22:e25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green KE, Vu BN, Phan HT, Tran MH, Ngo HV, Vo SH, et al. From conventional to disruptive: upturning the HIV testing status quo among men who have sex with men in Vietnam. J Int AIDS Soc. 2018;21:e25127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Department of Health & Family Welfare Ministry of Health & Family . Annual Report 2021–22. 2022.
- 20. Ashburn K, Antelman G, N'Goran MK, Jahanpour O, Yemaneberhan A, N'Guessan Kouakou B, et al. Willingness to use HIV self‐test kits and willingness to pay among urban antenatal clients in Cote d'Ivoire and Tanzania: a cross‐sectional study. Trop Med Int Health. 2020;25:1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thirumurthy H, Masters SH, Agot K. Willingness to pay for HIV self‐tests among women in Kenya: implications for subsidy and pricing policies. J Acquir Immune Defic Syndr. 2018;78:E8–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tonen‐Wolyec S, Kayembe Tshilumba C, Batina‐Agasa S, Marini Djang'eing'a R, Hayette MP, Belec L. Comparison of practicability and effectiveness between unassisted HIV self‐testing and directly assisted HIV self‐testing in the Democratic Republic of the Congo: a randomized feasibility trial. BMC Infect Dis. 2020;20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ngoc BV, Majam M, Green K, Tran T, Hung MT, Que AL, et al. Acceptability, feasibility, and accuracy of blood‐based HIV self‐testing: a cross‐sectional study in Ho Chi Minh City, Vietnam. PLOS Glob Public Health. 2023;3:e0001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. West RL, Freeman L, Pahe C, Momanyi H, Kidiga C, Malaba S, et al. Characterising the HIV self‐testing market in Kenya: awareness and usage, barriers and motivators to uptake, and propensity to pay. PLOS Glob Public Health. 2023;3:e0001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Unitaid, World Health Organization . Market and technology landscape: HIV rapid diagnostic tests for self‐testing (4th edition). Geneva; 2018. [Google Scholar]
- 26. Pandya AK, Redcay A. Access to health services: barriers faced by the transgender population in India. J Gay Lesbian Ment Health. 2021;25:132–154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: State and district‐wise HIVST distribution models implemented under the STAR III study in India
Table S2: Questions, responses and dichotomization of the primary outcomes
Figure S1: Directed acyclic graph (DAG) of ease of interpreting the HIV self‐test result.
Figure S2: Directed acyclic graph (DAG) of ease of use of HIV self‐testing kit.
Figure S3: Directed acyclic graph (DAG) of acceptance of HIV self‐testing for next use.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request and after approval from the ethics committee.


