Abstract
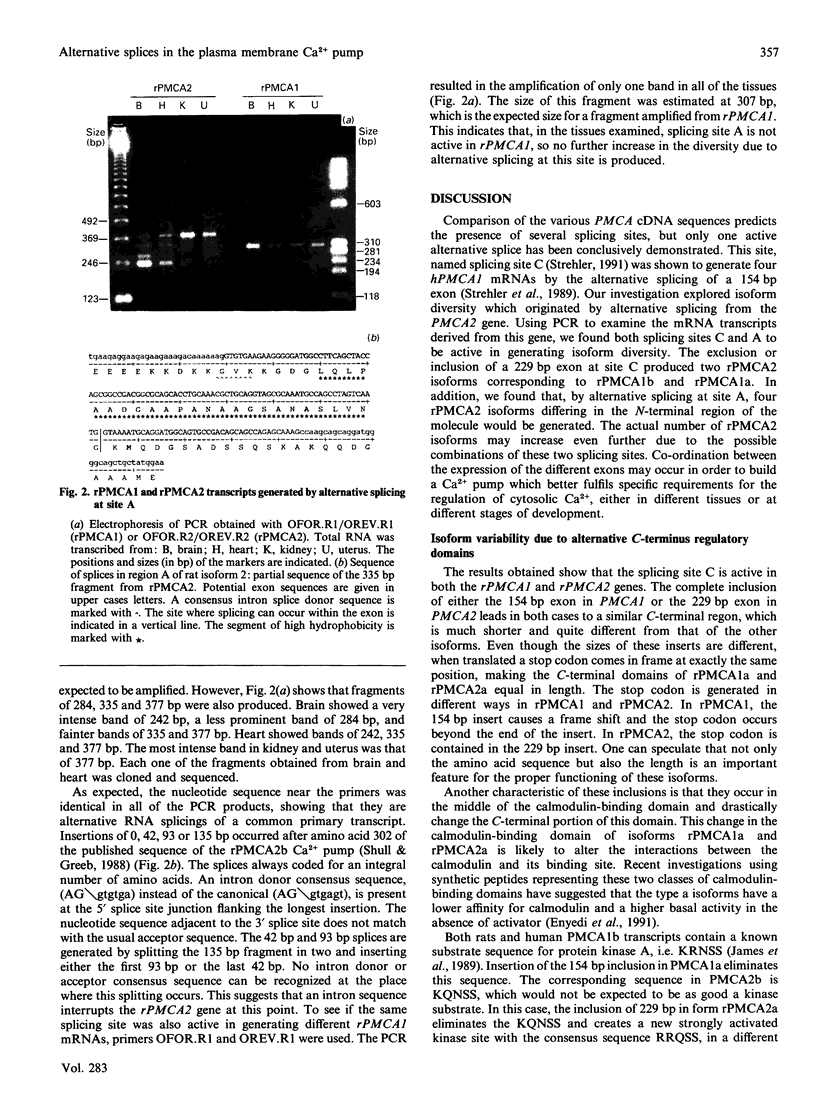
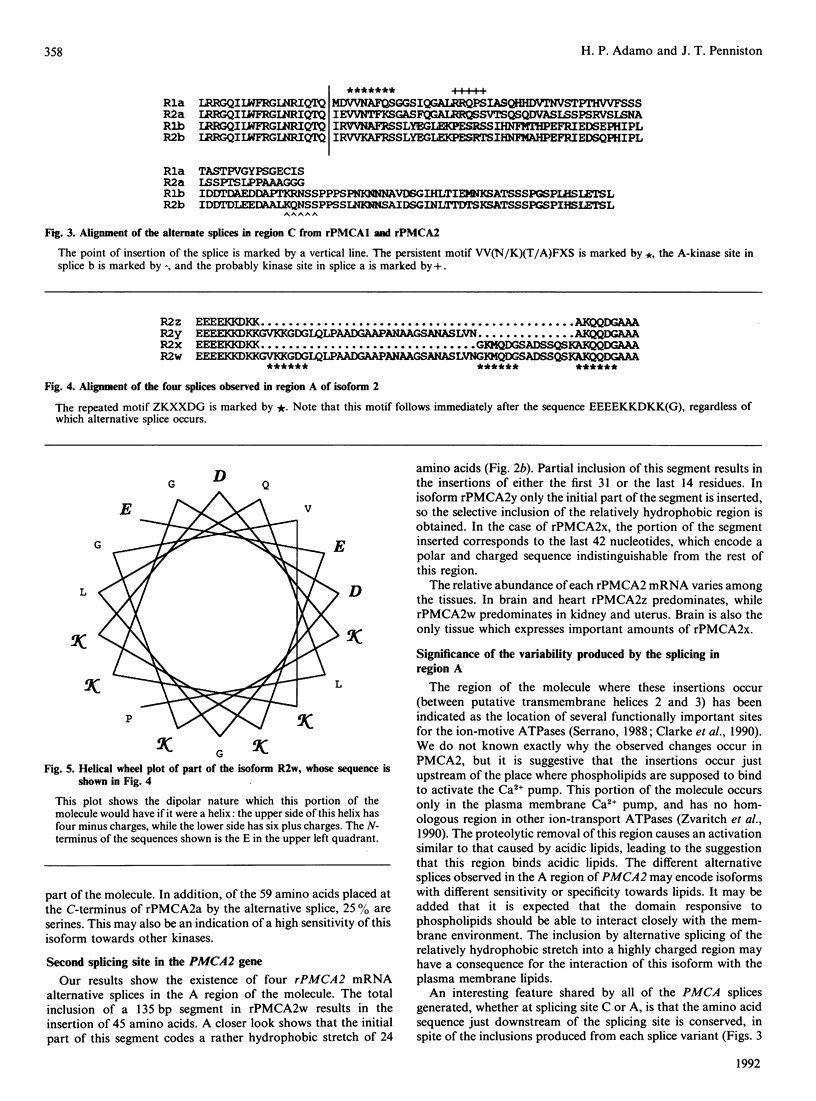
Alternative splices capable of generating proteins with altered functions were found (by PCR) in isoform 2 of the rat plasma membrane Ca2+ pump. These splices were concentrated in two hypervariable regions. One of these regions, near the N-terminus and the lipid-binding region, could be altered by the insertion of either or both of inserts x and y. Insertion of both x and y would add 45 amino acids to the molecule. The y insert causes the appearance of a rather hydrophobic stretch of amino acids in the middle of a highly polar region. The second variable region begins in the middle of the calmodulin-binding domain. Insertion of 229 nucleotides at this point of the message converts the b form to the a form, which has an altered (and shorter) C-terminus. The calmodulin-binding domain of this shortened form has a less basic character, which would decrease the affinity for calmodulin. The b form of isoenzyme 2 contains relatively weak protein kinase A substrate sequences, such as KQNSS and KNNS. These sequences are eliminated in form a, and a strongly activated kinase substrate sequence, RRQSS, appears in a different place. Different tissues use different combinations of alternative splices, with heart and brain showing the greatest diversity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke D. M., Loo T. W., MacLennan D. H. Functional consequences of mutations of conserved amino acids in the beta-strand domain of the Ca2(+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1990 Aug 25;265(24):14088–14092. [PubMed] [Google Scholar]
- De Jaegere S., Wuytack F., Eggermont J. A., Verboomen H., Casteels R. Molecular cloning and sequencing of the plasma-membrane Ca2+ pump of pig smooth muscle. Biochem J. 1990 Nov 1;271(3):655–660. doi: 10.1042/bj2710655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A., Filoteo A. G., Gardos G., Penniston J. T. Calmodulin-binding domains from isozymes of the plasma membrane Ca2+ pump have different regulatory properties. J Biol Chem. 1991 May 15;266(14):8952–8956. [PubMed] [Google Scholar]
- Greeb J., Shull G. E. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem. 1989 Nov 5;264(31):18569–18576. [PubMed] [Google Scholar]
- James P. H., Pruschy M., Vorherr T. E., Penniston J. T., Carafoli E. Primary structure of the cAMP-dependent phosphorylation site of the plasma membrane calcium pump. Biochemistry. 1989 May 16;28(10):4253–4258. doi: 10.1021/bi00436a020. [DOI] [PubMed] [Google Scholar]
- Khan I., Grover A. K. Expression of cyclic-nucleotide-sensitive and -insensitive isoforms of the plasma membrane Ca2+ pump in smooth muscle and other tissues. Biochem J. 1991 Jul 15;277(Pt 2):345–349. doi: 10.1042/bj2770345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988 Feb 24;947(1):1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988 Jun 25;263(18):8646–8657. [PubMed] [Google Scholar]
- Strehler E. E., James P., Fischer R., Heim R., Vorherr T., Filoteo A. G., Penniston J. T., Carafoli E. Peptide sequence analysis and molecular cloning reveal two calcium pump isoforms in the human erythrocyte membrane. J Biol Chem. 1990 Feb 15;265(5):2835–2842. [PubMed] [Google Scholar]
- Strehler E. E. Recent advances in the molecular characterization of plasma membrane Ca2+ pumps. J Membr Biol. 1991 Feb;120(1):1–15. doi: 10.1007/BF01868586. [DOI] [PubMed] [Google Scholar]
- Strehler E. E., Strehler-Page M. A., Vogel G., Carafoli E. mRNAs for plasma membrane calcium pump isoforms differing in their regulatory domain are generated by alternative splicing that involves two internal donor sites in a single exon. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6908–6912. doi: 10.1073/pnas.86.18.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. K., Filoteo A. G., Stanford D. R., Wieben E. D., Penniston J. T., Strehler E. E., Fischer R., Heim R., Vogel G., Mathews S. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem. 1988 Oct 5;263(28):14152–14159. [PubMed] [Google Scholar]
- Zvaritch E., James P., Vorherr T., Falchetto R., Modyanov N., Carafoli E. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry. 1990 Sep 4;29(35):8070–8076. doi: 10.1021/bi00487a012. [DOI] [PubMed] [Google Scholar]