Abstract
Zooplankton are critical indicators of pressures impacting freshwater ecosystems. We analyzed the response of zooplankton communities across different sub-catchment types—headwaters, natural, urban, urban-agricultural, and agricultural—within the Łyna river–lake system in Northern Poland. Using taxonomic groups and functional traits (body size, feeding strategies), we applied Partial Least Squares Regression (PLS-R) to elucidate the relationships between environmental conditions, land use, and zooplankton metacommunity structure. Two-Way Cluster Analysis (TWCA) identified local subsets with characteristic patterns, while Indicator Species Analysis (ISA) determined area-specific taxa. The natural river zone exhibited significant habitat heterogeneity and feeding niches, whereas urban areas created functional homogenization of zooplankton, dominated by small, broad-diet microphages. Agricultural areas promoted diversity among large filter feeders (Crustacea), active suctors (Rotifera), and amoebae (Protozoa). However, intensified agricultural activities, substantially diminished the zooplankton population, biomass, taxonomic richness, and overall ecosystem functionality. The impact of land cover change is more pronounced at small-scale sub-catchments than at the catchment level as a whole. Therefore, assessing these impacts requires detailed spatial and temporal analysis at the sub-catchment level to identify the most affected areas. This study introduces a new sub-catchment-based perspective on ecosystem health assessment and underscores the zooplankton's role as robust indicators of ecological change.
Keywords: Catchment area, Community traits, Lotic ecosystem, Partial least squares regression, Zooplankton assemblage matrix
Subject terms: Ecology, Ecology, Environmental sciences, Limnology
Introduction
Species distribution patterns and the structure of riverine zooplankton communities align with metacommunity theory, which posits interconnected local communities shaped by environmental and spatial processes1,2. Biological conditions in rivers are primarily shaped by water flow and watershed impacts, with flow being the strongest determinant of river biocoenosis, affecting habitat construction, food conditions, and species' functional traits3–5. The watershed environment also influences water chemistry, thermal conditions, and species composition6.
Zooplankton communities in lotic systems are less diverse than in standing waters due to food and reproductive limitations. Their presence in rivers often results from influxes from lakes, floodplains, or drift from tributaries, supported by the river continuum concept that describes gradual habitat and energy transformations along river courses3,7. Observations indicate increased zooplankton density in middle and lower river sections, with variations in crustacean presence, species diversity, and biomass across different zones influenced by environmental factors like flow and retention time4,8–10.
The rapid generational turnover of planktonic animals facilitates their adaptation to river environments, responding sensitively to changes including temperature, turbidity, and pollution, despite zooplankton not being standard indicators for water quality under the EU Water Framework Directive11–14. Their role in the trophic chain links primary producers with higher-order consumers, making them crucial for river ecosystem functioning15–18.
The taxonomic and functional diversity of zooplankton serves as "ecosystem-focused Essential Biodiversity Variables (EBVs)" which are essential for analyzing community structures and understanding ecological niches within ecosystems14,19,20. The ecosystem attributes, according to EBV framework—the basis of biomonitoring programs worldwide presented by Pereira et al.21, require priority attributes such as structural—(Ecosystem Structure) and functional attributes of the ecosystem (Ecosystem Function), as well as community-level abundance and diversity of organisms occurring within the ecosystem (Community Composition EBVs). The bioindicative value of zooplankton is significant in ecosystems facing anthropogenic threats like agriculture and urbanization, where they reflect impacts of physical and chemical changes on biocoenoses2,19,22,23.
Anthropogenic activities lead to increased nutrient concentrations, affecting autotroph activity and reducing zooplankton diversity, favoring species with high environmental tolerance ("generalists"). Increased organic pollutants and altered nutrient levels result in a shift towards smaller rotifers and protozoans, with fewer specialist species24,25. The use of pheopigments as indicators provides insights into algal cell condition and zooplankton food quality, reflecting the impact of these environmental changes26,27.
The aim of this study was to assess the variability in the zooplankton metacommunity of the Łyna River, the largest river in northeastern Poland, under the influence of diverse land use forms within its catchment. Prior studies have often focused on specific river segments impacted by environmental changes14,22,23. However, understanding the variability of zooplankton across the entire river course—encompassing a varied landscape influenced by a young glacial topography—presents a more complex and less explored challenge.
Our study required integration of multiparametric watershed-wide data according to the requirements of Water Framework Directive (WFD, 2000/60/EC), the primary legislation in EU setting out rules to halt deterioration in the status of water bodies and achieve good status for Europe’s rivers, lakes and groundwater. Thus, our objective as to comprehensively investigate the dynamics of zooplankton variability in a lowland river ecosystem for the first time. Utilizing Partial Least Square Regression (PLS-R), we analyzed how land use variability within the watershed affects the zooplankton metacommunity. This analysis spans a 200 km postglacial river course, evaluating both the direct impacts of watershed management on water quality and the indirect effects on zooplankton dynamics.
Our hypotheses deal with environmental impacts on zooplankton ecological niches. We predict that the diverse land uses in the river catchment —ranging from natural to urban and urban-agricultural areas—affect the ecological traits and distribution patterns of the zooplankton. Specifically, we hypothesize that: (1) zooplankton communities are composed of localized subsets, each characterized by distinct distribution and co-occurrence patterns that reflect their specific environmental contexts; (2) environmental factors such as land use and water flow significantly influence the abundance and diversity of specialized indicator taxa.
Drawing on the urban tolerance hypothesis, we anticipate that urbanized areas will predominantly support generalist species with broad environmental tolerances, resulting in functional homogenization. In contrast, areas characterized by natural landscapes or reduced water flow are expected to support a higher diversity of specialist species, indicative of more varied habitat niches. Additionally, increased flow intensity is likely to restrict the available niche space, favoring species that are more competitive.
This comprehensive study of the Łyna River's zooplankton aims to evaluate the zooplankton structures across varying watershed conditions and their responses to anthropogenic pressures. By doing so, we seek to highlight the bioindicative value of zooplankton in monitoring the ecological health and environmental changes of freshwater ecosystems.
Results
Environmental characteristics
Land use influenced the variation of certain physical and chemical water parameters across five zones (I–V) along the Łyna River. Significant differences were observed in pH, electrical conductivity (EC), total inorganic carbon (TIC), turbidity, nutrient and organic matter concentrations, and chlorophyll pigments (Supplementary Table S1). Headwaters (zone I) were rich in inorganic ionic forms correlated with EC and TIC. A distinct gradient of organic matter in the water was observed along with the increased catchment area, marked by significant increases in concentrations of biological oxygen demand (BOD), total organic carbon (TOC), total nitrogen (TN), and total phosphorus (TP) (Supplementary Table S1). A significantly different and several times higher than the average level in the waters of the Łyna River, high concentrations of N-NO3 and TN (3.15 mg/L and 3.43 mg/L, respectively) were observed in the headwaters. The average concentration of ammonia did not significantly differ across the individual catchment sections; however, increases in N-NH4, similar to orthophosphate, were associated with the urban section (zone III). The primary production size, measured by the total concentration of chlorophyll a, amounted to an average of 14.22 µg/L, varying along the river's course from 2.42 µg/L in the headwater section (zone I) to 20.66 µg/L in zone V. The proportion of pheophytin in the total chlorophyll a concentration ranged from 20% (zone II) to 80% (zone I), with an average of 44% for the Łyna River ecosystem. Water turbidity increased progressively along the river, from 1.61 NTU (zone I) to 7.34 NTU (zone V), with a sharp increase in the urban section (zone III) (Supplementary Table S1).
Zooplankton structure and diversity
In the zooplankton structure of the Łyna River, a total of 158 taxa and forms of zooplankton were identified, comprising 111 Rotifera, 23 Cladocera, 13 Copepoda (including juvenile stages of nauplii and copepodites), 10 Protozoa, and larval veliger stages of Dreissena polymorpha (Pallas, 1771). Rotifers constituted on average from 66.5% (zone I) to 85.3% (zone V) of the overall zooplankton density, and the most commonly were Keratella cochlearis (Gosse, 1851) and Polyarthra longiremis Carlin 1943, with average frequencies of 79.4% and 70.9%, respectively (Supplementary Table S2). Crustaceans accounted for an average of 16.7% (zone IV) to 68.6% (zone II) of the overall zooplankton biomass. The nauplii larval stages of Copepoda were characterised by the highest frequency, appearing in 69.1% of the samples. Protozoa were primarily represented by the amoebae Galeripora discoides (Ehrenberg, 1843), Codonella cratera Leidy 1887, and Difflugia spp., with frequencies of 75.4%, 34.9%, and 32.8%, respectively. The share of protozoans in the overall zooplankton density ranged from 5.1% (zone II) to 28.8% (zone I).
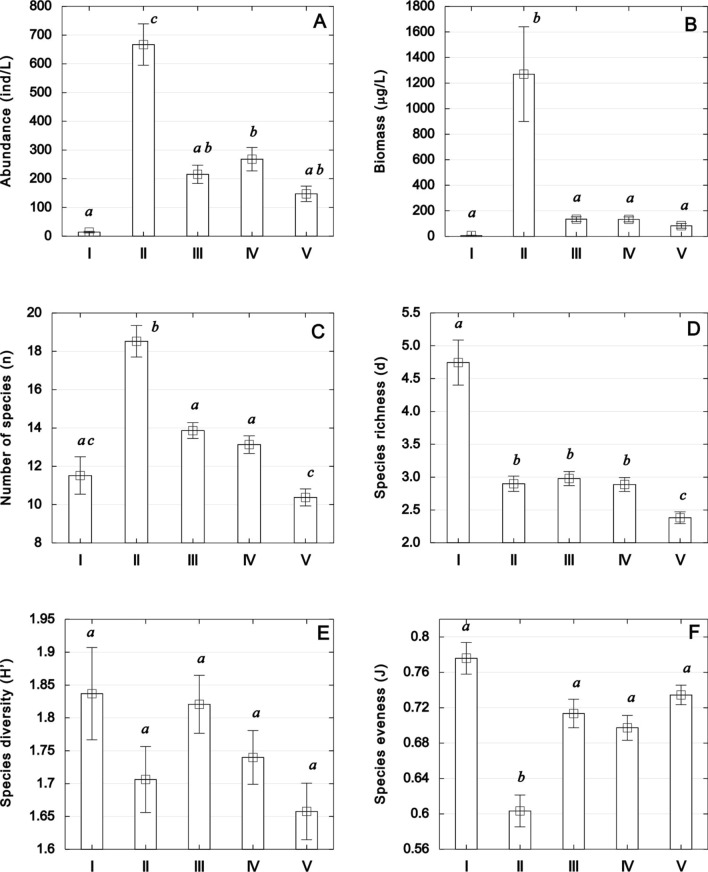
The highest statistically significant (p < 0.05) average abundance (667 ind/L), biomass (1280.8 µg/L), and number of species (19) of zooplankton were recorded in the natural section (zone II). In contrast, the lowest values of abundance and biomass were noted at site 1 (15 ind/L and 7.7 µg/L, respectively), while the fewest species (10) of zooplankton were found at the most downstream location (zone V). Measures of zooplankton taxonomic diversity, expressed by the Margalef’s species richness (d) index and Shannon’s diversity index (H’), were highest in zone I and lowest in zone V, with values of 4.74 and 1.84 (zone I) and 2.38 and 1.66 (zone V), respectively. The Pielou's evenness index (J’) reached the lowest average level of 0.603 in zone II, indicating the prolific development of a few species and an uneven quantitative proportion in the zooplankton communities of the natural zone (Fig. 1; Supplementary Table S2).
Figure 1.
Abundance (ind/L), biomass (µg/L), number of species (n), species diversity (H’), species richness (d), and species eveness (J) of zooplankton in five zones along the Łyna River course, distinguished by land use. Statistically significant differences, indicated by superscripts, were determined using Tukey's post-hoc test following one-way ANOVA (p < 0.05).
Habitat distribution patterns of zooplankton and characteristic species
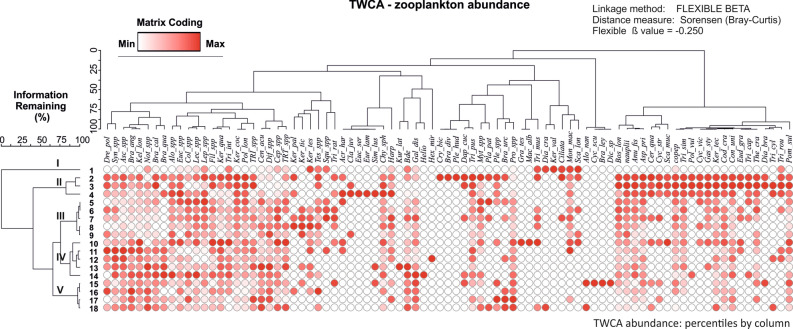
In the zooplankton metacommunity of the Łyna River ecosystem, we identified local subsets and species characteristic of specific habitat niches within the sub-catchment type. A total of 35 zooplankton taxa and two developmental forms of Copepoda showed significant affinity for the designated five watershed zones. Headwaters (zone I) formed the most distinct habitat assemblage from other zones, with top indicators being Scaridium longicaudum (Müller, 1786) (IndVal = 12.3; p = 0.0002), Monommata maculata Harring & Myers, 1924 (IndVal = 6.3; p = 0.015), and Ciliata (IndVal = 8.3; p = 0.014). The influence of the natural watershed (zone II), grouping sites 2–4, reflected the most numerous and diverse pattern of the zooplankton assembly, consisting of 17 taxa of Rotifera and 9 taxa and forms of Crustacea, significant for the habitat according to the IndVal index (Fig. 2; Table 1). Key indicators for the natural habitat (IndVal > 20; p ≤ 0.0016) included Keratella tecta (Gosse, 1851), Asplanchna priodonta Gosse, 1850, Conochilus unicornis (Rousselet, 1892), Trichocerca capucina (Wierzejski & Zacharias, 1893), T. similis (Wierzejski, 1893), T. pusilla (Lauterborn, 1898), Gastropus stylifer Imhof, 1891, Bosmina longirostris (O.F. Müller, 1785), Eudiaptomus graciloides (Lilljeborg, 1888), Thermocyclops crassus (Fischer, 1853) and nauplii and copepodite larvae of Copepoda. The urban zone (III) zooplankton community, forming a cohesive cluster of sites 5–9, included many taxa such as Keratella ticinensis (Callerio, 1920), K. paludosa (Lucks, 1912), Colurella spp., Lepadella spp., Squatinella spp., and Plationus patulus (Müller, 1786) (Fig. 2). However, none of the taxa had significant indicator value for this habitat. The urban-agricultural cluster (zone IV) grouped zooplankton assemblies of sites 10–14, represented by Kellicottia longispina (Kellicott, 1879), Synchaeta spp., Trichocerca musculus (Hauer, 1936), Brachionus calyciflorus Pallas, 1766, and Alona spp. (Fig. 2). Significant indicators (p < 0.05) characterizing the local habitat of zone 4 included rotifers of the genera Cephalodella (IndVal = 12.4), Trichotria (IndVal = 6.8), Ascomorpha (IndVal = 11.6), Lecane (IndVal = 9.5), and the protozoans A. discoides (IndVal = 37.0), Centropyxis aculeata (Ehrenberg, 1832) (IndVal = 7.7), and Difflugia spp. (IndVal = 9.0). The agricultural zone (V) zooplankton community included Brachionus angularis Gosse, 1851, B. leydigii Cohn, 1862, Trichocerca rattus (Müller, 1776), Proales sp., and Alonella nana (Baird, 1843), but only one species, Brachionus urceolaris (Müller, 1773) (IndVal = 6.1; p = 0.03), proved to be a characteristic and significant indicator of this habitat niche (Fig. 2; Table 1).
Figure 2.
TWCA matrix of zooplankton grouping in 5 catchment zones of the Łyna River. Zooplankton species name abbreviations are explained in Supplementary Table S2.
Table 1.
Taxa characteristic of the habitats of Łyna river catchment sections.
| Taxa | Functional group | Feeding guilds | Site | Catchment zone | IndVal x ± SD | p | M–K test p | Trend |
|---|---|---|---|---|---|---|---|---|
| Rotifera | ||||||||
| Monommata maculata | RAP | Suctor | 1 | I | 6.3 (2.8 ± 1.2) | 0.0152 | ||
| Scaridium longicaudum | RAP | Suctor | 1 | I | 12.3 (2.6 ± 1.3) | 0.0002 | ||
| Anuraeopsis fissa | SMC | Filtration | 2 | II | 13.3 (6.5 ± 2.4) | 0.0196 | ||
| Asplanchna priodonta | RAP | Predator | 2 | II | 31.7 (5.9 ± 2.1) | 0.0002 | 0.045 | ↓ |
| Brachionus diversicornis | SMC | Filtration | 2 | II | 8.3 (4.0 ± 1.0) | 0.0492 | ||
| Keratella cochlearis | SMC | Filtration | 2 | II | 15.4 (9.3 ± 1.6) | 0.0042 | 0.002 | ↓ |
| Mytilina spp. | SMC | Filtration | 2 | II | 7.4 (3.2 ± 1.3) | 0.0116 | 0.005 | ↑ |
| Ploesoma spp. | RAP | Suctor | 2 | II | 12.5 (2.6 ± 1.4) | 0.0028 | ||
| Polyarthra longiremis | RAP | Suctor | 2 | II | 17.1 (7.9 ± 1.3) | 0.0002 | 0.000 | ↓ |
| Trichocerca pusilla | RAP | Suctor | 2 | II | 21.4 (5.3 ± 2.0) | 0.0002 | ||
| Trichocerca musculus | RAP | Suctor | 2 | II | 10.2 (2.9 ± 1.4) | 0.0002 | ||
| Conochilus unicornis | SMC | Filtration | 3 | II | 33.8 (8.1 ± 3.3) | 0.0002 | 0.002 | ↓ |
| Gastropus stylifer | RAP | Suctor | 3 | II | 26.4 (3.6 ± 1.6) | 0.0002 | ||
| Keratella tecta | SMC | Filtration | 3 | II | 35.6 (12.1 ± 3.6) | 0.0002 | 0.001 | ↑ |
| Polyarthra vulgaris | RAP | Filtration | 3 | II | 16.7 (3.1 ± 1.5) | 0.0002 | ||
| Pompholyx sulcata | SMC | Filtration | 3 | II | 17.1 (4.9 ± 1.8) | 0.0006 | ||
| Trichocerca capucina | RAP | Suctor | 3 | II | 28.6 (3.0 ± 1.4) | 0.0002 | ||
| Trichocerca cylindrica | RAP | Suctor | 3 | II | 14.9 (4.9 ± 2.3) | 0.0030 | 0.006 | ↑ |
| Trichocerca similis | RAP | Suctor | 3 | II | 26.9 (5.6 ± 2.2) | 0.0002 | ||
| Cephalodella spp. | RAP | Suctor | 10 | IV | 12.4 (5.4 ± 1.2) | 0.0002 | ||
| Trichotria spp. | SMC | Filtration | 10 | IV | 6.8 (3.8 ± 1.5) | 0.0474 | ||
| Ascomorpha spp. | RAP | Suctor | 11 | IV | 11.6 (7.9 ± 1.8) | 0.0378 | < 0.0001 | ↓ |
| Lecane spp. | SMC | Filtration | 14 | IV | 9.5 (6.0 ± 1.2) | 0.0138 | 0.001 | ↑ |
| Brachionus urceolaris | SMC | Filtration | 17 | V | 6.1 (2.8 ± 1.5) | 0.0304 | ||
| Crustacea | ||||||||
| Ceriodaphnia quadrangula | LMC | Filtration | 2 | II | 9.9 (3.5 ± 1.8) | 0.0080 | ||
| Daphnia cucullata | LMC | Filtration | 2 | II | 15.4 (3.7 ± 2.0) | 0.0004 | ||
| Cyclops strenuus | RAP | Raptorial | 2 | II | 10.1 (2.6 ± 1.2) | 0.0012 | ||
| Bosmina longirostris | LMC | Filtration | 3 | II | 21.5 (8.9 ± 3.1) | 0.0016 | 0.007 | ↓ |
| Diaphanosoma brachyurum | LMC | Filtration | 3 | II | 17.2 (2.6 ± 1.5) | 0.0004 | ||
| Eudiaptomus graciloides | STA | Filtration | 3 | II | 34.9 (3.0 ± 1.4) | 0.0002 | ||
| Thermocyclops crassus | RAP | Raptorial | 3 | II | 52 (3.7 ± 1.4) | 0.0002 | ||
| Copepodites | LMC | Filtration | 3 | II | 53.6 (5.2 ± 1.6) | 0.0002 | ||
| Nauplii | SMC | Filtration | 3 | II | 35.2 (8.8 ± 1.7) | 0.0002 | 0.004 | ↓ |
| Protozoa | ||||||||
| Ciliata | 1 | I | 8.3 (2.9 ± 1.6) | 0.0146 | ||||
| Difflugia spp. | 13 | IV | 9 (5.5 ± 1.4) | 0.0230 | ||||
| Centropyxis aculeata | 13 | IV | 7.7 (4.5 ± 1.3) | 0.0280 | ||||
| Galeripora discoides | 14 | IV | 37 (15.8 ± 3.0) | 0.0002 | 0.001 | ↑ | ||
Indicator Value (IndVal), mean ± SD), and the trend of population size variability with the increasing area of the Łyna River catchment (Mann–Kendall test, p < 0.05).
Spatial variability of zooplankton metacommunities
The increase in flow intensity correlated with sub-catchment size significantly differentiated the population of 21 taxa and naupliar forms of Copepoda (Supplementary Table S3), of which 11 belonged to the set of indicators for habitats of individual sub-catchment types (Table 1). Most taxa characteristic of upper segment habitats (section I and II), showed a negative correlation in population numbers along the river course, or this variability was not significant. The variability in population numbers of individual taxa, which was important for the structure of the metacommunity, was diverse (Supplementary Fig. S1). Trends in population number growth with sub-catchment area and flow were shown by 9 taxa, and a decrease in population numbers was observed by 12 taxa and nauplii.
Environmental and spatial variability of zooplankton functionality
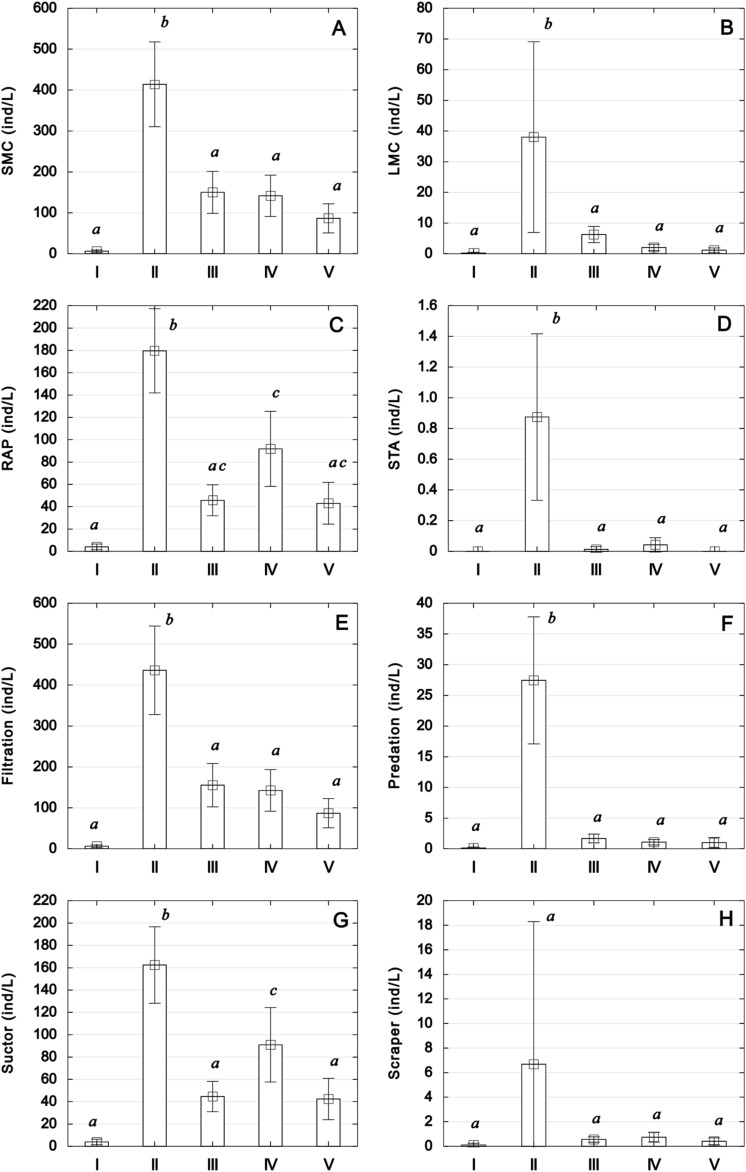
The natural zone (II) significantly differed from the other sections in terms of zooplankton abundance and biomass (Fig. 1 A, B), affecting the density of all functional groups and main feeding guilds of zooplankton (Fig. 3A–H). In the zooplankton metacommunity, the small microphages (SMC) group was dominant, ranging from 59% (zone I) to 74% (zone III), corresponded to the type of trophy in Rotifera (Supplementary Fig. S2; Table S2). The increase in significance of actively feeding RAP species, particularly from the group of suctor rotifers in the urban-agricultural zone (IV), was statistically significant and differed from other watershed sections (Fig. 3C,G). The largest share (6%), across the entire Łyna ecosystem, of large microphages (LMC), primarily represented by filter feeders and scrapers as well as predatory species, was noted in zone II.
Figure 3.
Functional groups (A–D) and feeding guilds of zooplankton (E–H) in five zones along the Łyna River course. Statistically significant differences, indicated by superscripts, were determined using Tukey's post-hoc test following one-way ANOVA (p < 0.05).
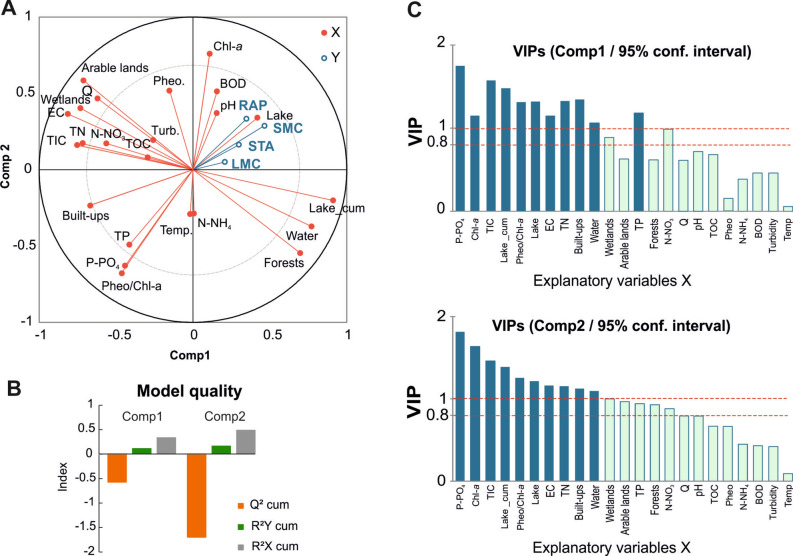
PLS-R analysis demonstrated the importance of explanatory variables significantly contributing to the response of zooplankton functional groups. The Variable Importance for Projection included phosphorus forms (P-PO4, TP), primary production (Chl-a, Pheo/Chl-a), inorganic ionic forms (EC, TIC), surface water areas (Lake, Lake_cum, Water), and urban areas in the catchment (Build-ups) (Fig. 4). The most important predictor, based on the highest VID scores for three of the four functional groups, was the share of areas with high water retention, primarily provided by lakes (Lake_cum): LMC (VID = 0.880), STA (VID = 0.790), and SMC (VID = 0.619). The key predictor with a positive VID score for the RAP group was Chl-a (VID = 0.584). EC, TN, and the share of wetlands in the direct catchment were adversely associated with LMC and STA (Table 2). A negative effect on SMC and RAP was detected for P-PO4 (VID = − 0.663 and − 0.691, respectively), Pheo/Chl-a (VID = − 0.563 and − 0.586, respectively), and TP (VID = − 0.541 and − 0.547, respectively), (Table 2). All functional zooplankton groups were adversely associated with the share of urbanized areas in the catchment, phosphorus compounds (P-PO4 and TP), and low phytoplankton quality (Pheo/Chl-a) (Fig. 4).
Figure 4.
Partial least squares (PLS) regression biplot reflecting the effect of land-use classes and water quality parameters as the explanatory variables (X) on the zooplankton groups (Y). Inner dashed circle denotes correlation coefficient r = 0.75 (A). PLS-R model quality (B). VIPs (Variable Influence on Projection) for each explanatory variable of Component 1 and Component 2. VIP diagrams show relative importance of predictors. VIPs > 0.8, based on Wold’s criteria69, indicate that the predictor variable is considered to be significantly important to the corresponding dependent variable (C).
Table 2.
Discriminative components for each zooplankton functional group selected through the Variable Identification (VID) procedure (PLS-R) and listed in decreasing order of VID score ( >|0.5|; n = 428).
| SMC | LMC | RAP | STA | |||||
|---|---|---|---|---|---|---|---|---|
| VID | Component | VID | Component | VID | Component | VID | Component | |
| Positive | 0.619 | Lake_cum | 0.880 | Lake_cum | 0.584 | Chl-a | 0.790 | Lake_cum |
| 0.737 | Water | 0.648 | Water | |||||
| 0.604 | Forests | |||||||
| Negative | − 0.663 | P-PO4 | − 0.732 | EC | − 0.691 | P-PO4 | − 0.638 | TN |
| − 0.563 | Pheo/Chl-a | − 0.689 | Wetlands | − 0.586 | Pheo/Chl-a | − 0.624 | EC | |
| − 0.541 | TP | − 0.686 | TN | − 0.547 | TP | − 0.600 | Wetlands | |
| − 0.525 | TN | − 0.619 | Arable lands | − 0.579 | P-PO4 | |||
| − 0.514 | Built-ups | − 0.589 | TIC | − 0.551 | Built-ups | |||
| − 0.567 | N-NO3 | − 0.518 | TIC | |||||
| − 0.551 | Built-ups | |||||||
| − 0.525 | Q | |||||||
Positive VID scores indicate positively associated variables with the response variables, while negative scores indicate variables negatively associated with the response variables. More negative or positive scores suggest a stronger influence.
Discussion
The results of this study demonstrated that watershed management conditions and water flow intensity are factors controlling the structural characteristics and accumulation patterns of zooplankton communities in the Łyna River, typical watercourse for postglacial areas of Mid-Eastern Europe. Rotifers predominated in the zooplankton metacommunity, a common trait in lotic environments due to their short generational cycles and ability to thrive in nutrient-poor river waters3,28. The qualitative structure of Rotifera was primarily composed of eurytopic species such as Keratella cochlearis and Polyarthra longiremis, which are often dominant in potamoplankton11,12,29,30. However, local subsets consisting of both the aforementioned generalists and specialist species showed a variability in their populations, correlated with land use form, catchment area, and flow intensity (Fig. 2, Supplementary Fig. S1).
The headwaters zone (I) differed from other river segments in terms of the lowest abundance, biomass, and species count of zooplankton. As emphasized by Ejsmont-Karabin and Kruk31, upper stream biotic communities are highly susceptible to adjacent land use due to the large surface area in contact with the narrow, shallow river channel. Additionally, the small water flow and fast current of headwater sections result in varied abundances and specific zooplankton assemblages30,31. The headwaters section of the studied Łyna River, characterized by a forested setting, low water temperature, and significant shading, despite good organic matter resources (BOD), limited primary production (Chl-a) and resulted in a high proportion of pheophytin26. A high Pheo/Chl-a ratio, indicative of poor physiological state of phytoplankton27,32 pointed to poor quality and/or availability of food, constraining all functional groups. The headwaters section of the Łyna River was represented by diverse but small populations of minute pelagic rotifers, aligning with findings by Ejsmont-Karabin and Kruk31. Specialised rotifers (RAP) Monommata maculata and Scaridium longicaudum, equipped with the best functionally active feeding methods and utilizing feeding guilds, best represented (IndVal) the upper river segment (Table 1).
The local zooplankton community in the natural zone (II) reflected a structure typically found in lentic—lacustrine ecosystems. High abundance and biomass of all taxonomic and functional groups of zooplankton, along with the subdominance of eutrophic species populations, are characteristics of potamoplankton communities in the outflow zones of lakes8,9,28. Lakes within river flow systems serve as refuges for numerous species with higher nutritional, thermal, and phenological requirements12,18,29 simultaneously supplying the outflow zone with organic matter and phytoplankton33. Therefore, the local subset of zooplankton in the natural section was abundant and diverse both taxonomically and functionally, with a characteristic pattern of co-occurrence of indicator taxa of specific feeding guilds (Fig. 2). Representatives of all functional groups showed a highly positive correlational relationship (PLS) with areas of the catchment having high hydrological retention (SMC, LMC and STA) and concentrations of Chl-a (with a low proportion of Pheo) in the water (RAP), characterizing the natural section (II). These included crustaceans from Cyclopoida (e.g., Cyclops spp.; RAP), Calanoida (Eudiaptomus graciloides; STA), and pelagic and littoral Cladocera (e.g., Simocephalus, Ceriodaphnia; LMC). A similar finding was reported by Thorp and Mantovani12, highlighting a positive correlation between crustacean density and hydrological retention (negative with flow velocity). The resource-rich outflow habitat (site 2 and 3) also provided optimal conditions for Rotifera species with varied functional traits (different trophic types), both from the "specialist" group (e.g. predator A. priodonta) and the common/"generalist" group (K. cochlearis, P. longiremis) (Supplementary Table S2). These characteristics of the zooplankton community in the outflow section are consistent with the findings of Braghin et al.34, where the availability of food resources (Chl-a) on one hand increased the functional diversity of zooplankton and on the other led to the intense development of populations of highly competitive species (generalists). The strong dominance of K. cochlearis (38%), P. longiremis (14%), K. tecta, and nauplii larvae of Copepoda resulted in a low assessment of taxonomic diversity in zone II.
Moving away from the natural habitat, zooplankton abundance, biomass, and species number decreased due to the river current's filtering impact, deteriorating habitat conditions, and fish predation8,9,17,28. In the urban zone (III), under the influence of the largest city on the river (Olsztyn), zooplankton abundance halved, and biomass nearly decreased tenfold. All functional groups were negatively correlated with urbanized areas, increased phosphorus and nitrogen compounds, decreased primary production, and increased Pheo/Chl-a ratio. This deterioration of habitat quality severely limits zooplankton in urbanized waters22,23. According to the urban tolerance hypothesis35,36, the zooplankton community of the urban section was dominated (75%) by small microphages (SMC) from a pool of common rotifers (generalists) with broad environmental tolerance. The largest share in this group comprised small filter feeders from the genera Keratella, Brachionus, Lecane, and Filinia, which corresponds with the results of Frau et al.19 and Mulani et al.24. The significance of LMC and RAP groups significantly declined, consistent with urbanization impacts on river catchment25. Thus, no indicators were found, characteristic of the local subset of section III (lack of IndVal indicators). Although these observations indicate an intensification of functional homogenization of zooplankton within the urban section, it should be noted that the dominant group of small filter feeders (SMC; Brachionus angularis, B. calyciflorus, Conochilus unicornis, Keratella spp., Filinia spp.) was "heterogeneous" in terms of a wide spectrum of food sources (detritus, bacteria, protozoans, algae). The ability to utilize various food sources, especially in the presence of poor quality or scarcity of phytoplankton, is a phenomenon observed in anthropogenically disturbed ecosystems37,38, including urbanised areas and results in the overlapping (interlocking) of niches25.
The increasing catchment area, flow intensity, and volume of water, as well as the growth in the proportion of agriculturally used areas in the lower sections, significantly influenced the increase in water turbidity, mineral compounds (TIC), and EC. High turbidity, limiting the euphotic zone, can impact feeding efficiency and development of filtering zooplankton species34,39,40. On the other hand, mineral suspension particles, determining water turbidity, can also enhance the food pool by accumulating organic forms, thereby increasing zooplankton diversity41,42. As in the experiments of Mulani et al.24 and Frau et al.19, these factors favored rotifers from the families Brachionidae, Lecanidae, Trichocercidae, and Gastropodidae, but not LMC and STA.
The lower section of the Łyna River (zone IV) responded to improved food conditions with restored Crustacea structure, possibly due to reduced fish predation on Cladocera and adult Copepoda in turbid waters and expanded littoral vegetation zones providing habitat for littoral species (e.g. Simocephalus, Graptoleberis, Kurzia, Pleuroxus; Supplementary Table S2)43–45. Additionally, zone IV was characterised by an increase in Protozoa, and Rotifera from the actively feeding suctors roup (RAP: Trichocerca, Polyarthra, Synchaeta) at the expense of filter feeders (SMC), likely due to competition with LMC—Cladocera46–49. Indicator taxa for urban-agricultural habitats included rotifers from the genera Ascomorpha, Cephalodella, Lecane, and Trichotria, as well as protozoans from the amoeba group (Galeripora, Difflugia, Centropyxis).
The lower sections (IV and V) showed dominance by Galeripora discoides most likely due to increasing turbidity (suspended solid concentration), agricultural areas, but also the presence of hydraulic structures. As shown by Endler et al.50, water levels, small hydropower plants, various elements, and structures impounding the river often become a substrate for the development of a "microbial film" and protozoans. Numerous populations of Protozoa are detached from the structural elements by the water current and complement the local zooplankton communities.
The study of the Łyna River highlights the pivotal role of watershed management and water flow intensity in shaping the health of its ecosystem, particularly through the structural characteristics and distribution patterns of zooplankton communities. These findings provide crucial insights for the assessment of ecosystem health, linking zooplankton community structure to environmental stressors. By addressing these key factors, it is possible to mitigate the adverse effects of anthropogenic pressures and promote the long-term health and sustainability of riverine environments.
Conclusions
The study of the Łyna River highlights the pivotal role of land use and water flow intensity in shaping the health of its ecosystem, particularly through the structural characteristics and distribution patterns of zooplankton communities. Our findings provide crucial insights for assessing ecosystem health by linking zooplankton community structure to environmental stressors. Increased primary production (rise in Chl-a), good quality food (decrease in Pheo/Chl-a), and enhanced water retention in the catchment fostered a heterogeneous habitat and diverse feeding guilds for all functional groups, particularly large microphages (LMC), the stationary suspension group (STA), and various specialized taxa. In contrast, urban watersheds limited habitat suitability for all functional groups, with only small filtering microphages from the generalist group, which have a broad food spectrum (detritophagous, bacteriophagous), showing resilience, thus supporting the urban tolerance hypothesis.
The semi-natural watershed (urban-agricultural) showed increased diversity in feeding guilds, greater variety of LMC, higher activity of suctor Rotifera (RAP), and more amoeboid Protozoa compared to the urban area. However, intensified agricultural pressure significantly reduced zooplankton abundance, biomass, and taxa diversity, impairing the habitat functionality of the lower river zone, which confirms our hypotheses. The impact of land cover change is more pronounced at small-scale sub-catchments than at the catchment level as a whole. Therefore, assessing land cover change impacts on both hydrological processes and biological components requires sufficient spatial and temporal detail at the sub-catchment level to identify the most impacted areas. While some existing conclusions are deepened, this study primarily presents a new sub-catchment-based perspective on ecosystem health assessment.
To preserve water quality and biodiversity, maintaining natural hydrological conditions and minimizing urban and agricultural runoff are priorities for sustainable water management. Effective watershed management strategies should focus on enhancing water retention in natural areas, reducing nutrient loading, and protecting riparian habitats to support resilient and healthy aquatic ecosystems. Addressing these key factors will help mitigate the adverse effects of anthropogenic pressures and promote the long-term health and sustainability of riverine environments.
Methods
Study area
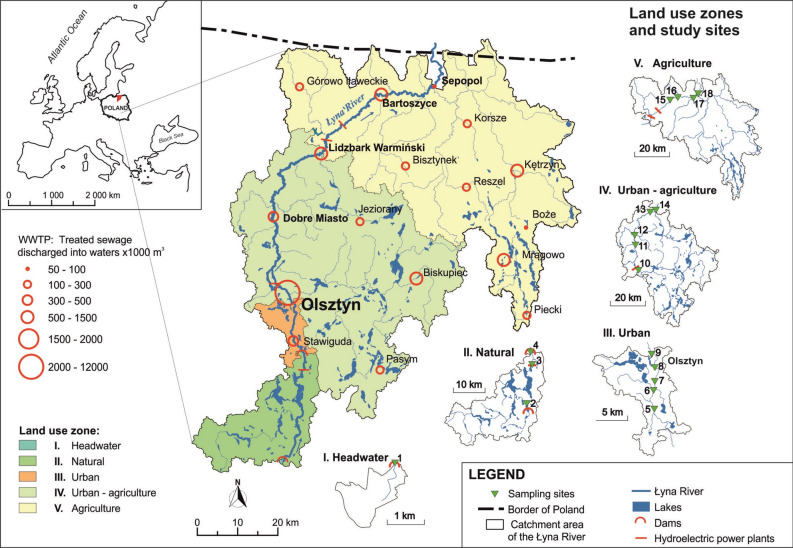
The Łyna River is the main watercourse in northeastern Poland (53°26′28.4″N, 20°24′48.6″E; 54°37′14.8″N; 21°13′35.6″E), with a flow regime typical for temperate climate zones on the Central European plains. The total catchment area of the river is 7126 km2, and its length is 264 km (Fig. 5). The geomorphology and hydrographic network of the area are products of the last glaciation, which occurred approximately 10,000 years before present (BP). The landscape is characterized by morainic hills and outwash plains, predominantly utilized for agriculture or covered with pine forests. Additionally, the region features numerous natural lakes of postglacial origin, which occupy 4.2% of the river catchment area. Flow-through lakes along the Łyna watercourse take as much as 15.9% of the total riverbed length, with a maximum of 54.11% in the upper part, contributing to the flow range attenuation and minimized the risk of floods. Average specific outflows amount to 5.5 L/s from 1 km251. Longitudinal riverbed gradients range from 0.05‰ to 10‰. Along the river's course, anthropogenic pressure increases51. In the south, the headwater catchment has a semi-natural character. Large areas are covered with forests growing on sandy outwash plains. The largest share of agriculturally used lands occurs in the lower part of the Łyna catchment, where the soils are primarily composed of moraine clays, as well as silty deposits suitable for agricultural development.
Figure 5.
Location of the study area on the background of the Łyna River catchment.
Due to the threats to the water quality of the Łyna catchment from land use, five zones (I-V) characteristic for various segments of the river were identified (Supplementary Table S4). Each zone was monitored for this hydrochemical and biological study. In total, 18 sampling sites were located along an approximately 200-km stretch of the river (Fig. 5). These sites represent characteristic sub-catchment types along the river: I: headwaters (site 1), II: natural (sites 2–4), III: urban (sites 5–9), IV: urban-agricultural (sites 10–14), and V: agricultural (sites 15–18).
Because the entire Łyna catchment, under EU Directive 91/271/EEC, is designated as an area sensitive to eutrophication due to pollution (N and P) from municipal sources, which cause deterioration of the riverine ecosystem health, maintaining a balance between nature conservation and economic needs is priority for the sustainable water management of the Warmia and Mazury region in NE Poland.
Sampling and analytical procedure
Zooplankton samples were collected one to two times per season—spring, summer, autumn, and winter—from 2019 to 2023. A total of 428 zooplankton samples were collected during the study period. Samples were collected using a 10-L sampler from a depth of approximately 20 cm below the water surface, at the central part of the riverbed.
Each collected sample of a volume of 20 L was filtered through a plankton net with 30 μm mesh size, preserved with Lugol’s solution, and fixed in 4% formalin solution. Each sample was analysed in triplicate (sub-samples). Each time, 1 mL of the sample was analysed in the Sedgewick-Rafter chamber. Zooplankton were identified to the lowest possible taxonomic level (with the exception of juvenile Crustacea stages) under a Zeiss AXIO Imager microscope, using the methods described by Błędzki and Rybak52, Radwan et al.53, Koste54, Rybak and Błędzki55, and von Flössner56. The number of individuals among the zooplankton (ind/L) was estimated according to the Hansen’s rule57. In order to determine the zooplankton biomass (µg/L), standard weights for rotifers were applied53. Regarding crustaceans and protozoa, particular organisms were measured under a microscope with a measuring lens at the maximum precision to 0.01 mm, using transmitted light. For the purpose of estimating biomass, it was assumed that the density of a zooplankton organism = 1. i.e. 1 mm3 = 1 mg58. Based on the results of the measurements, cubic volume of individuals was calculated, by comparing their shape to the basic geometrical solids.
Species diversity (Shannon diversity index, H′), and species evenness of zooplankton communities (Pielou’s evenness index, J′) were analyzed with the use of MVSP 3.22 software59 The species richness index (d) was calculated according to the formula of Margalef60.
The functional variability of the zooplankton metacommunities was assessed in relation to feeding guilds, dependent of feeding strategy and body size of rotifer and crustacean species. On this basis, rotifer and crustacean species were classified into four groups: small microphagous (SMC), large microphagous (LMC), raptorials (RAP), and stationary/suspended (STA) feeders (Supplementary Table S2), according methods61–64.
The SMC functional group included Rotifer’s species with a malleate, malleoramate, or ramate trophi, that collect (filtration) multiple food items (Anuraeopsis, Brachionus, Colurella, Conochilus, Euchlanis, Filinia, Hexarthra, Kellicottia, Keratella, Lecane, Lepadella, Mylitina, Notholca, Plationus, Platyias, Pompholyx, Proales, Squatinella, Testudinella, and Trichotria), and Copepod’s nauplii. The RAP functional group included Rotifer’s genera with forcipate, incudate, or virgate trophi that show an active action (suctor, predator) to catch single food items (Ascomorpha, Asplanchna, Cephalodella, Dicranophorous, Gastropus, Monommata, Ploesoma, Polyarthra, Scaridium, Synchaeta, and Trichocerca), and all adult Cyclopoida. The LMC functional group included Cladocera species and copepodites Copepoda (Supplementary Table S2).
The physical and chemical parameters of water were analyzed in each zooplankton sampling site during each sampling event. Water temperature (°C), pH, dissolved oxygen (DO), turbidity (NTU), electrical conductivity (EC), were measured with the YSI 6600R2 calibrated multiprobe (Yellow Springs, OH, USA). Water samples were collected for laboratory analyses of total nitrogen (TN), nitrate nitrogen (N-NO3), ammonium nitrogen (N-NH4,), total phosphorous (TP), orthophosphate (P-PO4), total organic carbon (TOC), total inorganic carbon (TIC), biochemical oxygen demand (BOD), chlorophyll a (Chl-a), and pheophytin (Pheo). Hydrochemical analyses were conducted in accordance with APHA-AWWA-WEF65.
Hydrological data—daily river flows (Q, m3/s) for the period from 2019 to 2023 for three gauging stations on the Łyna River: Olsztyn—Kortowo (site 7), Smolajny (site 12), and Sępopol (site 18) were provided by the Institute of Meteorology and Water Management (IMGW-PIB) in Poland. Flow data for other study sites was obtained using the Delft-3D wflow-sbm model, an open-source tool available at https://www.deltares.nl/en/software-and-data/products/wflow-catchment-hydrology66.
In five distinguished research zones along the Łyna River, the following shares of land use forms were determined: forests, wetlands, arable land, build-up areas (Build-ups), and lakes. The part (%) of the riverbed lengh occupied by lakes was also determined (Lake cum) (Table S4). The share of land use forms in various parts of the Łyna river catchment was determined based on information on land cover/land use provided by the CORINE Land Cover (CLC2018) using the SCALGO Live® platform (www.scalgo.com).
Statistical procedures
Before analyses, the dataset was checked for normality using the Shapiro–Wilk test at p < 0.05. To evaluate general differences among five zones of the Łyna River, distinguished by land use, in terms of zooplankton abundance, biomass, species diversity, and characteristics of functional groups, analysis of variance one-way ANOVA, followed by a post-hoc Tukey HSD test (p ≤ 0.05), was performed.
The specific zooplankton taxa for a given site were determined by indicator species analysis (ISA) using PC-ORD 6.0 (MjM Software, Gleneden Beach, Oregon, US). The ISA was calculated as the product of relative species abundance and frequency of occurrence to obtain a maximum indicator value (IndVal) for each species67. Indicator values range from 0 to 100. A value of 100 represents a perfect indicator species, i.e., a species that occurs exclusively in one group, is found in all samples in that group, and has a high relative abundance within that group. To group zooplankton species and sampling locations along the river course we employed Two-Way Cluster Analysis (TWCA) based on Bray–Curtis similarity. Prior to analysis, data were normalised to mitigate the influence of outliers.
Partial Least Squares Regression (PLS-R) analysis was performed to identify the impact of various environmental factors on the functional groups of zooplankton community along the river. PLS-R is a multivariate statistical technique that combines features from principal component analysis and multiple regression, particularly suited for ecological studies where the predictor variables are numerous and highly collinear68. It allowed us for the identification of the most influential factors on zooplankton distribution and abundance. We used rotifer and crustacean functional groups (SMC, LMC, RAP, and STA) as response (Y) variables, and environmental parameters (physical, chemical, and biological parameters) as predictor (X) variables.
To identify the most significant predictors, the Variable Importance in Projection (VIP) scores in the PLS-R model were used to assess the importance of each variable in explaining the variance in the response variables69. Variables X (predictors) with VIP scores > 1 were considered as more important than average in explaining the variance in the response variables across all the components of the PLS model. To enhance the selection of predictors (X) that are considered key drivers in the PLS-R model we applied Variable Identification (VID) technique. VID absolute value indicates the strength of the variable's influence on the model by identifying those that have a substantial impact on the response variable. In the interpretation of the PLS model, a VID score cut-off at 0.50 was considered indicative of an important variable, provided that the confidence interval for the standardized coefficient did not include zero. This condition ensures clarity regarding the direction and statistical significance of the variable's impact on the dependent variable. Positive VID scores indicate positively associated variables with particular zooplankton groups, while negative scores indicate an adverse relationship. The robustness of the PLS model was assessed through various diagnostic checks, including RMSE, MSE and the examination of the predictive relevance (Q2) of the model. The PLS-R model analysis was performed using XLSTAT software, MS Excel add-ins statistical tool (www.xlstat.com).
Supplementary Information
Acknowledgements
Funded by the Minister of Science under “The Regional Initiative of Excellence Program”. The results presented in this paper were obtained as part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn: Faculty of Geoengineering, Department of Tourism, Recreation and Ecology (Grant No. 29.610.022-110), and Faculty Agriculture and Forestry (Grant No. 30.610.008–110).
Author contributions
A.M.G. designed the research, conducted fieldwork, analyzed the zooplankton samples and water samples, planned and wrote the main manuscript text and prepared Figs. 1, 3, and Fig. S2. I.C. conducted fieldwork, analyzed hydrological and cachment data and prepared Fig. 5. K.G-L. conducted main statistical analysis (ISA, PLS-R, and TWCA) interpreted results and prepared Figs. 2, 4, and Fig. S1. All authors reviewed the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69577-z.
References
- 1.Leibold, M. A. et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett.7, 601–613. 10.1111/j.1461-0248.2004.00608.x (2004). 10.1111/j.1461-0248.2004.00608.x [DOI] [Google Scholar]
- 2.Zhao, K. et al. Metacommunity structure of zooplankton in river networks: Roles of environmental and spatial factors. Ecol. Ind.73, 96–104. 10.1016/j.ecolind.2016.07.026 (2017). 10.1016/j.ecolind.2016.07.026 [DOI] [Google Scholar]
- 3.Allan, J. D., Castillo, M. M. & Capps, K. A. Stream Ecology: Structure and Function of Running Waters (Springer Nature, 2021). [Google Scholar]
- 4.Ribeiro, B. I. O. et al. Environmental heterogeneity increases dissimilarity in zooplankton functional traits along a large Neotropical river. Hydrobiologia849, 3135–3147. 10.1007/s10750-022-04917-6 (2022). 10.1007/s10750-022-04917-6 [DOI] [Google Scholar]
- 5.Thorp, J. H., Thoms, M. C. & Delong, M. D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Res. Applic.22, 123–147. 10.1002/rra.901 (2006). 10.1002/rra.901 [DOI] [Google Scholar]
- 6.Bomfim, F. F., Deosti, S., Louback-Franco, N., Sousa, R. L. M. & Michelan, T. S. How are zooplankton’s functional guilds influenced by land use in Amazon streams?. PLoS ONE18(8), e0288385. 10.1371/journal.pone.0288385 (2023). 10.1371/journal.pone.0288385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vannote, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R. & Cushing, C. E. The river continuum concept. Can. J. Fish. Aquat. Sci.37, 130–137 (1980). 10.1139/f80-017 [DOI] [Google Scholar]
- 8.Chang, K.-H., Doi, H., Imai, H., Gunji, F. & Nakano, S. Longitudinal changes in zooplankton distribution below a reservoir outfall with reference to river planktivory. Limnology9, 125–133. 10.1007/s10201-008-0244-6 (2008). 10.1007/s10201-008-0244-6 [DOI] [Google Scholar]
- 9.Pourriot, R., Rougier, C. & Miquelis, A. Origin and development of river zooplankton: Example of the Marne. Hydrobiologia345, 143–148 (1997). 10.1023/A:1002935807795 [DOI] [Google Scholar]
- 10.Ramos, E. A. et al. Influence of spatial and environmental factors on the structure of a zooplankton metacommunity in an intermittent river. Aquat. Ecol.56, 239–249. 10.1007/s10452-021-09912-y.(012345678 (2022). 10.1007/s10452-021-09912-y.(012345678 [DOI] [Google Scholar]
- 11.Goździejewska, A. et al. Effects of lateral connectivity on zooplankton community structure in floodplain lakes. Hydrobiologia774, 7–21. 10.1007/s10750-016-2724-8 (2016). 10.1007/s10750-016-2724-8 [DOI] [Google Scholar]
- 12.Thorp, J. H. & Mantovani, S. Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshw. Biol.50, 1474–1491. 10.1111/j.1365-2427.2005.01422.x (2005). 10.1111/j.1365-2427.2005.01422.x [DOI] [Google Scholar]
- 13.Yang, Y. et al. Geographical distribution of zooplankton biodiversity in highly polluted running water ecosystems: Validation of fine-scale species sorting hypothesis. Ecol. Evol.8, 4830–4840. 10.1002/ece3.4037 (2018). 10.1002/ece3.4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan, D., Chen, L., Luan, L., Wang, Q. & Yang, Y. Effect of salinity on the zooplankton community in the pearl river estuary. J. Ocean Univ. China (Ocean. Coast. Sea Res.)19(6), 1389–1398. 10.1007/s11802-020-4449-6 (2020). 10.1007/s11802-020-4449-6 [DOI] [Google Scholar]
- 15.Du, X. et al. Analyzing the importance of top-down and bottom-up controls in food webs of Chinese lakes through modeling. Aquat. Ecol.49, 199–210. 10.1007/s10452-015-9518-3 (2015). 10.1007/s10452-015-9518-3 [DOI] [Google Scholar]
- 16.Lampert, W. Zooplankton research: The contribution of limnology to general ecological paradigms. Aquat. Ecol.31, 19–27. 10.1023/A:1009943402621 (1997). 10.1023/A:1009943402621 [DOI] [Google Scholar]
- 17.Sotton, B. et al. Trophic transfer of microcystins through the lake pelagic food web: Evidence for the role of zooplankton as a vector in fish contamination. Sci. Total Environ.466–467, 152–163. 10.1016/j.scitotenv.2013.07.020 (2014). 10.1016/j.scitotenv.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 18.Wang, Q. et al. Effects of land use and environmental gradients on the taxonomic and functional diversity of rotifer assemblages in lakes along the Yangtze River, China. Ecol. Ind.142, 109199. 10.1016/j.ecolind.2022.109199 (2022). 10.1016/j.ecolind.2022.109199 [DOI] [Google Scholar]
- 19.Frau, D., Gutierrez, M. F., Regaldo, L., Saigo, M. & Licursi, M. Plankton community responses in Pampean lowland streams linked to intensive agricultural pollution. Ecol. Ind.120, 106934. 10.1016/j.ecolind.2020.106934 (2021). 10.1016/j.ecolind.2020.106934 [DOI] [Google Scholar]
- 20.Junker, J. et al. D4.1. List and specifications of EBVs and EESVs for a European wide biodiversity observation network. ARPHA Preprints10.3897/arphapreprints.e102530 (2023). 10.3897/arphapreprints.e102530 [DOI] [Google Scholar]
- 21.Pereira, H. M. et al. Essential biodiversity variables. Science339, 277–278. 10.1126/science.1229931 (2013). 10.1126/science.1229931 [DOI] [PubMed] [Google Scholar]
- 22.Xiong, W. et al. Determinants of community structure of zooplankton in heavily polluted river ecosystems. Sci. Rep.6, 22043. 10.1038/srep22043 (2016). 10.1038/srep22043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong, W. et al. Biological consequences of environmental pollution in running water ecosystems: A case study in zooplankton. Environ. Poll.252, 1483–1490. 10.1016/j.envpol.2019.06.055 (2019). 10.1016/j.envpol.2019.06.055 [DOI] [PubMed] [Google Scholar]
- 24.Mulani, S. K., Mule, M. B. & Patil, S. U. Studies on water quality and zooplankton community of the Panchganga river in Kolhapur city. J. Environ. Biol.30(3), 455–459 (2009). [PubMed] [Google Scholar]
- 25.Pantel, J. H., Engelen, J. M. T. & De Meester, L. Niche use and co-occurrence patterns of zooplankton along a strong urbanization gradient. Ecography2022, e05513. 10.1111/ecog.05513 (2022). 10.1111/ecog.05513 [DOI] [Google Scholar]
- 26.Bhattacharya, R. & Osburn, C. L. Multivariate analyses of phytoplankton pigment fluorescence from a freshwater river network. Environ. Sci. Technol.51(12), 6683–6690. 10.1021/acs.est.6b05880 (2017). 10.1021/acs.est.6b05880 [DOI] [PubMed] [Google Scholar]
- 27.Siwek, H., Wybieralski, J. & Gałczyńska, M. Zawartość chlorofilu a i jego feopochodnych jako element monitoringu rzek. Zeszyty Problemowe Postępu Nauk Rolniczych476, 497–502 (2001). [Google Scholar]
- 28.Basu, B. K. & Pick, F. R. Phytoplankton and zooplankton development in a lowland, temperate river. J. Plankton Res.19(2), 237–253 (1997). 10.1093/plankt/19.2.237 [DOI] [Google Scholar]
- 29.Akopian, M., Garnier, J. & Pourriot, R. A large reservoir as a source of zooplankton for the river: Structure of the populations and influence of fish predation. J. Plankton Res.21(2), 285–297 (1999). 10.1093/plankt/21.2.285 [DOI] [Google Scholar]
- 30.Czerniawski, R. Zooplankton community changes between forest and meadow sections in small headwater streams, NW Poland. Biologia68(3), 448–458. 10.2478/s11756-013-0170-x (2013). 10.2478/s11756-013-0170-x [DOI] [Google Scholar]
- 31.Ejsmont-Karabin, J. & Kruk, M. Effects of contrasting land use on free-swimming rotifer communities of streams in Masurian Lake District, Poland. Hydrobiologia387(388), 241–249 (1998). 10.1023/A:1017081407452 [DOI] [Google Scholar]
- 32.Wang, L., Jiang, L., Xing, X., Chen, Y. & Meng, Q. The Effects of Pheophytin a on Absorption Properties of Phytoplankton in Dalian Bay, China. 7th Annual International Conference on Geo-Spatial Knowledge and Intelligence IOP Conf. Series: Earth and Environmental Science 428, 012048, IOP Publishing. 10.1088/1755-1315/428/1/012048 (2020).
- 33.Lehman, P. W., Mayr, S., Mecum, L. & Enright, C. The freshwater tidal wetland Liberty Island, CA was both a source and sink of inorganic and organic material to the San Francisco Estuary. Aquat. Ecol.44, 359–372. 10.1007/s10452-009-9295-y (2010). 10.1007/s10452-009-9295-y [DOI] [Google Scholar]
- 34.Braghin, L. S. M., Dias, J. D., Simőes, N. R. & Bonecker, C. C. Food availability, depth, and turbidity drive zooplankton functional diversity over time in a Neotropical floodplain. Aquat. Sci.83, 10. 10.1007/s00027-020-00763-7 (2021). 10.1007/s00027-020-00763-7 [DOI] [Google Scholar]
- 35.Preston, F. W. The canonical distribution of commonness and rarity: Part II. Ecology43(3), 410–432 (1962). 10.2307/1933371 [DOI] [Google Scholar]
- 36.Sih, A., Ferrari, M. C. O. & Harris, D. J. Evolution and behavioural responses to human-inducedrapid environmental change. Evol. Appl.4, 367–387. 10.1111/j.1752-4571.2010.00166.x (2011). 10.1111/j.1752-4571.2010.00166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goździejewska, A. M., Gwoździk, M., Kulesza, S., Bramowicz, M. & Koszałka, J. Effects of suspended micro- and nanoscale particles on zooplankton functional diversity of drainage system reservoirs at an open-pit mine. Sci. Rep.9, 16113. 10.1038/s41598-019-52542-6 (2019). 10.1038/s41598-019-52542-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goździejewska, A. M., Koszałka, J., Tandyrak, R., Grochowska, J. & Parszuto, K. Functional responses of zooplankton communities to depth, trophic status, and ion content in mine pit lakes. Hydrobiologia848, 2699–2719. 10.1007/s10750-021-04590-1(01234 (2021). 10.1007/s10750-021-04590-1(01234 [DOI] [Google Scholar]
- 39.Kirk, K. L. & Gilbert, J. J. Suspended clay and the population dynamics of planktonic rotifers and cladocerans. Ecology71(5), 1741–1755 (1990). 10.2307/1937582 [DOI] [Google Scholar]
- 40.Levine, S. N., Zehrer, R. F. & Burns, C. W. Impact of resuspended sediment on zooplankton feeding in Lake Waihola, New Zealand. Freshw. Biol.50, 1515–1536 (2005). 10.1111/j.1365-2427.2005.01420.x [DOI] [Google Scholar]
- 41.Bilotta, G. S. & Brazier, R. E. Understanding the influence of suspended solids on water quality and aquatic biota. Water Res.42, 2849–2861. 10.1016/j.watres.2008.03.018 (2008). 10.1016/j.watres.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 42.Goździejewska, A. M. & Kruk, M. Zooplankton network conditioned by turbidity gradient in small anthropogenic reservoirs. Sci. Rep.12, 3938. 10.1038/s41598-022-08045-y (2022). 10.1038/s41598-022-08045-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czerniawski, R. & Domagała, J. Similarities in zooplankton community between River Drawa and its two tributaries (Polish part of River Odra). Hydrobiologia638, 137–149. 10.1007/s10750-009-0036-y (2010). 10.1007/s10750-009-0036-y [DOI] [Google Scholar]
- 44.Kimbell, H. S. & Morrel, L. J. Turbidity weakens selection for assortment in body size in groups. Behav. Ecol.27, 545–552. 10.1093/beheco/arv183 (2016). 10.1093/beheco/arv183 [DOI] [Google Scholar]
- 45.Pithart, D. et al. Spatial and temporal diversity of small shallow waters in river Lužnice floodplain. Hydrobiologia584, 265–275. 10.1007/s10750-007-0607-8 (2007). 10.1007/s10750-007-0607-8 [DOI] [Google Scholar]
- 46.Burns, C. W. & Gilbert, J. J. Effects of daphnid size and density on in-terference between Daphnia and Keratella cochlearis. Limnol. Oceanogr.31(4), 848–858. 10.4319/lo.1986.31.4.0848 (1986). 10.4319/lo.1986.31.4.0848 [DOI] [Google Scholar]
- 47.Conde-Porcuna, J. M., Morales-Baquero, R. & Cruz-Pizarro, L. Effects of Daphnia longispina on rotifer populations in a natural environment: Relative importance of food limitation and interference competition. J. Plankton Res.16(6), 691–706. 10.1093/plankt/16.6.691 (1994). 10.1093/plankt/16.6.691 [DOI] [Google Scholar]
- 48.Gilbert, J. J. Suppression of rotifer populations by Daphnia: A re-view of the evidence, the mechanisms, and the effects on zooplankton community structure. Limnol. Oceanogr.33(6), 1286–1303. 10.4319/lo.1988.33.6.1286 (1998). 10.4319/lo.1988.33.6.1286 [DOI] [Google Scholar]
- 49.Goździejewska, A. M., Kruk, M. & Bláha, M. The zooplankton adaptation patterns along turbidity gradient in shallow water reservoirs. Ecohydr. Hydrobiol.24, 188–200. 10.1016/j.ecohyd.2023.08.005 (2024). 10.1016/j.ecohyd.2023.08.005 [DOI] [Google Scholar]
- 50.Endler, Z., Goździejewska, A., Jaworska, B. & Grzybowski, M. Wpływ małej elektrowni wodnej na organizmy planktonowe w wodzie rzecznej. Acta Sci. Pol. Form. Circumiectus5(2), 121–134 (2006). [Google Scholar]
- 51.Glińska-Lewczuk, K. et al. The impact of urban areas on the water quality gradient along a lowland river. Environ. Monit. Assess.188, 624. 10.1007/s10661-016-5638-z (2016). 10.1007/s10661-016-5638-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Błędzki, L. A. & Rybak, J. I. Freshwater crustacean zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida). In Key to Species Identification With Notes on Ecology, Distribution, Methods and Introduction to Data Analysis (eds Błędzki, L. A. & Rybak, J. I.) (Springer International Publishing, 2016). [Google Scholar]
- 53.Radwan, S., Bielańska-Grajner, I. & Ejsmont-Karabin, J. Rotifers. Monogononta–Atlas of Species. Polish Freshwater Fauna (Univ of Łódź, 2004). [Google Scholar]
- 54.Koste, W. Rotatoria. Die Rädertiere Mitteleuropas. Überordnung Monogononta. I Textband, II Tafelband. 52–570. (Gebrüder Borntraeger, Berlin, 1978).
- 55.Rybak, J. I. & Błędzki, L. A. Freshwater Planktonic Crustaceans (Warsaw University Press, 2010). [Google Scholar]
- 56.von Flössner, D. Krebstiere, Crustacea. Kiemen-und Blattfüsser, Branchiopoda, Fischläuse, Branchiura (VEB Gustav Fischer Verlag, 1972). [Google Scholar]
- 57.Starmach, K. Metody Badania Planktonu (PWRiL, 1955). [Google Scholar]
- 58.Hernroth, L. Recommendations on methods for marine biological studies in the Baltic Sea. Mesozooplankton biomass assessment. The Baltic Biologists Publication10 (1985).
- 59.Kovach, W. L. MVSP—A Multivariate Statistical Package for Windows, Ver. 3.2 (Kovach Computing Services Pentraeth, 2015). [Google Scholar]
- 60.Margalef, R. Information theory in ecology. Int. J. Gen. Syst. 36–71 (1958).
- 61.Obertegger, U., Smith, H. A., Flaim, G. & Wallace, R. L. Using the guild ratio to characterize pelagic rotifer communities. Hydrobiologia662, 157–162. 10.1007/s10750-010-0491-5 (2011). 10.1007/s10750-010-0491-5 [DOI] [Google Scholar]
- 62.Bertani, I., Ferrari, I. & Rossetti, G. Role of intra-community biotic interactions in structuring riverine zooplankton under low-flow, summer conditions. J. Plankton Res.34, 308–320. 10.1093/plankt/fbr111 (2012). 10.1093/plankt/fbr111 [DOI] [Google Scholar]
- 63.Vogt, R. J., Peres-Neto, P. R. & Beisner, B. E. Using functional traits to investigate the determinants of crustacean zooplankton community structure. Oikos122, 1700–1709. 10.1111/j.1600-0706.2013.00039.x (2013). 10.1111/j.1600-0706.2013.00039.x [DOI] [Google Scholar]
- 64.Moreira, F. W. A. et al. Assessing the impacts of mining activities on zooplankton functional diversity. Acta Limnol. Brasil.10.1590/S2179-975X0816 (2016). 10.1590/S2179-975X0816 [DOI] [Google Scholar]
- 65.APHA-AWWA-WEF. Standard methods for the examination of water and wastewater 20th ed. (American Public Health Association, American Water Works Association, Water Environment Federation. Washington DC, 1999)
- 66.van Verseveld, W. J. et al. Wflow_sbm v0.7.3, a spatially distributed hydrological model: From global data to local applications. GMD17, 3199–3234. 10.5194/gmd-17-3199-2024 (2024). 10.5194/gmd-17-3199-2024 [DOI] [Google Scholar]
- 67.Dufręne, M. & Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr.67, 345–366 (1997). [Google Scholar]
- 68.Shawul, A. A., Chakma, S. & Melesse, A. M. The response of water balance components to land cover change based on hydrologic modeling and partial least squares regression (PLSR) analysis in the Upper Awash Basin. J. Hydrol. Reg.26, 100640. 10.1016/j.ejrh.2019.100640 (2019). 10.1016/j.ejrh.2019.100640 [DOI] [Google Scholar]
- 69.Wold, S., Sjöström, M. & Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab.58(2), 109–130 (2001). 10.1016/S0169-7439(01)00155-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.