Abstract
Background
Bronchiectasis is a major respiratory complication in patients with common variable immunodeficiency (CVID) and is associated with recurrent pulmonary infections. However, it is unclear whether other infections or non-infectious respiratory conditions are related to its development.
Objective
To identify respiratory comorbidities associated with bronchiectasis in patients with CVID.
Methods
A total of 1470 CVID patients enrolled in the USIDNET registry were included in a cross-sectional analysis. The primary outcome of our study was to determine the clinical characteristics and other respiratory conditions associated with respiratory comorbidities and physician-reported bronchiectasis.
Results
One hundred ninety-seven CVID patients were noted to have bronchiectasis (13.4%). Affected patients were significantly older than patients without bronchiectasis (median age 54 years vs. 49 years, p = 0.0004). These patients also had lower serum IgA (13 mg/dL IQR 60 mg/dL vs. 28.4 mg/dL IQR 66 mg/dL, p = 0.000). Notably, chronic rhinosinusitis (OR = 1.69 95%CI 1.05–2.75), sinusitis (OR = 2.06 95%CI 1.38–3.09), pneumonia (OR = 2.70 95%CI 1.88–3.88), COPD (OR = 2.66 95%CI 1.51–4.67), and interstitial lung disease (OR = 2.34 95%CI 1.41–3.91) were independently associated with the development of bronchiectasis in this population.
Conclusion
These data suggest that lower and upper respiratory infections, chronic lower airway disease, and interstitial lung diseases are independently associated with bronchiectasis in CVID patients. Further study into predisposing conditions related to the development of bronchiectasis in CVID patients may allow prediction and early intervention strategies to prevent the development of this complication.
Keywords: Primary immunodeficiency diseases, Common variable immunodeficiency, Bronchiectasis, Respiratory disease, Pneumonia, Sinusitis, Interstitial lung diseases, Inborn errors of immunity, Antibody deficiency
Introduction
Common variable immunodeficiency (CVID) is a heterogeneous group of inborn errors of immunity characterized by a predisposition to infections, defective antibody production, and in many, immune dysregulation [1, 2]. Although with some regional variations [3], CVID is the most frequent symptomatic primary antibody deficiency diagnosed in adults worldwide [4, 5]. Sinopulmonary infections are the most frequent complication in patients with CVID. In one study, over 70% of CVID patients presented with upper respiratory tract infections [6], and about 60% had lower respiratory infections [7]. Notably, many CVID patients develop chronic lung complications. For instance, bronchiectasis affected approximately 34% of CVID patients; in the same study, about a third of CVID patients may present with asthma and chronic obstructive lung disease [6]. Parenchymal lung disease also occurs involving the interstitium (e.g., granulomatous and lymphocytic interstitial lung disease-GLILD, organizing pneumonia, malignancies) [8, 9]. The development of chronic lung disease in CVID patients is particularly problematic, considering that inflammatory lung lesions may progress despite immunoglobulin replacement therapy [9].
Bronchiectasis, characterized by irreversible dilatation and bronchi damage, remains a leading cause of lung damage and healthcare utilization among patients with CVID and other predominantly antibody deficiencies [10–12]. Clinically, bronchiectasis may be asymptomatic [13] or be diagnosed in patients with lower respiratory and/or constitutional symptoms such as malaise and weight loss [14]. The bronchial damage is considered sequelae of recurrent and prolonged infections with inflammation [15]. Nonetheless, the origin in CVID and other antibody deficiencies is not fully understood, as this may occur even when optimal immunoglobulin replacement therapy is utilized [16]. Accordingly, multiple efforts have been directed to elucidate risk factors, underlying mechanisms, and biological markers that predict its appearance [17–20].
While the role of frequent lower respiratory tract infections in the development of bronchiectasis is well-recognized [21, 22], whether other airway diseases are linked with bronchiectasis in CVID patients is unclear. In this study, we aimed to identify predictors of bronchiectasis in CVID patients enrolled in the USIDNET registry [23]. Specifically, we defined the main clinical characteristics and respiratory comorbidities of patients with CVID in the registry, evaluated the differences between CVID patients with bronchiectasis and those unaffected, and examined associations between bronchiectasis and several types of respiratory disease.
Methods
Study Population
All CVID cases reported in the USIDNET registry [23] by September 2022 were included. Enrollment in the USIDNET registry requires patients’ consent and institutional research board approval. Patients are registered by their physician at their enrolling center, and the data is entered using an established data collection form. Sociodemographic and main clinical characteristics, respiratory comorbidities, and immunoglobulin levels data were extracted from the registry’s primary dataset. One patient with cystic fibrosis and two with immotile cilia syndromes were excluded because these conditions are associated with the development of bronchiectasis.
Variable Definitions
The primary outcome, bronchiectasis, was defined as a binary variable based on physician reports as recorded in the registry’s database. The information recorded in the registry was extracted and contrasted in CVID patients with and without bronchiectasis. Respiratory comorbidities evaluated as predictors of bronchiectasis are also physician-reported outcomes in the registry’s records and are comprised of infectious and non-infectious respiratory conditions grouped by anatomical compartments (Tables 1–3) [24]. These conditions included upper respiratory tract disorders, lung airways, lung parenchyma and pleural abnormalities, anatomical abnormalities of the chest, and obstructive sleep apnea. Associations between bronchiectasis, serum IgA and IgM levels, and basic lymphocyte phenotype (T and B cell counts) were also evaluated. These laboratory parameters were analyzed as continuous values and categorized. IgG levels were not assessed as most patients received IgG replacement, and baseline measurements were unavailable.
Table 1.
Demographics and baseline characteristics
| Variable | CVID with bronchiectasis (n = 197) | CVID w/o bronchiectasis (n = 1273) | p value |
|---|---|---|---|
|
| |||
| Sex (n, %) | |||
| Male | 78 (39.6) | 494 (38.8) | .83 |
| Female | 119 (60.4) | 779 (61.2) | |
| Age (median, IQR) | 54 (31) | 49 (38) | .00 |
| Race (n, %) | |||
| White | 156 (79.2) | 980 (77.0) | .27 |
| Hispanic | 3 (1.52) | 16 (1.26) | |
| Black | 5 (2.54) | 18 (1.41) | |
| Asian | 1 (0.51) | 6 (0.47) | |
| Native American/other | 1 (0.51) | 2 (0.16) | |
| Mixed | 6 (3.1) | 20 (1.57) | |
| Unknown | 25 (12.7) | 231 (18.2) | |
| Alive (n, %) | |||
| Yes | 178 (90.4) | 1152 (90.5) | .00 |
| No | 14 (7.11) | 34 (2.7) | |
| Unknown | 5 (2.54) | 87 (6.8) | |
| Family history of PI (n, %) | |||
| Yes | 22 (11.2) | 163 (12.8) | .18 |
| No | 106 (53.8) | 595 (46.7) | |
| Unknown | 69 (35.0) | 515 (40.5) | |
Data are presented as the absolute number of patients and proportions in each group. For age, median and interquartile ranges (IQR) are shown. Comparisons were made using chi-square or Wilcoxon rank-sum tests. Bold indicates statistical significance (p ≤ .05)
Table 3.
Respiratory infections in patients with bronchiectasis
| Organ/compartment | Infection | CVID with bronchiectasis (n = 197) | CVID w/o bronchiectasis (n = 1273) | p value |
|---|---|---|---|---|
|
| ||||
| Upper airways | Sinusitis (n, %) | 160 (81.22) | 803 (63.08) | 0.00 |
| Other upper respiratory tract infection (n, %) | 81 (41.12) | 482 (37.86) | 0.38 | |
| Ear | Otitis media (n, %) | 74 (37.56) | 411 (32.29) | 0.14 |
| Mastoiditis (n, %) | 4 (2.03) | 11 (0.86) | 0.13 | |
| Lung airways | Bronchitis (n, %) | 68 (34.52) | 404 (31.64) | 0.46 |
| Bronchiolitis (n, %) | 4 (2.03) | 41 (3.22) | 0.37 | |
| Lung parenchyma | Pneumonia (n, %) | 146 (74.11) | 599 (47.05) | 0.00 |
| Lung abscess (n, %) | 6 (3.05) | 7 (0.55) | 0.00 | |
Data are presented as the absolute number of patients and proportions in each group. Comparisons were made using chi-square. Bold indicates statistical significance (p ≤ .05)
Statistical Analyses
Data were analyzed using STATA version 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.) and GraphPad Prism version 9.0.0 for Windows (GraphPad Software, San Diego, CA, USA). Sociodemographic, clinical, and laboratory values were extracted from the USIDNET database [23]. Continuous variables are summarized as medians and interquartile ranges (IQRs) as distributions were non-Gaussian and were compared using the Wilcoxon rank-sum test. Categorical variables are presented as frequencies and proportions. Demographic and clinical characteristics were compared using chi-square tests as indicated. Single and multiple logistic regression models were built to evaluate the relationship between bronchiectasis and other respiratory comorbidities and laboratory markers. Every variable included in the final model was selected through a stepwise logistic regression, and selected covariables were added if deemed clinically relevant. Model fitness was evaluated using the Akaike information criterion (AIC).
Results
Baseline Clinical Characteristics
There were 1473 patients in the USIDNET registry diagnosed with CVID who were screened for eligibility. Out of them, two patients with immotile cilia syndromes and one patient with cystic fibrosis were excluded. A total of 1470 patients were included in our final analysis. Bronchiectasis were identified in 197 patients (13.4%). The median age of patients in the analysis was 50 years (IQR 37 years). Patients with bronchiectasis had a median age of 54 years (IQR 31 years) and were significantly older than those unaffected, who had a median age of 49 years (IQR 38 years, p = 0.00). Additionally, 61.13% were females (n = 898), and no significant sex differences were identified between patients with and without bronchiectasis (Table 1). Regarding ethnicity, 77.3% of patients were White-Caucasian (n = 1135), other ethnic groups comprised 5.31% of the included patients (n = 78) and there were no significant differences in ethnicity between the two study groups. About 17.46% of patients (n = 256) had no information on race or ethnicity.
Respiratory Symptoms Associated with Bronchiectasis in CVID Patients
Symptoms previously reported in association with bronchiectasis [25] were compared between affected and unaffected patients. Respiratory symptoms were reported in 173 out of 1470 CVID patients (Table 2). The proportion of patients with respiratory symptoms was higher in CVID patients with bronchiectasis than those unaffected (22.8% vs. 10.05% p = 0.00). Importantly, although cough, hemoptysis, dyspnea, and digital clubbing were significantly more frequent in patients with bronchiectasis, these symptoms were not specific as they were also reported in unaffected patients (Table 2). Notably, the proportion of patients with weight loss or underweight was nearly twice as high among those with bronchiectasis (26/197 patients, 13.2%) compared with those in the unaffected group (87/1274 patients, 6.83%). Conversely, no significant differences in chest pain, restrictive lung disease, or incident respiratory failure were found between the two groups. Importantly, most CVID patients in our analysis did not report any of the above symptoms (n = 1207, 82.1%).
Table 2.
Differences in respiratory symptoms reported in patients according to bronchiectasis status
| Symptom | Frequency and % of pts with bronchiectasis (n = 197) | Frequency and % of pts w/o bronchiectasis (n = 1273) | p value |
|---|---|---|---|
|
| |||
| Cough | 24 (12.18) | 89 (6.99) | .01 |
| Hemoptysis | 4 (2.03) | 2 (0.16) | .00 |
| Clubbing | 3 (1.52) | 3 (0.24) | .03 |
| Pain | 2 (1.02) | 5 (0.39) | .24 |
| Dyspnea | 21 (10.66) | 44 (3.46) | .00 |
| Respiratory failure | 2 (1.02) | 6 (0.47) | .29 |
| Restrictive lung disease | 3 (1.52) | 7 (0.55) | .14 |
This table summarizes the most frequent respiratory symptoms in the cohort. Data are presented as frequencies and proportions of patients affected in the two groups. Comparisons were made using the chi-square test. Bold indicates statistical significance (p ≤ .05)
Infections Were the Most Frequent Respiratory Comorbidities in CVID Patients with Bronchiectasis
In this cohort, there were 1266 patients (86.1%) with respiratory infections on record (Tables 3, 4). Notably, sinusitis was more frequently reported in CVID patients with bronchiectasis (n = 160, 81.2%) compared with the group of unaffected patients (n = 803, 63.1%) (p = 0.00) (Table 3). Lung infections were also significantly more common among patients with bronchiectasis. Specifically, among 197 patients with CVID and bronchiectasis, pneumonia was reported in 146 (74.1%) compared with 599 patients (47.1%) out of 1273 CVID patients without bronchiectasis (p = 0.000) (Tables 3, 4). Additionally, although the number of patients with lung abscesses was small, these lesions were more common among patients with bronchiectasis (n = 6, 3.05%) in comparison with those without (n = 7, 0.55%) (p = 0.004). In contrast, there was no significant difference in the frequency of other upper respiratory infections and ear infections or in the frequency of lower airway infections or pleural empyema between patients with and without bronchiectasis (Tables 3, 4).
Table 4.
Chronic respiratory conditions in patients with CVID with and without bronchiectasis
| Organ/compartment | Condition | CVID with bronchiectasis (n = 197) | CVID w/o bronchiectasis (n = 1273) | p value |
|---|---|---|---|---|
|
| ||||
| Nasal | Allergic rhinitis (AR) | 16 (8.12) | 78 (6.13) | .28 |
| Chronic rhinosinusitis (CRS) | 27 (13.71) | 98 (7.70) | .01 | |
| Nasal polyps | 3 (1.52) | 7 (0.55) | .14 | |
| Ear | Hearing loss | 4 (2.03) | 15 (1.18) | 0.31 |
| Chronic otitis | 1 (0.51) | 13 (1.02) | 0.71 | |
| Eustachian tube dysfunction | 2 (1.02) | 6 (0.47) | .29 | |
| Other upper airway | Vocal cord dysfunction | 1 (0.51) | 7 (0.55) | 1.00 |
| Lower airway | Chronic bronchitis | 3 (1.52) | 17 (1.34) | .74 |
| COPD | 25 (12.69) | 49 (3.85) | .00 | |
| Asthma | 98 (49.75) | 512 (40.22) | .01 | |
| Lung alveoli | PAP | 1 (0.51) | 4 (0.31) | .51 |
| ARDS | 3 (1.52) | 1 (0.08) | .01 | |
| Lung Interstitium | ILD (ALL) | 32 (16.24) | 71 (5.58) | .00 |
| ILD ns | 24 (12.18) | 48 (3.77) | .00 | |
| OP | 4 (2.03) | 7 (0.55) | .048 | |
| FIB | 4 (2.03) | 6 (0.47) | .03 | |
| IP | 2 (1.02) | 8 (0.63) | .63 | |
| LIP | 6 (3.05) | 11 (0.86) | .02 | |
| NLH | 2 (1.02) | 3 (0.24) | .14 | |
| BO | 4 (2.03) | 2 (0.16) | .00 | |
| FB | 5 (2.54) | 8 (0.63) | .02 | |
| Nodular lesions (ALL) | 8 (4.06) | 34 (2.67) | .26 | |
| Single nodule | 1 (0.51) | 10 (0.79) | 1.00 | |
| Multiple nodules | 7 (3.55) | 24 (1.89) | .18 | |
| Lung granulomas | 10 (5.08) | 14 (1.10) | .00 | |
| Lung vasculature | Pulmonary embolism (PE) | 2 (1.02) | 9 (0.71) | .65 |
| Pulmonary arterial hypertension (PAH) | 2 (1.02) | 6 (0.47) | .29 | |
| Pleura | Pleural compromise (ALL) | 11 (5.58) | 26 (2.04) | .01 |
| Empyema | 1 (0.51) | 10 (0.79) | 1.00 | |
| Pleural ns | 10 (5.08) | 17 (1.34) | .00 | |
| Other | Chest Anatomy | 5 (2.54) | 18 (1.41) | .22 |
| OSA | 10 (5.08) | 56 (4.40) | .71 | |
This table summarizes the details of non-infectious respiratory conditions by compartment. Data are presented as frequency and percentage of patients in each group. Comparisons were made using the chi-square test. Bolding indicates statistical significance (p ≤ .05)
Chronic Respiratory Disorders Associated with Bronchiectasis in CVID Patients
Chronic rhinosinusitis was significantly more frequent in patients with bronchiectasis (n = 27, 13.7%) compared with those unaffected (n = 98, 7.70%) (p = 0.005). In contrast, no significant differences were found in the frequency of allergic rhinitis, ear disorders (Table 3), or vocal cord dysfunction between patients with and without bronchiectasis (Table 4). Additionally, bronchiectasis was associated with several types of lung airway disorders [24]. Specifically, COPD and asthma were more frequent among CVID patients with bronchiectasis. Conversely, chronic bronchitis did not differ significantly between CVID patients with and without bronchiectasis (Table 4).
The lung parenchyma and the pleura were also affected, and lesions in the lung interstitium including organizing pneumonia (OP), lymphoid interstitial pneumonia (LIP), bronchiolitis obliterans (BO), follicular bronchiolitis (FB), pulmonary fibrosis (FIB), and lung granulomas were significantly more frequent in patients with bronchiectasis (Table 4). In the alveoli, although rare, there were three cases of acute respiratory distress syndrome (ARDS) (1.52%) among CVID patients with bronchiectasis and one case (0.08%) among patients without. In addition, pleural compromise occurred in 5.58% of the patients with bronchiectasis (n = 11), compared with 2.04% of CVID patients without (n = 26) (p = 0.003). Additional respiratory comorbidities found in CVID patients included obstructive sleep apnea (OSA) and anatomical abnormalities of the chest. Specifically, sixty-six patients had OSA as comorbidity, occurring at similar proportions between groups: 5.1% (n = 10) in patients with bronchiectasis group and 4.4% (n = 56) in CVID patients without bronchiectasis. In addition, anatomical abnormalities of the chest were not associated with bronchiectasis in this population (Table 4).
To further evaluate respiratory comorbidities associated with bronchiectasis in CVID, we built a multiple logistic regression model, including respiratory comorbidities associated with bronchiectasis in our exploratory analysis (sinusitis, pneumonia, chronic rhinosinusitis, asthma, COPD, ILD), other clinically important respiratory disorders in patients with CVID (ear infections, bronchitis, allergic rhinitis) and basic demographic variables (age and sex) as predictors (Fig. 1). Our multivariate analysis revealed a 2.06-fold increase in the odds of bronchiectasis in patients with sinusitis independent of age, gender, pneumonia, and additional respiratory comorbidities in the model (OR 2.06, 95% CI 1.38–3.09) (Fig. 1). Notably, a history of pneumonia was also independently associated with increased odds of bronchiectasis (OR 2.70, 95% CI 1.88–3.88). In contrast, ear and other upper respiratory tract infections were not significantly associated with bronchiectasis.
Fig. 1.
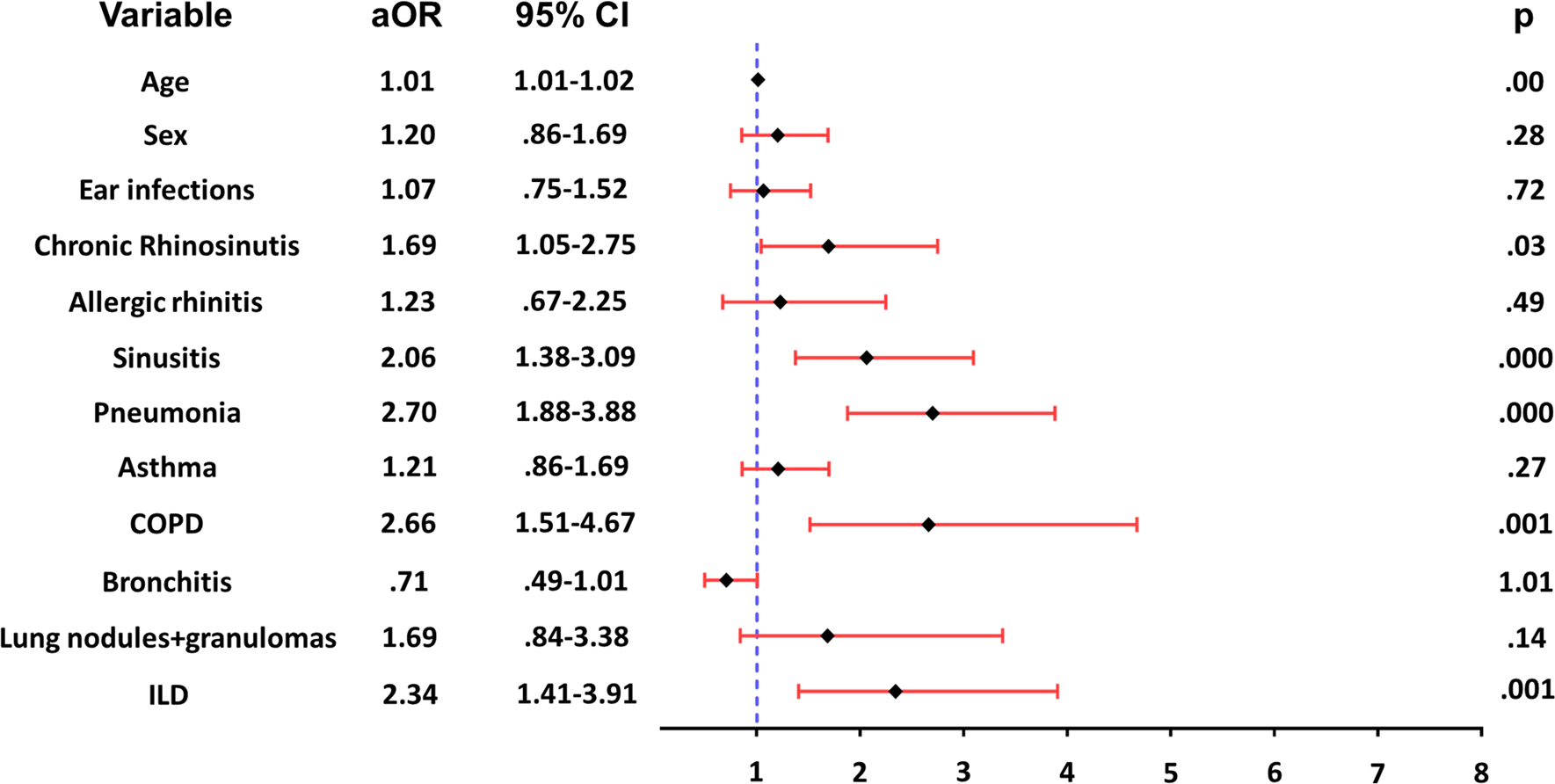
Respiratory comorbidities associated with bronchiectasis in CVID patients. Multivariate logistic regression analysis showed that chronic rhinosinusitis, sinusitis, pneumonia, COPD and ILD were independently associated with increased odds of bronchiectasis in CVID patients. aOR = adjusted odds ratios, 95% CIs, and p values obtained in the final adjusted model are shown
Several non-infectious chronic respiratory disorders were also linked to bronchiectasis. Specifically, chronic rhinosinusitis was associated with 1.69-fold increased odds of bronchiectasis (OR 1.69, 95% CI 1.05–2.75) independently of age, sex, and other respiratory conditions in the analysis (Fig. 1). In addition, in the lower airways, COPD was independently associated with a 2.66-fold increase in the odds of bronchiectasis (OR 2.66, 95% CI 1.51–4.67). Furthermore, CVID patients with interstitial lung diseases comprising non-specific ILD, OP, FIB, IP, LIP, BO, FB, interstitial pneumonia (IP) and pulmonary nodular lymphoid hyperplasia (NLH), had 2.34-fold increased odds of having bronchiectasis compared with patients without those lesions (OR 2.34, 95% CI 1.41–3.91) (Fig. 1). Interestingly, asthma was not associated with bronchiectasis in the adjusted model.
Low Serum IgA Levels Are Associated with Bronchiectasis in CVID
Serum IgA and IgM measurements with the date of sample collection (to estimate age at the time of measurement) were available in 1075 and 1069 patients, respectively. Notably, serum IgA values in patients with bronchiectasis (median 13 mg/dL, IQR 60 mg/dL) were significantly lower compared with patients without bronchiectasis (median 28.4 mg/dL, IQR 66 mg/dL) (Fig. 2). To further explore this association, we divided serum IgA levels into two categories above or below the median in our dataset (≤ 26 mg/dL vs. > 26 mg/dL). Notably, patients with serum IgA levels equal to or less than 26 mg/dL had a 1.97-fold increase in the odds of bronchiectasis compared with CVID patients with IgA levels above 26 mg/dL, adjusting by age at the time of the measurement (OR 1.97, 95% CI 1.39–2.79) (Fig. 2). A similar analysis was conducted to examine the relationship between IgM levels and bronchiectasis. In contrast with serum IgA, the serum IgM values did not significantly differ between patients with bronchiectasis (median 29 mg/dL, IQR 53 mg/dL) and those without (median 36 mg/dL, IQR 57.1 mg/dL) (Fig. 2). In addition, there were no significant associations between serum IgM categories (≤ 35 mg/dL vs. >35 mg/dL) in an analysis adjusted by age at the time of the test.
Fig. 2.
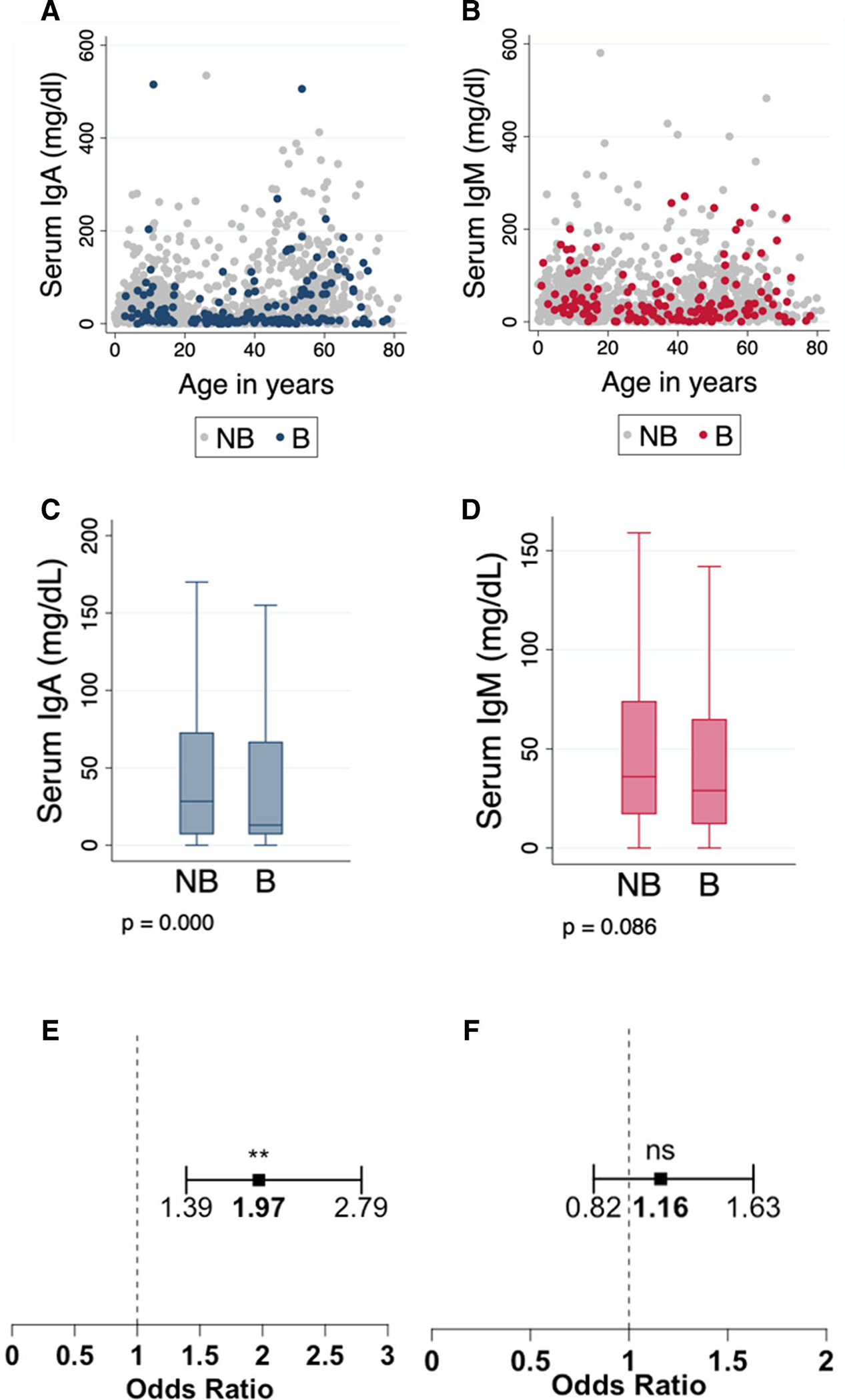
Serum immunoglobulin levels between CVID patients with and without bronchiectasis. (A) Serum IgA and (B) IgM levels by age and disease subgroup. (C) Comparison of IgA and (D) IgM levels between patients with and without bronchiectasis using a median test. (E) Forest plots showing the odds of bronchiectasis if IgA levels ≤26mg/dL and (F) IgM levels ≤35mg/dL adjusted by age at the time of testing. Abbreviations: NB=No bronchiectasis, B=Bronchiectasis. Outliers are not shown in plots for visualization. p-value < 0.001(**), p-value > 0.05(ns)
We also evaluated the relationship between lymphocyte counts (CD4 +, CD8 +, and CD19 + cells) and bronchiectasis in CVID patients with available lymphocyte phenotype and date of measurement data. There were 662 CVID patients with available CD4 + counts, 638 with available CD8 + data, and 632 with CD19 + B-cells measurements. Using these data, no median differences were identified for any of the values in patients with bronchiectasis compared to unaffected CVID patients (Fig. 3). However, due to age-related variation in lymphocyte counts, the analysis was stratified accordingly [26]. In this comparison, lower CD4 + values were seen in CVID patients with bronchiectasis older than 16 years, compared with those unaffected (Table E5).
Fig. 3.
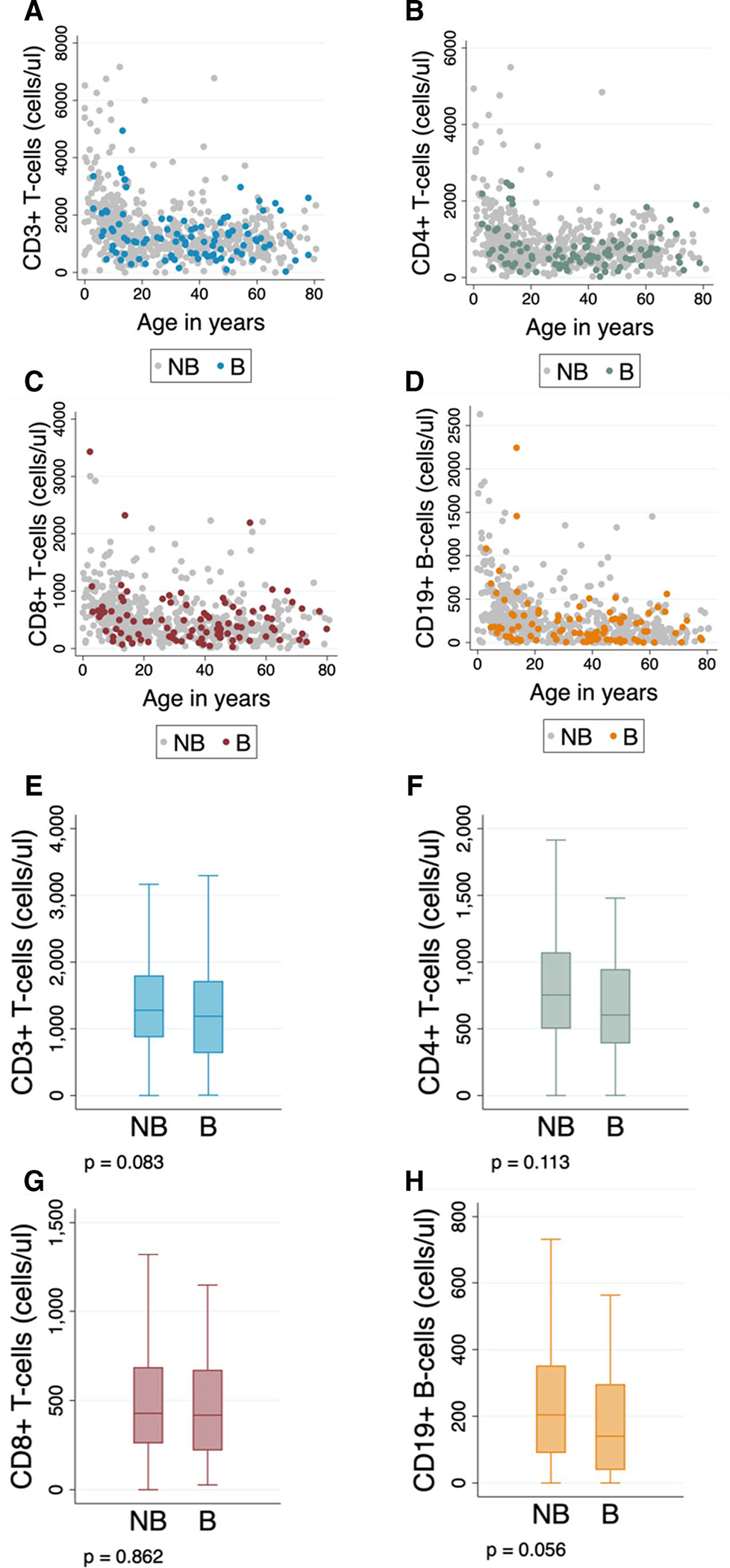
Lymphocyte counts between CVID patients with and without bronchiectasis. (A) CD3+, (B) CD4+, and (C) CD8+ T-cell counts by age and disease subgroup. (D) CD19+ B-cell counts by age and disease subgroup. (E) Comparison of CD3+, (F) CD4+, and (G) CD8+ T-cell counts between CVID patients with and without bronchiectasis. (H) Comparison of CD19+ B-cell counts between patients with and without bronchiectasis. Differences between CVID subgroups (patients with and without bronchiectasis) were evaluated using the median test. Abbreviations: NB=No bronchiectasis, B=Bronchiectasis. Outliers are not shown in plots for visualization
Discussion
This study includes 1470 CVID patients from the USIDNET Registry and examines clinical respiratory comorbidities associated with the development of bronchiectasis. We identified a prevalence of bronchiectasis of 13.4% among CVID patients in this population, in line with previous reports from the same cohort [17]. Most patients were adults, and those with CVID and bronchiectasis were older than CVID patients without bronchiectasis. This finding may reflect the cumulative effect of recurrent respiratory infections over time [27] as this is a major determinant of the emergence of bronchiectasis in CVID [28, 29]. Alternatively, senescence may affect both epithelial and immune cell responses in the respiratory system [30, 31]. For example, studies indicate that aging impairs the alveoli’s local innate immune defenses, reducing alveolar fluid hydrolytic capacity, and increasing local oxidative stress and inflammation [32]. Additionally, aging may diminish systemic and respiratory mucosal innate and adaptive immune cell responses, making the elderly more prone to infections [33] and potentially contributing to bronchiectasis development [34–36]. Notably, no sex differences between CVID patients with and without bronchiectasis were identified, a finding aligned with previous studies [37]. Additionally, no significant differences in ethnicity were found; however, our study included predominantly White-Caucasian patients (77.28%), and differences between ethnic groups may not have been detected due to the smaller number of individuals of other ethnicities (Table 1).
As previously described [25], most patients with bronchiectasis in this study were asymptomatic underscoring the need for routine screening of pulmonary complications in CVID patients. In symptomatic patients, their respiratory symptoms were not specific, but some were significantly more common in patients with bronchiectasis. Specifically, chronic cough was the most common respiratory symptom in patients with CVID and bronchiectasis, in line with previous reports in non-CVID patients [25, 38]. In addition, dyspnea, hemoptysis, and clubbing were also noted more commonly in patients with bronchiectasis (10.7%, 2.03 and 1.52%, respectively). Importantly, weight loss was also more frequent in the bronchiectasis group. In non-CVID patients, weight loss and growth delay are associated with chronic lung disease and are often multifactorial (e.g., poor intake, muscle loss due to decreased physical activity) [39–41] and are an important prognostic factor for conditions such as ILD and COPD [42–44]. In CVID, extrapulmonary conditions may also precipitate these changes (e.g., inflammatory bowel disease) [45]. Nonetheless, since chronic lung disease can be the standalone cause of growth delay and unexpected weight loss, pulmonary involvement should be excluded when these abnormalities are detected in CVID patients.
Our study confirmed previously described associations between respiratory infections and the development of bronchiectasis [46, 47]. Specifically, a history of sinusitis and pneumonia were independently associated with 2.06-fold and 2.70-fold increased odds of bronchiectasis, respectively. These findings are aligned with previous work documenting the link between pneumonia and lower respiratory tract infections and bronchiectasis in patients with primary antibody immunodeficiencies [29, 48, 49]. The association between sinusitis and bronchiectasis has also been described in non-CVID patients [47, 50]. It has been attributed to impaired airway clearance of pathogens, leading to chronic bacterial infection and inflammation of the upper and lower airways [47]. Of note, our study did not find a link between bronchitis and bronchiectasis. This is in contrast with other studies indicating that protracted or recurrent bacterial bronchitis, increases the risk of bronchiectasis [51]. However, our data was limited and did not include details about types of bronchitis, such as whether it was acute or chronic, or how often exacerbations ocurred. Therefore, we cannot conclusively exclude associations between bronchiectasis and certain subtypes of bronchitis in patients with CVID. In summary, our findings reinforce the notion that sinusitis and pneumonia are two of the main risk factors for the development of bronchiectasis in CVID patients and support the idea that routine surveillance for bronchiectasis should be conducted among all CVID patients who present with sinopulmonary infections.
Chronic respiratory conditions in all anatomical respiratory compartments [24] were reported in both groups of CVID patients (Table 4) and CRS, COPD, and ILD were associated with bronchiectasis (Fig. 1). Specifically, CVID patients with CRS had a 1.69-fold increase in odds of bronchiectasis, findings similar to those described in non-CVID patients [52, 53]. Chronic rhinosinusitis (CRS) is also associated with subclinical lower airway flow limitation even in the absence of underlying lung disease and can be considered as a biomarker of pan-airway inflammation [54]. In the lower airways, patients with COPD had a 2.66-fold increase in the odds of bronchiectasis, a finding consistent with previous studies in immunocompetent adults describing up to 40% prevalence of bronchiectasis in COPD patients [55]. This co-occurrence of conditions is associated with poorer outcomes and increased mortality [55]. Notably, a significant number of CVID patients in our cohort had asthma (41.5%), highlighting the common coexistence of CVID with this condition [56–58]. Nonetheless, asthma was not independently associated with bronchiectasis in our adjusted analysis.
Notably, in the lungs, patients with interstitial lung disease (a composite variable that groups OP, FIB, IP, LIP, BO, FB, and NLH) had a 2.34-fold increase in the odds of bronchiectasis in patients independent of age, gender, and additional respiratory comorbidities (Fig. 1). This finding is in line with previous descriptions of a prevalence of bronchiectasis of 17.4% in patients with CVID and ILD [59]. This association suggests that patients with immune dysregulation manifestations affecting the lung interstitium are more prone to develop bronchiectasis and motivates further investigation into the possible airway immune abnormalities underlying this association.
We also tested whether serum IgM and IgA, and peripheral blood lymphocyte counts were related to the development of bronchiectasis in our cohort. Previous work has revealed that low levels of serum IgM, IgA, and low CD4 + T-cell counts are linked to a higher prevalence of bronchiectasis in predominantly antibody deficiency patients [37, 60]. In this study, we found no significant associations between serum IgM levels and the presence of bronchiectasis in patients with CVID. This diverges from a previous study by Sperlich et al., who found that higher long-term average serum IgM concentrations were associated with a lower risk of bronchiectasis in a retrospective cohort of 110 CVID patients [37]. Their study had the advantage of serial IgM measurements, albeit a smaller sample size, potentially accounting for the discrepancy [61]. However, since IgM cooperates with mucosal IgA and IgG to provide mucosal protection, and IgM has a compensatory role in IgA deficient patients [62], the involvement of IgM in the development of bronchiectasis cannot be excluded and further research focusing on the role of IgM on local airway immunity in CVID patients is needed.
In our study, CVID patients with bronchiectasis had significantly lower levels of IgA when compared to those without, aligning with previous reports in patients with antibody deficiencies [6, 50, 60]. Schnell et al. further demonstrated that patients with humoral immunodeficiencies had reduced levels of serum and sputum IgA compared to healthy subjects, and showed that IgA levels in sputum correlate with serum levels of IgA in patients with immunodeficiency [63]. These findings shifts the focus to the role of IgA in the airways and align with our findings, as we observed lower IgA serum levels in CVID patients with bronchiectasis. Together, these studies suggest that not only IgG deficiency but defective production of IgA contribute to bronchiectasis in CVID patients [16]. This is particularly compelling given that bronchiectasis can progress despite appropriate IgG replacement therapy [16]. This work may have diagnostic and prognostic implications, indicating a need for future studies on systemic and airway IgA levels as potential bronchiectasis risk markers in CVID patients. Our results also motivate additional investigation into how local antibody responses in the airway microenvironment determine susceptibility or resilience to bronchiectasis.
We found that CD4 + T-cells were significantly lower in adult CVID patients with bronchiectasis (Table E5). These findings are in agreement with what was reported by Maglione et al. [20] and Sperlich et al. [37], who independently identified an increased risk of bronchiectasis in CVID patients with low CD4 + levels. Conversely, we did not identify statistically significant associations between CD3 +, CD8 +, or CD19 + lymphocyte counts and the development of bronchiectasis in CVID patients (Table E5).
Our study has several limitations. First, since this is based on physician-reported outcomes recorded in a registry database, there is a risk of recall, selection, and reporting bias limiting our results. The USIDNET registry only includes individuals with CVID selectively reported from participating centers in the USA; thus, the findings of this analysis do not necessarily apply to all patients with this disease. Additionally, we could not establish temporal relationships between time-varying variables (e.g., Ig levels, treatments) and the development of CVID-associated bronchiectasis. Similarly, the time lapsed between disease onset and diagnosis and time of development of bronchiectasis could not be established due to the cross-sectional nature of this analysis. Finally, there were variable amounts of missing data, in certain variables (lymphocyte counts). Nonetheless, this study describes the spectrum of respiratory complications occurring in a cohort of 1470 CVID patients and defines clinical risk factors associated with the development of bronchiectasis associated in these population.
In summary, this is the most extensive US study to date examining the link between bronchiectasis and various respiratory conditions in patients with CVID. Remarkably, our findings show that not only respiratory infections like sinusitis and pneumonia but also chronic inflammatory conditions such as chronic rhinosinusitis, COPD, and ILD are associated with the development of bronchiectasis in these patients. Additionally, our study confirms that low IgA levels increase the risk of bronchiectasis among CVID patients. Our findings offer key insights that facilitate the identification of CVID patients at high risk for bronchiectasis, allowing for the development of early detection and prevention methods for this severe CVID complication. Future studies, both prospective and focused on underlying mechanisms, are needed to deepen our understanding the assocaitions between bronchiectasis and other respiratory comorbidities in patients CVID.
Supplementary Material
Acknowledgements
We appreciate the invaluable assistance of Julieann Magnusson, B.A., USIDNET project manager, who supported us in obtaining the information from the registry. Additionally, we thank Dr. Diana De La Hoz for facilitating the use of analytical software during this study. Finally, we also acknowledge the contribution of all members of the USIDNET Consortium who enrolled the patients included in this study.
Funding
The U.S. Immunodeficiency Network (USIDNET), a program of the Immune Deficiency Foundation (IDF) is supported by cooperative agreements, U24AI86837 and 1R24AI155390-01A1, from the National Institute of Allergy and Infectious Diseases (NIAID).
Abbreviations
- CVID
Common variable immunodeficiency
- PI
Primary immunodeficiency
- FTT
Failure to thrive
- AR
Allergic rhinitis
- CRS
Chronic rhinosinusitis
- COPD
Chronic obstructive pulmonary disease
- BO
Bronchiolitis obliterans
- FB
Follicular bronchiolitis
- PAP
Pulmonary alveolar proteinosis
- ARDS
Acute respiratory distress syndrome
- ILD
Interstitial lung disease
- OP
Organizing pneumonia
- FIB
Pulmonary fibrosis
- IP
Interstitial pneumonia
- LIP
Lymphoid interstitial pneumonia
- NLH
Pulmonary nodular lymphoid hyperplasia
- OSA
Obstructive sleep apnea
- URI
Upper respiratory tract infection
- PE
Pulmonary embolism
- PAH
Pulmonary arterial hypertension
Footnotes
Competing Interests The authors declare no competing interests.
Ethics Approval This study will be conducted according to U.S. and International Standards of Good Clinical Practice (FDA regulations 21 CFR 312 for IND studies and FDA guidance E6) and in line with the principles of the Declaration of Helsinki. USIDNET has developed a clinical protocol and associated informed consent documents that have received IRB approval.
Consent to Participate Not applicable.
Consent for Publication Not applicable.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10875-023-01593-6.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Gupta S, Pattanaik D, Krishnaswamy G. Common variable immune deficiency and associated complications. Chest. 2019;156(3):579–93. [DOI] [PubMed] [Google Scholar]
- 2.Ameratunga R, Brewerton M, Slade C, Jordan A, Gillis D, Steele R, et al. Comparison of diagnostic criteria for common variable immunodeficiency disorder. Front Immunol. 2014;5:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weifenbach N, Schneckenburger AAC, Lotters S. Global distribution of common variable immunodeficiency (CVID) in the light of the UNDP Human Development Index (HDI): a preliminary perspective of a rare disease. J Immunol Res. 2020;2020:8416124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gathmann B, Mahlaoui N, Ceredih, Gerard L, Oksenhendler E, Warnatz K, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134(1):116–26. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramzi N, Jamee M, Bakhtiyari M, Rafiemanesh H, Zainaldain H, Tavakol M, et al. Bronchiectasis in common variable immunodeficiency: a systematic review and meta-analysis. Pediatr Pulmonol. 2020;55(2):292–9. [DOI] [PubMed] [Google Scholar]
- 7.Zainaldain H, Rizvi FS, Rafiemanesh H, Alizadeh M, Jamee M, Mohammadi S, et al. Infectious complications reporting in common variable immunodeficiency: a systematic review and meta-analysis. Oman Med J. 2020;35(4): e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen LMA, van der Flier M, de Vries E. Lessons learned from the clinical presentation of common variable immunodeficiency disorders: a systematic review and meta-analysis. Front Immunol. 2021;12: 620709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghamohammadi A, Allahverdi A, Abolhassani H, Moazzami K, Alizadeh H, Gharagozlou M, et al. Comparison of pulmonary diseases in common variable immunodeficiency and X-linked agammaglobulinaemia. Respirology. 2010;15(2):289–95. [DOI] [PubMed] [Google Scholar]
- 10.Ameratunga R, Jordan A, Cavadino A, Ameratunga S, Hills T, Steele R, et al. Bronchiectasis is associated with delayed diagnosis and adverse outcomes in the New Zealand Common Variable Immunodeficiency Disorders cohort study. Clin Exp Immunol. 2021;204(3):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Periselneris J, Schelenz S, Loebinger M, Macedo P, Adhya Z, Armstrong-James D, et al. Bronchiectasis severity correlates with outcome in patients with primary antibody deficiency. Thorax. 2021;76(10):1036–9. [DOI] [PubMed] [Google Scholar]
- 12.Patrawala M, Cui Y, Peng L, Fuleihan RL, Garabedian EK, Patel K, et al. Pulmonary disease burden in primary immune deficiency disorders: data from USIDNET Registry. J Clin Immunol. 2020;40(2):340–9. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Jung YJ, Ko MS, Lee SW, Lee JS, Oh YM. Prevalence of asymptomatic bronchiectasis and associations among the health screening population in South Korea. ERJ Open Res. 2021;7(3):00188–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalmers JD, Chang AB, Chotirmall SH, Dhar R, McShane PJ. Bronchiectasis. Nat Rev Dis Primers. 2018;4(1):45. [DOI] [PubMed] [Google Scholar]
- 15.Gaffar S, Lee WW, Harrington JW. Bronchiectasis. Pediatr Rev. 2021;42(2):103–5. [DOI] [PubMed] [Google Scholar]
- 16.Stubbs A, Bangs C, Shillitoe B, Edgar JD, Burns SO, Thomas M, et al. Bronchiectasis and deteriorating lung function in agammaglobulinaemia despite immunoglobulin replacement therapy. Clin Exp Immunol. 2018;191(2):212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberger T, Fuleihan R, Cunningham-Rundles C, Maglione PJ. Factors beyond lack of antibody govern pulmonary complications in primary antibody deficiency. J Clin Immunol. 2019;39(4):440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez AL, Paolini MV, Fernandez Romero DS. Lung disease in patients with common variable immunodeficiency. Allergol Immunopathol (Madr). 2020;48(6):720–8. [DOI] [PubMed] [Google Scholar]
- 19.Gregersen S, Aalokken TM, Mynarek G, Fevang B, Holm AM, Ueland T, et al. Development of pulmonary abnormalities in patients with common variable immunodeficiency: associations with clinical and immunologic factors. Ann Allergy Asthma Immunol. 2010;104(6):503–10. [DOI] [PubMed] [Google Scholar]
- 20.Maglione PJ, Overbey JR, Radigan L, Bagiella E, Cunningham-Rundles C. Pulmonary radiologic findings in common variable immunodeficiency: clinical and immunological correlations. Ann Allergy Asthma Immunol. 2014;113(4):452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383–93. [DOI] [PubMed] [Google Scholar]
- 22.Uzunhan Y, Jeny F, Kambouchner M, Didier M, Bouvry D, Nunes H, et al. The lung in dysregulated states of humoral immunity. Respiration. 2017;94(5):389–404. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan KE, Puck JM, Notarangelo LD, Fuleihan R, Caulder T, Wang C, et al. USIDNET: a strategy to build a community of clinical immunologists. J Clin Immunol. 2014;34(4):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez MJ, Nino G, Sun D, Restrepo-Gualteros S, Sadreameli SC, Fiorino EK, et al. The lung in inborn errors of immunity: from clinical disease patterns to molecular pathogenesis. J Allergy Clin Immunol. 2022;150(6):1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell AE. Bronchiectasis - A Clinical Review. N Engl J Med. 2022;387(6):533–45. [DOI] [PubMed] [Google Scholar]
- 26.Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130(3):388–93. [DOI] [PubMed] [Google Scholar]
- 27.Schussler E, Beasley MB, Maglione PJ. Lung disease in primary antibody deficiencies. J Allergy Clin Immunol Pract. 2016;4(6):1039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goussault H, Salvator H, Catherinot E, Chabi ML, Tcherakian C, Chabrol A, et al. Primary immunodeficiency-related bronchiectasis in adults: comparison with bronchiectasis of other etiologies in a French reference center. Respir Res. 2019;20(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall LA, Wisner EL, Gipson KS, Sorensen RU. Bronchiectasis in primary antibody deficiencies: a multidisciplinary approach. Front Immunol. 2020;11:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrance BL, Haynes L. Cellular senescence is a key mediator of lung aging and susceptibility to infection. Front Immunol. 2022;13:1006710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010;126(4):690–9; quiz 700–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moliva JI, Rajaram MV, Sidiki S, Sasindran SJ, Guirado E, Pan XJ, et al. Molecular composition of the alveolar lining fluid in the aging lung. Age (Dordr). 2014;36(3):9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray MA, Chotirmall SH. The impact of immunosenescence on pulmonary disease. Mediators Inflamm. 2015;2015: 692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Olive I, Radua J, Sanchez-Berenguer D, Hernandez-Biette A, Raya-Marquez P, Stojanovic Z, et al. Association between environmental factors and hospitalisations for bronchiectasis in Badalona, Barcelona, Spain (2007–2015). Med Clin (Barc). 2018;150(7):257–61. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Olive I, Stojanovic Z, Radua J, Rodriguez-Pons L, Martinez-Rivera C, Ruiz MJ. Effect of air pollution on exacerbations of bronchiectasis in Badalona, Spain, 2008–2016. Respiration. 2018;96(2):111–6. [DOI] [PubMed] [Google Scholar]
- 36.Lacoma A, Mateo L, Blanco I, Mendez MJ, Rodrigo C, Latorre I, et al. Impact of host genetics and biological response modifiers on respiratory tract infections. Front Immunol. 2019;10:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperlich JM, Grimbacher B, Soetedjo V, Workman S, Burns SO, Lowe DM, et al. Predictive factors for and complications of bronchiectasis in common variable immunodeficiency disorders. J Clin Immunol. 2022;42(3):572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7–22. [DOI] [PubMed] [Google Scholar]
- 39.Broekhuizen R, Grimble RF, Howell WM, Shale DJ, Creutzberg EC, Wouters EF, et al. Pulmonary cachexia, systemic inflammatory profile, and the interleukin 1beta -511 single nucleotide polymorphism. Am J Clin Nutr. 2005;82(5):1059–64. [DOI] [PubMed] [Google Scholar]
- 40.Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501. [DOI] [PubMed] [Google Scholar]
- 41.De Brandt J, Beijers R, Chiles J, Maddocks M, McDonald MN, Schols A, et al. Update on the etiology, assessment, and management of COPD cachexia: considerations for the clinician. Int J Chron Obstruct Pulmon Dis. 2022;17:2957–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pugashetti J, Graham J, Boctor N, Mendez C, Foster E, Juarez M, et al. Weight loss as a predictor of mortality in patients with interstitial lung disease. Eur Respir J. 2018;52(3):1801289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comes A, Wong AW, Fisher JH, Morisset J, Johannson KA, Farrand E, et al. Association of BMI and change in weight with mortality in patients with fibrotic interstitial lung disease. Chest. 2022;161(5):1320–9. [DOI] [PubMed] [Google Scholar]
- 44.Kwan HY, Maddocks M, Nolan CM, Jones SE, Patel S, Barker RE, et al. The prognostic significance of weight loss in chronic obstructive pulmonary disease-related cachexia: a prospective cohort study. J Cachexia Sarcopenia Muscle. 2019;10(6):1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pikkarainen S, Martelius T, Ristimaki A, Siitonen S, Seppanen MRJ, Farkkila M. A high prevalence of gastrointestinal manifestations in common variable immunodeficiency. Am J Gastroenterol. 2019;114(4):648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solarat B, Perea L, Faner R, de La Rosa D, Martinez-Garcia MA, Sibila O. Pathophysiology of chronic bronchial infection in bronchiectasis. Arch Bronconeumol. 2023;59(2):101–8. [DOI] [PubMed] [Google Scholar]
- 47.Loebinger MR, Bilton D, Wilson R. Upper airway 2: bronchiectasis, cystic fibrosis and sinusitis. Thorax. 2009;64(12):1096–101. [DOI] [PubMed] [Google Scholar]
- 48.Baumann U, Routes JM, Soler-Palacin P, Jolles S. The lung in primary immunodeficiencies: new concepts in infection and inflammation. Front Immunol. 2018;9:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolles S Subclinical infection and dosing in primary immunodeficiencies. Clin Exp Immunol. 2014;178 Suppl 1(Suppl 1):67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berbers RM, Mohamed Hoesein FAA, Ellerbroek PM, van Montfrans JM, Dalm V, van Hagen PM, et al. Low IgA associated with oropharyngeal microbiota changes and lung disease in primary antibody deficiency. Front Immunol. 2020;11:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wurzel DF, Marchant JM, Yerkovich ST, Upham JW, Petsky HL, Smith-Vaughan H, et al. Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest. 2016;150(5):1101–8. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Xu Y, Jin J, Li R, Liu X, Sun Y. Chronic rhinosinusitis is associated with higher prevalence and severity of bronchiectasis in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters AT, Bose S, Guo A, Li N, Benjamin M, Prickett M, et al. Prevalence of bronchiectasis in patients with chronic rhinosinusitis in a tertiary care center. J Allergy Clin Immunol Pract. 2021;9(8):3188–95 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SY, Yoon SH, Song WJ, Lee SH, Kang HR, Kim SS, et al. Influence of chronic sinusitis and nasal polyp on the lower airway of subjects without lower airway diseases. Allergy Asthma Immunol Res. 2014;6(4):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobala R, De Soyza A. Bronchiectasis and chronic obstructive pulmonary disease overlap syndrome. Clin Chest Med. 2022;43(1):61–70. [DOI] [PubMed] [Google Scholar]
- 56.Akaba T, Kondo M, Toriyama M, Kubo A, Hara K, Yamada T, et al. Common variable immunodeficiency diagnosed during the treatment of bronchial asthma: unusual cause of wheezing. Respir Med Case Rep. 2015;16:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milota T, Bloomfield M, Parackova Z, Sediva A, Bartunkova J, Horvath R. Bronchial asthma and bronchial hyper-responsiveness and their characteristics in patients with common variable immunodeficiency. Int Arch Allergy Immunol. 2019;178(2):192–200. [DOI] [PubMed] [Google Scholar]
- 58.Kotsiou OS, Gourgoulianis KI, Daniil Z. Common variable immunodeficiency and asthma: coexistence or coincidence? Ann Allergy Asthma Immunol. 2020;124(6):635. [DOI] [PubMed] [Google Scholar]
- 59.Lopes JP, Ho HE, Cunningham-Rundles C. Interstitial lung disease in common variable immunodeficiency. Front Immunol. 2021;12: 605945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodkinson JP, Bangs C, Wartenberg-Demand A, Bauhofer A, Langohr P, Buckland MS, et al. Low IgA and IgM is associated with a higher prevalence of bronchiectasis in primary antibody deficiency. J Clin Immunol. 2017;37(4):329–31. [DOI] [PubMed] [Google Scholar]
- 61.Serdar CC, Cihan M, Yucel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1): 010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen K, Magri G, Grasset EK, Cerutti A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat Rev Immunol. 2020;20(7):427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnell A, Davrandi M, Saxenhofer M, Leboreiro C, Graeter S, Moreira F, et al. Airway inflammation and dysbiosis in antibody deficiency despite the presence of IgG. J Allergy Clin Immunol. 2022;149(6):2105–15 e10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


