ABSTRACT.
Novel methods are required to aid the monitoring of schistosomiasis control and elimination initiatives through mass drug administration. Portable digital and mobile phone microscopy is a promising tool for this purpose. This cross-sectional study evaluated the diagnostic operating characteristics of a converted mobile phone microscope (the SchistoScope) for the detection of Schistosoma haematobium eggs, as determined by community-based field workers and expert microscopists, compared with a field gold standard of light microscopy. Three hundred sixty-five urine samples were evaluated by conventional light microscopy, with 49 (13.4%) positive for S. haematobium. Compared with light microscopy, the sensitivity and specificity of S. haematobium detection by field microscopists trained to use the SchistoScope were 26.5% (95% CI: 14.9–41.1%) and 98.4% (95% CI: 96.3–99.5%), respectively. The sensitivity and specificity of S. haematobium detection by expert microscopists using the SchistoScope was 74% (95% CI: 59.7–85.4%) and 98.1% (95% CI: 95.9–99.3%), respectively, compared with light microscopy. The sensitivity rose to 96.1% and 100% when evaluating for egg counts greater than five and 10 eggs per 10 mL, respectively. A point-of-care circulating cathodic anion (POC CCA) test was used to evaluate Schistosoma mansoni; however, there were too few positive samples to reliably comment on diagnostic characteristics. This study demonstrated that a “urine-only” approach to rapidly screen for schistosomiasis at the point of sample collection can be conducted with mobile phone microscopy (S. haematobium) coupled with POC CCA (S. mansoni). Such an approach may aid in streamlined schistosomiasis control and elimination initiatives.
INTRODUCTION
Schistosomiasis infects approximately 200 million people globally, with the majority of infections occurring in African countries.1 Chronic infection with schistosomiasis leads to considerable morbidity impacting the gastrointestinal and genitourinary systems,2 including bladder cancer, pelvic pain, infertility, and complications of portal hypertension.3 The WHO recommends preventive chemotherapy via mass drug administration (MDA) campaigns, which involve empiric treatment of entire communities when the prevalence of infection is greater than 10%.4 Mass drug administration campaigns aim to reduce morbidity from schistosomiasis with the ultimate goal of parasite control and elimination.4
In 2022, the WHO highlighted the need for effective surveillance tools to monitor and evaluate schistosomiasis control programs. Such tools should be able to identify when transmission has been interrupted or when MDA requirements are met.5 Mobile phone microscopy is an emerging and promising screening tool for schistosomiasis and other neglected tropical diseases. Mobile phone microscopes are portable, handheld, battery-powered devices that can be used to rapidly identify infection at the point of sample collection. They have been successfully used to screen for Schistosoma haematobium6–8 using only 10 mL of urine. Combining mobile phone microscopy with point-of-care circulating cathodic antigen (POC CCA), a test that evaluates for Schistosoma mansoni infection with only a few drops of urine,9–11 may facilitate more rapid screening for both genitourinary schistosomiasis (S. haematobium) and gastrointestinal schistosomiasis (S. mansoni) in African settings. This proposed method requires only 10 mL of urine and bypasses the time-consuming, more expensive, and cumbersome process of stool collection and analysis. We evaluated the combination of mobile phone microscopy and POC CCA for S. haematobium and S. mansoni screening in central Côte d’Ivoire.
MATERIALS AND METHODS
This cross-sectional study was conducted in Koubi village near the Tiébissou district between November 7, 2021 and December 20, 2021. Ethical permission was granted by both the local health district officer, from the Comité National d’Éthique des Sciences de la Vie et de la Santé, Abidjan, Côte d’Ivoire (REB #186-21) and the University Health Network, Toronto, Canada (REB #21-5582). Community members over 5 years old were asked to participate in this study and provide one stool and one urine sample. Adults provided written consent, and children were included if they assented and had written consent from a parent or guardian. Schistosomiasis infections that were identified by field gold standard microscopy were treated with praziquantel (40 mg/kg).
Urine was collected between 10:00 and 14:00 in sterile containers and processed on the day of collection. Urine containers were first shaken, and then 10 mL was extracted for evaluation by the mobile phone microscope, 10 mL was extracted for conventional microscopy, and several drops were extracted for POC CCA testing using a commercially available tool (Rapid Medical Diagnostics, Pretoria, Republic of South Africa). For conventional light microscopy analysis, the 10 mL of urine was pressed through filter paper with 20-µm pores (Sefar AG, Heiden, Switzerland) with a syringe. The filter paper was then transferred to a standard glass microscope slide, and one drop of Lugol’s iodine was applied. The slide was then examined on-site by laboratory personnel with light microscopes under ×10 and ×20 magnification.12 The microscopists recorded the presence or absence of S. haematobium eggs and quantified the egg burden if eggs were present. Ten percent of the samples were reanalyzed by expert microscopists for quality control.
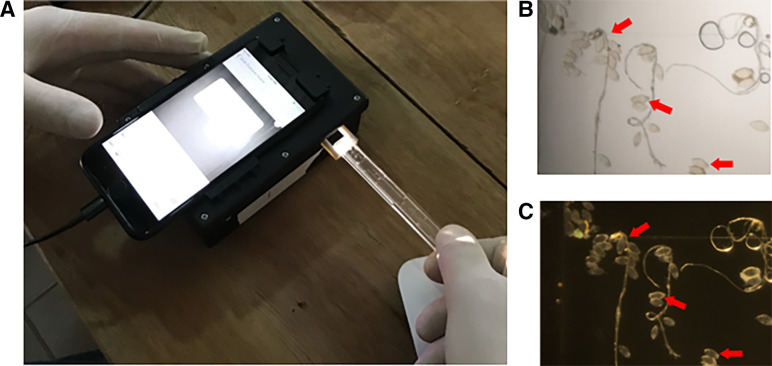
Simultaneous to this process, 10 mL of urine was evaluated using the mobile phone microscope (referred to as the “SchistoScope”). Urine was pressed via a syringe through a plastic capillary tube designed with a tapering structure to trap S. haematobium eggs (Figure 1). The capillary tube was then placed in the mobile phone microscope for imaging. This device is modified from the LoaScope13,14 and has an iPhone 8 (Apple, Cupertino, CA) nested in a custom-designed plastic docking unit that illuminates and magnifies images of the capillary tube. Brightfield and darkfield images of the capillary tube were captured with the iPhone camera and could be further magnified using the digital zoom (Figure 1). Microscopists were trained to use the device and had opportunities to practice prior to the onset of this study. Community-based field microscopists evaluated images of the urine capillaries captured by the mobile phone microscope and were blinded to results from conventional light microscopy. These same images captured on the mobile phone microscopes were also evaluated by expert microscopists who were blinded to results from both field microscopists and conventional microscopy. All microscopists documented the presence or absence of S. haematobium eggs and quantified the egg burden, if present.
Figure 1.
Mobile phone microscope imaging process. (A) Inserting a capillary tube with 10 mL of urine filtered into the mobile phone microscope (SchistoScope); resulting (B) brightfield and (C) darkfield images of the capillary tube–captured Schistosoma haematobium eggs present (red arrows).
The POC CCA tests (Lot #210811080) were processed per manufacturer’s instructions. A drop of urine was placed on the cassette followed by a drop of reagent. After 20 minutes, cassettes were evaluated for bands appearing on the test and/or control lines. Cassettes were reviewed by two people to determine if the test was positive or negative, and a third person adjudicated if there was any discrepancy.
Two Kato-Katz thick smears were made from a single stool sample using the 41.7-mg template per standardized protocols.15 Stool was examined under light microscopy at ×5 and ×20 lenses for S. mansoni and soil-transmitted helminth infections. The presence or absence of eggs was noted, reordered, and quantified if present.
All results were entered into a Microsoft Excel file (Microsoft, Redmond, WA) and analyzed using R, v. 4.1.3. We evaluated the accuracy of the SchistoScope performed by expert microscopists and field microscopists compared with light microscopy, reporting sensitivity and specificity with exact 95% binomial intervals. We further evaluated sensitivity when restricted to samples with ≥5 or ≥10 eggs/mL by conventional microscopy. We used Cohen’s κ to test the correlation between expert and field microscopists.
RESULTS
Four hundred fourteen individuals submitted a single urine specimen that was evaluated by POC CCA. Of these, 365 samples were also evaluated by conventional light microscopy and the SchistoScope. Of the 365 urine samples, 49 (13.4%) were positive for S. haematobium by conventional light microscopy, with 4 (8.2%) quantified as being a heavy burden infection with 50 or more eggs per 10 mL of urine.16 Compared with conventional light microscopy, the sensitivity and specificity of S. haematobium detection by field microscopists trained to use the SchistoScope was 26.5% (95% CI: 14.9–41.1%) and 98.4% (95% CI: 96.3–99.5%), respectively (Table 1). The sensitivity rose to 44% and 47.1% when evaluating for egg counts greater than 5 and 10 eggs per 10 mL, respectively, via conventional microscopy. Compared with conventional light microscopy, the sensitivity and specificity of S. haematobium detection by expert microscopists was 74% (95% CI: 59.7–85.4%) and 98.1% (95% CI: 95.9–99.3%), respectively (Table 1). The sensitivity rose to 96.1% and 100% when evaluating for egg counts greater than 5 and 10 eggs per 10 mL, respectively, via conventional microscopy. The κ coefficient comparing S. haematobium identification by field microscopists and expert microscopists using the SchistoScope was 0.46.
Table 1.
Sensitivity and specificity of the SchistoScope compared with conventional light microscopy in the identification of Schistosoma haematobium eggs by field microscopists and expert microscopists
| Sensitivity and Specificity | Field Microscopists | Expert Microscopists |
|---|---|---|
| Overall Specificity (95% CI) | 98.4% (96.3–99.5%) | 98.1% (95.9–99.3%) |
| Overall Sensitivity (95% CI) | 26.5% (14.9–41.1%) | 74.0% (59.7–85.4%) |
| Sensitivity by Egg Count | ||
| Samples with >5 Eggs per 10 mL | 44.0% | 96.1% |
| Samples with >10 Eggs per 10 mL | 47.0% | 100% |
In all, 382 individual stool samples were collected with two Kato-Katz thick smears processed from each sample. Three individuals (0.8%) had evidence of S. mansoni by light microscopy, and all infections were deemed to be low intensity.16 Four hundred fifteen POC CCA tests were conducted with two (0.5%) positive tests. Only one of the two participants with a positive POC CCA test had Kato-Katz studies performed, and this was negative for S. mansoni.
DISCUSSION
Handheld digital microscopy combined with POC CCA has significant potential to facilitate the monitoring of existing WHO-endorsed schistosomiasis control and elimination programs.
Mobile phone and portable microscopes have many applications, including several recent field studies screening for schistosomiasis, malaria, Loa, and other infections of public health significance.7,17,18 Over the past decade, iterative advancements in mobile phone and handheld microscopes have significantly enhanced their diagnostic capabilities, such that there is now markedly improved sample processing and analytic capability.8,19 The WHO has outlined schistosomiasis control and elimination efforts as a major priority and called for innovations to monitor ongoing control programs.5 Combining mobile phone microscopy with POC CCA screening appears to be a potential pragmatic solution; this process requires only 10 mL of urine per person and is a less expensive and more rapid method of evaluating larger cohorts given that it does not require stool examination. Specimens can be rapidly processed at the point of sample collection, enabling a timely and actionable response, such as making the decision to treat individuals or whole communities based on emerging data in real time.
A high-burden S. haematobium infection is defined as having more than 50 eggs per milliliter of urine.16 When a 10-mL urine sample was examined, the sensitivity of mobile phone microscopy for S. haematobium for expert microscopists exceeded 96% when five or more eggs were present. However, in cases of very low egg counts (e.g., one or two eggs per 10-mL sample), there is a reasonable chance that a divided urine sample may contain an uneven number of eggs per 10 mL extracted. For instance, a 10-mL sample evaluated by conventional microscopy may reveal one or two eggs, which are correctly identified as true positives, whereas a 10-mL sample extracted from the same shaken urine container and evaluated by mobile phone microscopy may show no eggs, resulting in a true negative sample; however, this negative result would be mistakenly labeled as a false-negative compared with conventional microscopy. As a result, the overall sensitivity of mobile phone microscopy is likely underestimated here.
A current key issue with handheld digital microscopy is consistent and accurate identification of a pathogen. In mobile phone microscopy, even though images of urine capillaries are digitized, manual detection of the pathogen present on the image has been the primary method of identification in most field studies to date. This study demonstrated considerable differences in the sensitivity of manual detection of S. haematobium eggs when comparing field microscopists to expert microscopists with significantly more experience using mobile phone microscopes. Although field microscopists were very adept in accurately identifying S. haematobium with conventional light microscopy, there was a marked reduction in sensitivity when using mobile phone microscopy. This may be due to inadequate training or less experience using the devices. A better approach to ensure consistency and diagnostic accuracy is to incorporate artificial intelligence, computer vision, and machine learning algorithms into mobile phone microscopy to automate pathogen detection and quantification. Our team has successfully identified S. haematobium eggs with this approach,13 and we are currently adapting this technology and integrating it into devices capable of processing samples for schistosomiasis, soil-transmitted helminths, Loa, and a growing number of neglected tropical diseases.
Lastly, there is recent concern that the POC CCA test may have some inconsistent sensitivity between batches, particularly in regions with lower S. mansoni prevalence.20 It is unclear what the future utility of POC CCA testing will be if these sensitivity issues are real and are not addressed; however, there are other promising urine-based diagnostics for field evaluation of S. mansoni that are in development, such as the up-converting phosphor lateral flow circulating anodic antigen (UCP-LF CAA) assay.11
Combining handheld automated microscopes for S. haematobium screening with POC CCA for S. mansoni screening is a promising method to evaluate the community burden of schistosomiasis. This combined, urine-only approach could help rapidly and inexpensively monitor schistosomiasis control and elimination programs.
REFERENCES
- 1. King CH, Galvani AP, 2018. Underestimation of the global burden of schistosomiasis. Lancet 391: 307–308. [DOI] [PubMed] [Google Scholar]
- 2. Colley DG, Bustinduy AL, Secor WE, King CH, 2014. Human schistosomiasis. Lancet 383: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warren KS, 1978. The pathology, pathobiology and pathogenesis of schistosomiasis. Nature 5664: 609–612. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization , 2022. WHO Guideline on Control and Elimination of Human Schistosomiasis. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 5. World Health Organization , 2021. Diagnostic Target Product Profiles for Monitoring, Evaluation and Surveillance of Schistosomiasis Control Programmes. Geneva, Switzerland: WHO. [Google Scholar]
- 6. Coulibaly JT, Ouattara M, D’Ambrosio MV, Fletcher DA, Keiser J, Utzinger J, N’Goran EK, Andrews JR, Bogoch II, 2016. Accuracy of mobile phone and handheld light microscopy for the diagnosis of schistosomiasis and intestinal protozoa infections in Côte d’Ivoire. PLoS Negl Trop Dis 10: e0004768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasiman A, Stothard JR, Bogoch II, 2019. Mobile phone devices and handheld microscopes as diagnostic platforms for malaria and neglected tropical diseases (NTDs) in low-resource settings: A systematic review, historical perspective and future outlook. Adv Parasitol 103: 151–173. [DOI] [PubMed] [Google Scholar]
- 8. Coulibaly JT, Silue KD, Armstrong M, de León Derby MD, D’Ambrosio MV, Fletcher DA, Keiser J, Fisher K, Andrews JR, Bogoch II, 2023. High sensitivity of mobile phone microscopy screening for Schistosoma haematobium in Azaguié, Côte d’Ivoire. Am J Trop Med Hyg 108: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coulibaly JT, N’Gbesso YK, Knopp S, N’Guessan NA, Silué KD, van Dam GJ, N’Goran EK, Utzinger J, 2013. Accuracy of urine circulating cathodic antigen test for the diagnosis of Schistosoma mansoni in preschool-aged children before and after treatment. PLoS Negl Trop Dis 7: e2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assaré RK, Tra MBI, Ouattara M, Hürlimann E, Coulibaly JT, N’Goran EK, Utzinger J, 2018. Sensitivity of the point-of-care circulating cathodic antigen urine cassette test for diagnosis of Schistosoma mansoni in low-endemicity settings in Côte d’Ivoire. Am J Trop Med Hyg 99: 1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Assare RK, Tra-Bi MI, Coulibaly JT, Corstjens PLAM, Ouattara M, Hurlimann E, van Dam GJ, Utzinger J, N’Goran EK, 2021. Accuracy of two circulating antigen tests for the diagnosis and surveillance of Schistosoma mansoni infection in low-endemicity settings of Côte d’Ivoire. Am J Trop Med Hyg 105: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters PA, Mahmoud AAF, Warren KS, Ouma JH, Siongok TK, 1976. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ 54: 159–162. [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong M, Harris AR, D’Ambrosio MV, Coulibaly JT, Essien-Baidoo S, Ephraim RKD, Andrews JR, Bogoch II, Fletcher DA, 2022. Point-of-care sample preparation and automated quantitative detection of Schistosoma haematobium using mobile phone microscopy. Am J Trop Med Hyg 106: 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Ambrosio MV. et al. , 2015. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med 7: 286re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katz N, Chaves A, Pellegrino J, 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop São Paulo 14: 397–400. [PubMed] [Google Scholar]
- 16. World Health Organization , 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 912: i–vi, 1–57. [PubMed] [Google Scholar]
- 17. Rajchgot J, Coulibaly JT, Keiser J, Utzinger J, Lo NC, Mondry MK, Andrews JR, Bogoch II, 2017. Mobile-phone and handheld microscopy for neglected tropical disease. PLoS Negl Trop Dis 11: e0005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamgno J. et al. , 2017. A test-and-not-treat strategy for onchocerciasis in Loa –endemic areas. N Engl J Med 377: 2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bogoch II, Andrews JR, Speich B, Utzinger J, Ame SM, Ali SM, Keiser J, 2013. Short report: Mobile phone microscopy for the diagnosis of soil-transmitted helminth infections: A proof-of-concept study. Am J Trop Med Hyg 88: 626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peralta JM, Cavalcanti MG, 2018. Is POC-CCA a truly reliable test for schistosomiasis diagnosis in low endemic areas? The trace results controversy. PLoS Negl Trop Dis 12: e0006813. [DOI] [PMC free article] [PubMed] [Google Scholar]