Abstract
The herpesvirus saimiri open reading frame (ORF) 57 is homologous to genes identified in all classes of herpesviruses. It has previously been shown to regulate gene expression through a posttranscriptional mechanism. We demonstrate in this report that the expression of the ORF 57 protein leads to the cytoplasmic accumulation of glycoprotein B and capsid mRNAs. We also demonstrate that ORF 57 has the ability to specifically bind viral RNA transcripts. Utilizing an interspecies heterokaryon assay, we show that ORF 57 has the ability to shuttle between the nucleus and the cytoplasm. Furthermore, we show that ORF 57 contains a relatively leucine-rich sequence which shares some homology with nuclear export signals (NES) found in a number of proteins with the ability to shuttle between the nucleus and the cytoplasm. Moreover, we demonstrate that the ORF 57 NES enables the nuclear export of a heterologous protein and that mutation of the conserved leucine residues contained within the ORF 57 NES signal abrogates the ability of the ORF 57 protein to shuttle between the nucleus and cytoplasm. These results suggest that ORF 57 is involved in mediating the nuclear export of viral transcripts.
Herpesvirus saimiri (HVS) is a lymphotrophic herpesvirus of squirrel monkeys (Saimiri sciureus), which persistently infects its natural host asymptomatically but which can cause fatal T-cell lymphomas and lymphoproliferative diseases in other species of New World primates (9). Analysis of the HVS (strain A11) genome indicates that it shares significant homology, at a colinear level, with the gammaherpesviruses, including Epstein-Barr virus (EBV), Kaposi’s sarcoma-associated herpesvirus, and murine gammaherpesvirus 68 (1, 28, 34). Gene expression during the HVS lytic replication cycle is regulated by the products of the two major transcriptional regulating genes containing the open reading frames (ORFs) 50 and 57 (22, 23, 38). ORF 50 produces two gene products which are homologous to the EBV BRLF1 protein (Rta protein) (22, 36) and function as sequence-specific transactivators (35). ORF 57 is homologous to genes identified in all classes of herpesviruses, including the EBV Mta protein transactivator encoded by BMLF1 and IE63 or ICP27 of herpes simplex virus type 1 (HSV-1) (1, 15, 22).
The ORF 57 gene product is a 52-kDa multifunctional protein. We have previously shown that transactivation of late viral genes by ORF 57 occurs at a posttranscriptional level, whereas repression of gene expression appears to correlate with the presence of introns (37, 38). In addition, ORF 57 is responsible for the redistribution of the U2 and SC-35 splicing factors during an HVS infection (6). This suggests that ORF 57 plays a role in RNA processing and is functionally homologous to the more widely studied HSV-1 IE63 protein (30, 37). In addition to the above properties, the more widely characterized IE63 protein also contributes to the shutoff of host cell protein synthesis and to a decrease in cellular mRNA levels during infection. Infections utilizing IE63 deletion mutants result in increased levels of cellular protein synthesis and mRNA levels compared to wild-type infections (11, 12).
Analysis of IE63 has shown that it contains many functional domains, including an RGG box required for RNA binding (19), an N-terminal nuclear localization signal (NLS) (13, 18), and C-terminal transactivation and repression domains (31). Recent analysis has shown it also expresses a leucine-rich nuclear export signal (NES) that is homologous to sequences found in a number of shuttle proteins, including human immunodeficiency virus type 1 (HIV-1) Rev (14, 21). Further analysis of IE63 has shown that it has the ability to shuttle between the nucleus and the cytoplasm (20, 25), thus promoting the nuclear export of HSV-1 intronless RNAs (29, 33).
In this report, we further investigate the role of the HVS ORF 57 protein. We demonstrate that the ORF 57 protein is required for the cytoplasmic accumulation of virus mRNA. In addition, we show that ORF 57 has the ability to shuttle between the nucleus and the cytoplasm. Furthermore, we demonstrate that ORF 57 encodes an NES which enables the rapid nuclear export of a heterologous protein. These results suggest that ORF 57 plays a role in mediating the nuclear export of viral transcripts.
ORF 57 increases cytoplasmic transport of viral mRNA.
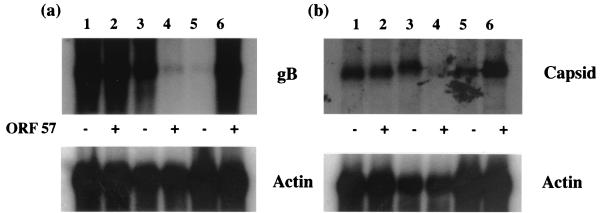
It has been previously shown that ORF 57 transactivates a range of HVS promoters, including glycoprotein B (gB) and capsid, but does not significantly alter the level of mRNA, suggesting that ORF 57 acts via a posttranscriptional mechanism (37). In order to determine if ORF 57 affects the nuclear cytoplasmic transport of viral mRNAs, Northern blot analysis was performed. To assess the effect of ORF 57 on gB mRNA, a transfer vector containing the full-length coding region and the promoter of gB was constructed. This region was PCR amplified using the primers 5′-GCG GGA TCC GTT ACA TGA TGC GCA TGC TAG and 5′-GCG GGA TCC CCA TGT CAA GAC AGC AAC TC. These oligonucleotides incorporated BamHI restriction sites for convenient cloning of the PCR product. This fragment was inserted into the polylinker region of pUC18 to derive pUCgB. In addition, to assess the effect of ORF 57 on capsid mRNA a transfer vector, pEcoB, containing the full-length coding region and promoter of the capsid gene was also utilized (16). Total, nuclear, and cytoplasmic RNA was then isolated separately from Cos-7 cells transfected with pUCgB or pEcoB in the absence or the presence of pRSVORF57, a eukaryotic expression vector expressing the ORF 57 coding region (37). The RNA was then separated by electrophoresis on a 1% denaturing formaldehyde agarose gel, transferred to Hybond-N membranes, and hybridized with 32P-radiolabelled random primed probes specific for the HVS gB or the capsid coding sequences (Fig. 1). The results of the Northern blot analysis suggest that ORF 57 does not affect the quantities of gB or capsid transcripts but is required for the efficient cytoplasmic accumulation of gB and capsid transcripts.
FIG. 1.
ORF 57 increases cytoplasmic transport of viral mRNA. Cos-7 cells transfected with pUCgB (a) and pEcoB (b) in the absence or presence of pRSVORF57. Total (lanes 1 and 2), nuclear (lanes 3 and 4), and cytoplasmic (lanes 5 and 6) RNA was then isolated and separated by elecrophoresis on a 1% denaturing formaldehyde agarose gel. The RNA was transferred to Hybond-N membranes and hybridized with 32P-radiolabelled randomly primed probes specific for the HVS gB and the capsid coding sequence.
The ORF 57 protein binds viral mRNA.
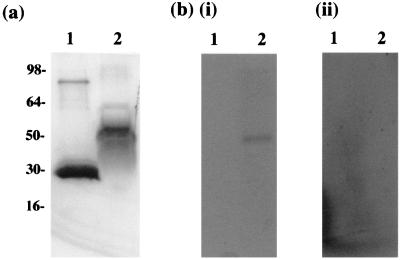
In order to determine whether ORF 57 binds viral RNA, Northwestern analysis was performed. Recombinant ORF 57 protein was produced and purified as an amino-terminal glutathione S-transferase (GST) fusion protein. A PCR product of the coding region of ORF 57 containing 78,394 to 79,684 bp of the previously published sequence (1) was generated by PCR, using the primers 5′-CGC GGA TCC GGA ATT TCA TCA GAT GAT GAC and 5′-GCG GGA TCC CTG AGT AGG TAA GAA AAA CAG CCC; these oligonucleotides incorporated BamHI restriction sites to facilitate subcloning into the expression vector, pGEX-2T (Pharmacia Biotech), thus deriving pGEX57. The ORF 57 fragment was expressed as a GST fusion protein in Escherichia coli DH5α and purified from the crude lysate by incubation with glutathione-Sepharose 4B affinity beads according to the manufacturer’s specification (Pharmacia Biotech). The molecular mass of the GST-ORF 57 fusion protein was predicted to be 81 kDa. However, analysis demonstrated that the expressed GST-ORF 57 protein produced was a stable breakdown product containing the amino-terminal portion of the protein of ca. 55 kDa (Fig. 2a). This smaller product may have been due to premature translational termination; however, DNA sequencing confirmed the integrity of the DNA sequence, further suggesting a stable breakdown product. In order to determine whether this expressed protein had the ability to bind RNA, the purified GST-ORF 57 recombinant protein and a control, GST alone, were separated on a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel and transferred onto nitrocellulose (Amersham). The nitrocellulose-bound proteins were denatured by the addition of 6 M guanidine-HCl in binding buffer (100 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM EDTA) for 10 min. The proteins were then renatured by using six 50% stepwise dilutions of the guanidine-HCl in binding buffer. The filter was rinsed in binding buffer and blocked by preincubation in 2% (wt/vol) nonfat milk powder–1 mM dithiothreitol for 2 h at 20°C. The filter-bound protein was incubated with 32P-radiolabelled RNA probes specific for HVS gB or actin. The gB RNA probe was synthesized by in vitro transcription of a PCR product containing a T7 transcription start site in the 5′ primer in the presence of [32P]UTP (Ambion). The gB coding region was amplified with primers 5′-TAA TAC GAC TCA CTA TAG GGA ATG GTA CCT AAT AAA CAC TTA CTG and 5′-TGA ACT GCA CAG ATC CTA TAT. The actin probe was generated using the control vector, pTriplescript, according to the manufacturer’s directions (Maxiscript Kit; Ambion). The labelled probes were hybridized with the filter-bound ORF 57 protein for 16 h at 20°C. The results demonstrate that the amino-terminal portion of ORF 57 can specifically bind the viral gB RNA transcripts but did not interact with the control cellular RNA (Fig. 2b).
FIG. 2.
The ORF 57 protein binds viral mRNA. (a) Coomassie blue-stained gel of GST (lane 1) and GST-ORF 57 (lane 2) proteins expressed in E. coli DH5α and purified from the crude lysate by incubation with glutathione-Sepharose 4B beads according to manufacturer’s specifications. (b) RNA-binding assay of the ORF 57 protein. GST and GST-ORF 57 proteins were separated on an SDS–12% polyacrylamide gel and transferred onto nitrocellulose (Amersham). The nitrocellulose bound proteins were denatured by using 6 M guanidine-HCl and then renatured by using six 50% stepwise dilutions of the guanidine-HCl in binding buffer. The filter-bound ORF 57 protein was then hybridized with 32P-radiolabelled RNA probes specific for HVS gB (i) and actin (ii).
The ORF 57 protein shuttles between the nucleus and cytoplasm.
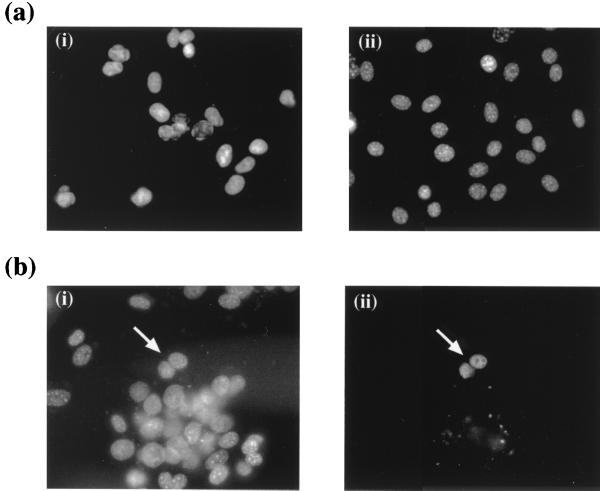
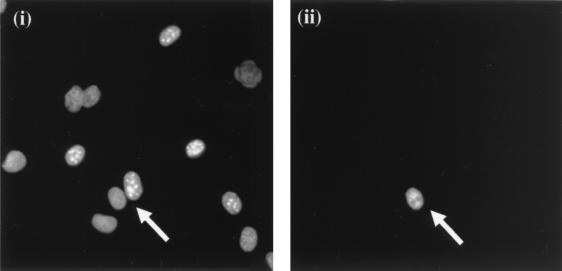
In order to determine whether the ORF 57 protein shuttles between the nucleus and cytoplasm, an interspecies heterokaryon assay was utilized (3, 20, 26). Cos-7 cells seeded at 2 × 105 cells per 35-mm-diameter petri dish were transiently transfected with 2 μg of pRSVORF57. After 18 h, mouse 3T3 cells (5 × 105 cells/well) were plated onto the Cos-7 cells in medium containing 50 μg of cycloheximide per ml. Four hours later the cells were washed in phosphate-buffered saline (PBS) and fused by the addition of 2 ml of 50% polyethylene glycol (wt/wt) in PBS. After 2 min the cells were washed extensively in PBS. After this washing, the cells were returned to medium containing 50 μg of cycloheximide per ml for 60 min. Cells were then fixed with 4% formaldehyde in PBS, washed in PBS three times, and permeabilized in 0.5% Triton X-100 for 5 min. The cells were rinsed in PBS and blocked by preincubation with 1% (wt/vol) nonfat milk powder for 1 h at 37°C. A 1:100 dilution of ORF 57 antibody (27) was layered over the cells and incubated for 1 h at 37°C. Fluorescence-conjugated anti-mouse immunoglobulin (Dako) at a 1:50 dilution and a costain of 0.5 μg of Hoechst dye (Sigma) per ml were added for 1 h at 37°C. After each incubation step, cells were washed extensively with PBS. Hoechst dye allowed the differentiation between monkey and mouse nuclei. As previously reported (20), monkey cells stained diffusely throughout the nuclei, whereas mouse nuclei stained with a distinctive speckled pattern (Fig. 3a). The immune fluorescence slides were observed by using a Zeiss Axiovert 135TV inverted microscope with a Neofluar 40× oil immersion lens. Analysis of the interspecies heterokaryons demonstrated that ORF 57 was expressed in both monkey and mouse cell nuclei. This indicates that ORF 57 in transfected cells is able to shuttle between the nucleus and cytoplasm (Fig. 3b).
FIG. 3.
(a) Hoechst dye staining of monkey and mouse cell nuclei. Hoechst dye allowed differentiation between monkey (i) and mouse (ii) nuclei. Monkey cells stained diffusely throughout the nuclei, whereas mouse nuclei stained with a distinctive speckled pattern. (b) The ORF 57 protein shuttles between the nucleus and cytoplasm. Cos-7 cells seeded at 2 × 105 cells per 35-mm-diameter petri dish were transiently transfected with 2 μg of pRSVORF57. After 18 h, mouse 3T3 cells (5 × 105 cells/well) were plated onto the Cos-7 cells in medium containing 50 μg of cycloheximide per ml. Four hours later the cells were washed in PBS and fused by the addition of 2 ml of 50% polyethylene glycol (wt/wt) in PBS. After being washed, the cells were returned to medium containing 50 μg of cycloheximide per ml for 60 min. Cells were incubated with a 1:100 dilution of ORF 57 antibody and then costained with 0.5 μg of Hoechst dye (i) and fluorescein-conjugated anti-mouse immunoglobulin (ii) per ml.
The ORF 57 gene product expresses an NES.
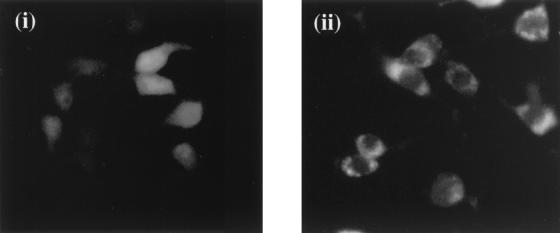
In order for a protein to shuttle between the nucleus and cytoplasm it requires both an NLS and an NES. An increasing number of viral proteins, including HIV-1 Rev, human T-cell lymphotropic virus type 1 Rex, HSV-1 IE63, and EBV Mta, have been found to contain a leucine-rich NES sequence enabling the protein to shuttle between the nucleus and cytoplasm (2, 14, 20, 21, 25, 29, 33). Analysis of the ORF 57 coding region identified a relatively leucine-rich region containing a sequence of amino acids resembling the consensus NES sequence. In order to determine whether the putative NES signal (ILPKSGEPKLFL) expressed by ORF 57 enables nuclear export of a heterologous protein, the sequence was fused with the green fluorescent protein (GFP). Synthetic oligonucleotides encoding the putative ORF 57 NES (nucleotides 78835 to 78870 of the published sequence) were synthesized. These oligonucleotides incorporated XhoI and BamHI restriction sites for convenient cloning. The oligonucleotides were annealed and ligated with pEGFP-C1 (Clontech) to create an in-frame carboxy-terminal fusion of the NES sequence and GFP, yielding pEGFP-57NES. Cos-7 cell monolayers were transfected with 2 μg of either pEGFP-C1 or pEGFP-57NES, and the subcellular localization of GFP was observed by fluorescence microscopy. Results showed that cells transfected with pEGFP-C1 displayed a fluorescence pattern throughout the cell in both the nucleus and cytoplasm. However, the fluorescence pattern observed in pEGFP-57NES-transfected cells was confined to the cytoplasm (Fig. 4), suggesting that the ORF 57-expressed NES is sufficient to direct the fusion protein from the nucleus to the cytoplasm.
FIG. 4.
The ORF 57 gene product expresses a nuclear export signal. Cos-7 cell monolayers were transfected with 2 μg of either pEGFP-C1 (i) or pEGFP-57NES (ii). After 24 h, the subcellular localization of GFP was observed by using fluorescence microscopy.
The NES signal is required for ORF 57 to shuttle between the nucleus and cytoplasm.
To further demonstrate that the NES sequence enables the ORF 57 protein to shuttle between the nucleus and the cytoplasm, site-directed mutagenesis of the NES was performed. The ORF 57-NES mutant was generated by a PCR-based method which incorporated the alteration of the conserved leucine residues at bp 78863 and 78869 of the published sequence. Two PCR products of the ORF 57 coding region containing the sequences bp 78291 to 78872 and bp 78872 to 79624 of the published sequence were generated by using the primers NES1 (5′-CCC AAG CTT AAC TGC CCA ATT GGA AGA TAT AAT TG), NES2 (5′-CCC CCG CGG GCT TTT GGC TCT CCA GAT TTA GGC AA), NES3 (5′-CGC CCG CGG GCC TGT ACC TTC GTT GCC TTG CCA A), and NES4 (5′-CCG CTC GAG CTG AGT AGG TAA GAA AAA CAG CCC TGT). Primers NES1 and NES4 contained HindIII and XhoI restriction sites to facilitate the subcloning into the expression vector pcDNA3.1 (Invitrogen), whereas NES2 and NES3 contained SstII restriction sites to facilitate ligation of the PCR products but also mutated the two conserved leucine residues contained in the ORF 57-NES sequence. The PCR products were ligated with pcDNA3.1 to derive the ORF 57-NES mutant, p57-NES. DNA sequencing was performed to confirm the mutation of the specified residues (data not shown). In order to assess the effect of the NES mutation on the ability of ORF 57 to shuttle between the nucleus and the cytoplasm, a heterokaryon assay was performed using p57-NES as previously described (Fig. 5). Analysis of heterokaryons which contained the mutated ORF 57 demonstrated that this protein was only expressed in the monkey nuclei. This indicates that mutation of the conserved leucine residues contained within the NES sequence abrogated the ability of the ORF 57 protein to shuttle between the nucleus and the cytoplasm.
FIG. 5.
Mutation of the ORF 57 NES abrogated the ability of the ORF 57 protein to shuttle between the nucleus and cytoplasm. Cos-7 cells seeded at 2 × 105 cells per 35-mm-diameter petri dish were transiently transfected with 2 μg of p57-NES. After 18 h, mouse 3T3 cells (5 × 105 cells/well) were plated onto the Cos-7 cells in medium containing 50 μg of cycloheximide per ml. Four hours later the cells were washed in PBS and fused by the addition of 2 ml of 50% polyethylene glycol (wt/wt) in PBS. After being washed, the cells were returned to medium containing 50 μg of cycloheximide per ml for 60 min. Cells were then incubated with a 1:100 dilution of ORF 57 antibody and costained with 0.5 μg of Hoechst dye (i) and fluorescein-conjugated anti-mouse immunoglobulin (ii) per ml.
We have previously demonstrated that the ORF 57 gene product transactivates a range of HVS promoters but does not significantly alter the level of mRNA, suggesting a posttranscriptional mechanism. In addition, the effect of ORF 57 is independent of either the promoter which drives transcription or the temporal class of this promoter. However, we were unable to determine the effect of ORF 57: whether it affects the mRNA processing, transport, or translational efficiency. In this report, we demonstrate that the ORF 57 protein is required for the cytoplasmic accumulation of virus mRNA. In addition, we show that ORF 57 can bind viral RNA and shuttle between the nucleus and cytoplasm. Furthermore, we demonstrate that ORF 57 expresses an NES which is required for ORF 57’s ability to shuttle between the nucleus and the cytoplasm and that it enables the rapid nuclear export of a heterologous protein. These results suggest that ORF 57 mediates the nuclear export of viral transcripts.
ORF 57 is a virus-encoded nuclear cytoplasmic shuttle protein, as are HIV-1 Rev, HTLV-1 Rex, HSV-1 IE63, EBV Mta, and adenovirus E4 34-kDa oncoprotein (2, 7, 14, 20, 21, 25, 29, 32, 33). Probably the best characterized of these proteins is HIV-1 Rev, which is involved in promoting the export of intron-containing mRNAs, which is mediated through the binding of the Rev protein to the Rev response elements (RRE) contained within the introns of these mRNAs (8). Moreover, HSV-1 IE63 selectively binds intronless RNAs, and export of these intronless RNAs is greatly reduced during IE63 mutant virus infections, suggesting that IE63 mediates the export of intronless RNAs (29, 33). All of these proteins contain leucine-rich NESs that enable rapid nuclear export. Similar sequences which function as NESs have been identified in a wide range of proteins, including PK1, transcription factor IIIA, the yeast protein Gle 1, and MDM2 (reviewed in reference 17). Recently, CRM1 (chromosomal region maintenance 1) or exportin 1, a protein that shares homology with members of the importin-karyopherin nuclear transport pathway, has been identified as a nuclear export receptor for proteins carrying a leucine-rich NES in a process that also requires the GTP-bound form of Ran (10, 24). Furthermore, exportin 1 has been shown to interact with nuclear pore complex proteins, namely, the nucleoporins CAN/Nup214 and Nup88 (24), suggesting that exportin 1 is the bridging protein for the interactions of NES-containing proteins and the nuclear pore complex. Recent analysis has demonstrated that exportin 1 mediates the function and intracelluar localization of the EBV Mta protein (4). It will be of interest to determine whether ORF 57 interacts with any components of this cellular pathway.
In addition, virally encoded nuclear cytoplasmic shuttle proteins have the ability to bind RNA. As previously mentioned, the HIV-1 Rev protein binds to the RRE contained within the introns of these mRNAs mediated by an arginine-rich region (8). Moreover, RNA binding by HSV-1 IE63 is mediated by an arginine- and glycine-rich sequence resembling an RGG box motif, a putative RNA-binding determinant found in a number of cellular nuclear proteins involved in mRNA and rRNA metabolism (19). However, ORF 57 and other IE63 homologues, including EBV Mta, do not contain a homologous arginine- and glycine-rich RGG box motif. Recent analysis of the EBV Mta protein has suggested two regions which may contain alternative RNA-binding determinants. The EBV Mta and ORF 57 both have relatively arginine-rich amino termini. RNA-binding analysis of an EBV Mta-GST fusion product suggested that this arginine-rich region in the amino terminus of Mta is likely to mediate RNA binding (32). In addition, EBV Mta also contains an Arg-X-Pro tripeptide repeat homologous to an RNA-binding determinant in the HSV-1 US11 protein. However, deletion mutants of Mta have demonstrated this was not required for RNA binding (5). Analysis of the ORF 57 coding region shows this protein does not express the Arg-X-Pro RNA-binding domain. Nevertheless, data described in this report, utilizing the GST-ORF 57 fusion protein, suggest that the amino terminus of ORF 57 contains the RNA-binding determinant. However, it cannot be excluded that the degraded carboxy-terminal portion of the ORF 57 protein may also include RNA-binding determinants. Therefore, further analysis is now required to identify the functional domain(s) contained within ORF 57 which are responsible for RNA binding.
Acknowledgments
This work was supported in part from grants from the Medical Research Council (MRC) and Yorkshire Cancer Research. A.W. and D.J.G. are recipients of an MRC fellowship and an MRC studentship, respectively.
We thank Rick Randall for providing the SB monoclonal antibody.
REFERENCES
- 1.Albrecht J C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittman S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogerd H P, Fridell R A, Benson R E, Hua J, Cullen B R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borer R A, Lehner C F, Eppenberger H M, Nigg E A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 4.Boyle S M, Ruvolo V, Gupta A K, Swaminathan S. Association with the cellular export receptor CRM 1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J Virol. 1999;73:6872–6881. doi: 10.1128/jvi.73.8.6872-6881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buisson M, Hans F, Kusters I, Duran N, Sergeant A. The C-terminal region but not the Arg-X-Pro repeat of Epstein-Barr virus protein EB2 is required for its effect on RNA splicing and transport. J Virol. 1999;73:4090–4100. doi: 10.1128/jvi.73.5.4090-4100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper M, Goodwin D J, Hall K T, Stevenson A J, Meredith D M, Markham A F, Whitehouse A. The gene product encoded by ORF 57 of herpesvirus saimiri regulates the redistribution of the splicing factor, SC-35. J Gen Virol. 1999;80:1311–1316. doi: 10.1099/0022-1317-80-5-1311. [DOI] [PubMed] [Google Scholar]
- 7.Dobblestein M, Roth J, Kimberly W T, Levine A J, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like sequence. EMBO J. 1997;16:4276–4282. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer U, Meyer S, Teufel M, Heckel C, Luhrmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Press; 1982. pp. 253–332. [Google Scholar]
- 10.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 11.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and the regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbard M K, Sandri-Goldin R M. Arginine-rich regions succeeding the nuclear localization region of the herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J Virol. 1995;69:4656–4667. doi: 10.1128/jvi.69.8.4656-4667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalland K-H, Szilvay A M, Brokstad K A, Sætrevik W, Haukenes G. The human immunodeficiency virus type 1 Rev protein shuttles between the cytoplasm and nuclear compartments. Mol Cell Biol. 1994;14:7436–7444. doi: 10.1128/mcb.14.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenney S, Holley-Guthrie E, Mar E-C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knust E, Schirm S, Dietrich W, Bodemer W, Kolb E, Fleckenstein B. Cloning of the herpesvirus saimiri DNA fragments representing the entire L-region of the genome. Gene. 1983;25:281–289. doi: 10.1016/0378-1119(83)90232-9. [DOI] [PubMed] [Google Scholar]
- 17.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 18.Mears W E, Lam V, Rice S A. Identification of nuclear and nucleolar localization signal sequences in the herpes simplex virus regulatory protein ICP27. J Virol. 1995;69:935–947. doi: 10.1128/jvi.69.2.935-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mears W E, Rice S A. The RGG box of the herpes simplex virus ICP27 protein mediates RNA binding activity and determines in vivo methylation. J Virol. 1996;70:7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mears W E, Rice S A. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242:128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- 21.Meyer B E, Malim M H. The HIV-1 rev transactivator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 22.Nicholas J, Coles L S, Newman C, Honess R W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991;65:2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholas J, Gompels U A, Craxton M A, Honess R W. Conservation of sequence and function between the product of the 52-kilodalton immediate-early gene of the herpesvirus saimiri and the BMLF1-encoded transcriptional effector (EB2) of the Epstein-Barr virus. J Virol. 1988;62:3250–3257. doi: 10.1128/jvi.62.9.3250-3257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:767–775. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 25.Phelan A, Clements J B. Herpes simplex virus type 1 immediate early protein IE63 shuttles between the nuclear compartments and the cytoplasm. J Gen Virol. 1997;78:3327–3331. doi: 10.1099/0022-1317-78-12-3327. [DOI] [PubMed] [Google Scholar]
- 26.Pinol-Roma S, Dreyfuss G. hnRNP proteins: localisation and transport between the nucleus and cytoplasm. Trends Cell Biol. 1993;3:151–155. doi: 10.1016/0962-8924(93)90135-n. [DOI] [PubMed] [Google Scholar]
- 27.Randall R E, Honess R W, O’Hare P. Proteins specified by herpesvirus saimiri: identification and properties in virus-specific polypeptides in productively infected cells. J Gen Virol. 1983;64:19–35. doi: 10.1099/0022-1317-64-1-19. [DOI] [PubMed] [Google Scholar]
- 28.Russo J J, Bohenzhy R A, Chein M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequences of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandri-Goldin R M. ICP27 mediates HSV nuclear export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;6:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 31.Sandri-Goldin R M, Hibbard M K, Hardwicke M A. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J Virol. 1995;69:6063–6076. doi: 10.1128/jvi.69.10.6063-6076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semmes O J, Chen L, Sarisky R T, Gao Z, Zhong L, Hayward S D. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene RNA. J Virol. 1998;72:9526–9534. doi: 10.1128/jvi.72.12.9526-9534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soliman T M, Sandri-Goldin R M, Silverstein S J. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Virgin H W, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitehouse A, Stevenson A J, Cooper M, Meredith D M. Identification of a cis-acting element within the herpesvirus saimiri ORF6 promoter that is responsive to the HVS.R transactivator. J Gen Virol. 1997;78:1411–1415. doi: 10.1099/0022-1317-78-6-1411. [DOI] [PubMed] [Google Scholar]
- 36.Whitehouse A, Carr I M, Griffiths J C, Meredith D M. The herpesvirus saimiri ORF 50 gene, encoding a major transcriptional activator homologous to the Epstein-Barr virus R protein, is transcribed from two distinct promoters of different temporal phases. J Virol. 1997;71:2550–2554. doi: 10.1128/jvi.71.3.2550-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehouse A, Cooper M, Meredith D M. The IE gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level. J Virol. 1998;72:857–861. doi: 10.1128/jvi.72.1.857-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehouse A, Cooper M, Hall K T, Meredith D M. The open reading frame (ORF) 50a gene product regulates ORF 57 gene expression in herpesvirus saimiri. J Virol. 1998;72:1967–1973. doi: 10.1128/jvi.72.3.1967-1973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]