Abstract
Background.
Tenofovir disoproxil fumarate (tenofovir) has been associated with renal dysfunction in people infected with human immunodeficiency virus (HIV) receiving combination antiretroviral therapy. We reviewed data from an HIV preexposure prophylaxis trial to determine if tenofovir use was associated with changes in renal function in an HIV-uninfected population.
Methods.
During the trial, 2413 HIV-uninfected people who inject drugs were randomized to receive tenofovir or placebo. We assessed the renal function of trial participants with the Cockcroft-Gault, Modification of Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations using t tests for cross-sectional analysis and linear regression for longitudinal analysis.
Results.
Creatinine clearance and glomerular filtration rate (GFR) results were lower at 24, 36, 48, and 60 months in the tenofovir group compared with the placebo group. Results declined more in the tenofovir group than in the placebo group during follow-up using the Cockcroft-Gault (P < .001) and CKD-EPI (P = .007) equations, but not MDRD (P = .12). Creatinine clearance measured when study drug was stopped was lower in the tenofovir group than the placebo group (P < .001), but the difference resolved when tested a median of 20 months later (P = .12).
Conclusions.
We found small but significant decreases in cross-sectional measures of creatinine clearance and GFR in the tenofovir group compared with the placebo group and modest differences in downward trends in longitudinal analysis using the Cockcroft-Gault and CKD-EPI equations. These results suggest that with baseline assessments of renal function and routine monitoring of creatinine clearance during follow-up, tenofovir can be used safely for HIV preexposure prophylaxis.
Clinical Trials Registration.
Keywords: creatinine clearance, glomerular filtration rate, tenofovir disoproxil fumarate
Tenofovir disoproxil fumarate (tenofovir), a nucleotide reverse transcriptase inhibitor used in the treatment of human immunodeficiency virus (HIV) infection [1–3], is excreted by the kidneys using a combination of glomerular filtration and active tubular secretion [4]. Tenofovir is structurally similar to the nucleotide analogues adefovir and cidofovir, and these drugs are associated with nephrotoxicity [5, 6]. Large randomized clinical trials among people infected with HIV on combination antiretroviral therapy have not shown a clear association between the use of tenofovir and renal dysfunction [3, 7, 8]. However, as use of tenofovir has increased, there have been reports of tenofovir-associated renal dysfunction including proximal tubular dysfunction, Fanconi syndrome, nephrogenic diabetes insipidus, and acute renal failure [9–12]. Several studies have also found tenofovir-associated decreases in creatinine clearance and/or glomerular filtration rate (GFR) [13–18], although a study in Thailand did not [19]. These changes in renal function are likely multifactorial and may be due, in part, to interactions with transport proteins in the proximal tubule [20, 21].
HIV preexposure prophylaxis trials have demonstrated that daily use of the combination antiretroviral tenofovir-emtricitabine can reduce HIV transmission among men who have sex with men [22] and heterosexual men and women [23], and that tenofovir and tenofovir-emtricitabine can reduce sexual transmission among heterosexual HIV-discordant partners [24]. We recently completed the Bangkok Tenofovir Study showing that daily tenofovir can reduce HIV transmission among people who inject drugs [25]. The World Health Organization and the US Centers for Disease Control and Prevention have published guidelines for the use of preexposure prophylaxis [26–29] and, based on the results of these trials, use of tenofovir is likely to expand to people at high risk of HIV infection.
Preexposure prophylaxis trials conducted among HIV-uninfected participants without preexisting renal impairment have found similar rates of creatinine elevation and renal-associated adverse events among participants randomized to tenofovir or tenofovir-emtricitabine and placebo [22–25, 30]. Nonetheless, given reports of tenofovir-associated renal dysfunction [9–12] and decreases in GFR [13–18], and recognizing that a higher threshold of safety may be demanded by people using tenofovir to prevent HIV infection than by those using tenofovir to treat HIV, we reviewed Bangkok Tenofovir Study data to determine if tenofovir use was associated with changes in renal function. Another preexposure prophylaxis trial, the iPrEx study [31], conducted among 2499 men and transgender women who have sex with men who contributed an average of 81 weeks of follow-up time, recently reported that once-daily tenofovir-emtricitabine was associated with a small but statistically significant decrease in creatinine clearance. The Bangkok Tenofovir Study provided an opportunity to assess the impact of tenofovir on the renal function of 2413 HIV-uninfected participants randomly assigned to receive daily tenofovir or placebo with up to 60 months of follow-up.
METHODS
The Bangkok Tenofovir Study, a randomized, double-blind, placebo-controlled trial, was conducted at 17 Bangkok Metropolitan Administration (BMA) drug treatment clinics in densely populated urban communities of Bangkok. People who were HIV-uninfected, reported injecting drugs in the previous year, had a creatinine clearance rate ≥60 mL/minute by the Cockcroft-Gault formula [32], and met other inclusion criteria [33] were eligible for the study. Volunteers meeting all eligibility criteria could enroll after providing written informed consent. We randomly assigned participants in a 1:1 ratio to receive daily oral tenofovir 300 mg or placebo.
Procedures
At enrollment and monthly (28 days) visits, participants were weighed, assessed for adverse events, and provided individualized adherence and risk-reduction counseling. Oral fluid was collected for HIV antibody testing (OraQuick Rapid HIV-1/2 Antibody Test, OraSure Technologies, Bethlehem, Pennsylvania). Participants chose daily directly observed therapy (DOT) or monthly visits without DOT and could switch at monthly visits. Adherence was assessed daily at DOT visits and monthly at non-DOT visits using a study drug diary. We collected blood for hematologic, hepatic, and renal safety assessment, including creatinine clearance, at enrollment; months 1, 2, and 3; and every 3 months thereafter. Urine was not collected for analysis.
Serum creatinine measurements were performed at the BMA Public Health Laboratory. Creatinine levels were determined by an enzymatic colorimetric assay based on the Jaffé alkaline picrate reaction, using an automated bioanalyzer (Modular P800, Roche Diagnostics, Indianapolis, Indiana) calibrated using control samples standardized by isotope-dilution mass spectrometry (Roche Diagnostics Traceability and Uncertainty, catalog number 10759350190). Negative and positive controls were performed prior to each run. We graded serum creatinine results using a modified National Institutes of Health, Division of AIDS Table for Grading the Severity of Adverse Events [25].
Participants with grade 1 results (≥0.5 mg/dL increase in serum creatinine from baseline) were allowed to continue study drug, and creatinine results were monitored as clinically indicated (weekly in most cases) until serum creatinine value declined to <0.5 mg/dL above baseline. Participants with grade 2 (2.1–3.0 mg/dL), grade 3 (3.1–6.0 mg/dL), and grade 4 (>6.0 mg/dL) results permanently discontinued study drug and were monitored as clinically indicated (weekly in most cases) until serum creatinine value declined to <0.5 mg/dL above baseline. Study drug (placebo or tenofovir) dose was adjusted based on creatinine clearance measured using the Cockcroft-Gault equation [32] according to manufacturer guidelines [34].
Several formulas have been developed to estimate creatinine clearance and GFR. We used the Cockcroft-Gault formula [32] to determine participant eligibility and monitor renal function. We also used the 4-variable Modification of Diet in Renal Disease (MDRD) equation [35] that was developed to provide a more accurate estimate of GFR among people with kidney disease, the MDRD equation modified for Thai adults (ie, multiplying the MDRD result by 1.129) [36], and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation that was developed to provide a more accurate estimate of GFR, particularly when GFR is >60 mL/minute/1.73 m2, to assess renal function [37]. Renal function declines in most people with age due to vascular changes and the development of age-associated glomerulosclerosis [38]. The creatinine clearance and GFR formulas account for this by including age in the equations.
Statistical Analyses
We used a 2-sample t test to determine if there was a difference in cross-sectional mean estimates of creatinine clearance between participants in the tenofovir and placebo groups at enrollment and 12-monthly visits through month 60 using the Cockcroft-Gault formula, and GFR using the MDRD and CKD-EPI formulas. We used marginal longitudinal linear regression to determine if there was a difference in mean creatinine clearance results in tenofovir and placebo groups and if the difference changed over time, and to determine if there was a difference in creatinine clearance results in demographic and risk subgroups [39]. The time trend in creatinine clearance and GFR results was assessed using a Lowess scatterplot smoother [40]. We compared graded creatinine results by group using a Poisson model with robust standard error.
To determine if changes in creatinine clearance among participants taking tenofovir were reversible, we examined creatinine clearance results of 749 study participants who opted to take daily tenofovir once trial results were announced using a paired t test. Participants had been off study drug (ie, tenofovir or placebo) for an average of 23 months and blood was collected before participants started tenofovir to calculate posttrial creatinine clearance.
Ethical Review
The study protocol, consent, and other materials were approved by the BMA and Thailand Ministry of Public Health ethical review committees and the institutional review board of the US Centers for Disease Control and Prevention. An independent data and safety monitoring board conducted annual safety reviews and 1 interim efficacy review. We used SAS software version 9.3 (SAS Institute, Cary, North Carolina) for statistical analyses.
RESULTS
We have described trial results in previous publications [25, 33]. In brief, from June 2005 through July 2010, we screened 4094 volunteers; 2413 (59%) were deemed eligible and enrolled. A total of 1204 participants were randomly assigned to receive tenofovir, contributing 4843 person-years of follow-up time; 1209 were randomly assigned to receive placebo, contributing 4823 person-years of follow-up time. Their median age was 31 years (mean, 32.4 years; range, 20–59) and 1924 (79.7%) were men. Based on study drug diaries, participants took study drug an average (mean) of 83.8% of days (median, 94.1%; interquartile range [IQR], 79.2%–98.7%), and adherence did not differ by treatment group (P = .16) or by time on study (P = .22). Fifty participants became infected with HIV during follow-up: 17 in the tenofovir group and 33 in the placebo group, indicating a 48.9% reduction in the HIV incidence (95% confidence interval [CI], 9.6–72.2; P = .01) among participants randomized to tenofovir.
The frequency of deaths, serious adverse events, grade 3 and 4 laboratory results, and elevated creatinine results was similar in each group [25]. A total of 65 participants had grade 1 creatinine results: 37 (3.1%) in the tenofovir group, 28 (2.3%) in the placebo group (P = .27). Details of participants with grade 2–4 creatinine results are provided in Table 1. Two (<0.5%) participants in the tenofovir group and none in the placebo group had grade 2 creatinine results (P = .25). Six participants had grade 3 or 4 results: 3 in the tenofovir group, 1 of whom also had a grade 2 result, and 3 in the placebo group (P = .99). A total of 71 (2.9%) participants were found to have a creatinine clearance (Cockcroft-Gault) rate <50 mL/minute during study follow-up: 26 (2.2%) in the placebo group and 45 (3.7%) in the tenofovir group (P = .01).
Table 1.
Details of Bangkok Tenofovir Study Participants With Grade 2, 3, and 4 Creatinine Results
| Sex | Age at Enrollment | Study Drug | Adherence 30 d Prior to Graded Result | Maximum Serum Creatinine | Cause | Outcome |
|---|---|---|---|---|---|---|
| Grade 2 (2.1–3.0 mg/dL) | ||||||
| Male | 39 y | Tenofovir | Adherent 30 of 30 d prior to graded result | 2.9 mg/dL | Unknown | Creatinine returned to baseline (0.9 mg/dL) in 21 d |
| Male | 34 ya | Tenofovir | Adherent 15 of 30 d prior to graded result | 4.7 mg/dL | Diagnosed with diabetes and hypertension | Creatinine remained above baseline |
| Grade 3 (3.1–6.0 mg/dL) | ||||||
| Male | 34 ya | Tenofovir | Adherent 15 of 30 d prior to graded result | 4.7 mg/dL | Diagnosed with diabetes and hypertension | Creatinine remained above baseline |
| Male | 55 y | Placebo | Adherent 30 of 30 d prior to graded result | 3.6 mg/dL | Unknown, possible error | Creatinine returned to baseline (0.9 mg/dL) in 6 d |
| Female | 38 y | Tenofovir | Adherent 0 of 30 d prior to graded result | 4.2 mg/dL | Unknown, possible error | Creatinine returned to normal (0.9 mg/dL) in 2 d |
| Male | 41 y | Placebo | Adherent 15 of 30 d prior to graded result | 3.2 mg/dL | Endocarditis | Creatinine declined to 2.3 mg/dL in 7 d and 1.5 mg/dL in 6 wks |
| Grade 4 (>6.0 mg/dL) | ||||||
| Male | 27 y | Tenofovir | Adherent 30 of 30 d prior to graded result | 32.2 mg/dL | Acute tubular necrosis during period of intense drug use | Creatinine declined to 1.4 mg/dL in 3 mo (baseline 1.2 mg/dL) |
| Male | 37 y | Placebo | Adherent 30 of 30 d prior to graded result | 6.3 mg/dL | Rhabdomyolysis | Creatinine declined to 1.3 in 6 d |
Same participant.
Two participants were diagnosed with acute renal failure: 1 participant in the tenofovir group was diagnosed with acute tubular necrosis following several days of intense drug use; a second participant, in the placebo group, was diagnosed with rhabdomyolysis and acute tubular necrosis following physical exertion. All participants (n = 7) with grade 2, 3, and 4 creatinine results permanently stopped taking study drug. Serum creatinine levels returned to normal in all participants except 1 participant receiving tenofovir who was diagnosed with diabetes and hypertension during the study.
Cross-sectional Analyses
To assess differences in estimated creatinine clearance and GFR in the tenofovir and placebo groups, we examined cross-sectional results (Table 2). The demographic characteristics of participants contributing creatinine clearance results were similar through follow-up, although the proportion aged 40–59 years increased modestly, from 19.7% at baseline to 26.3% at month 60 (Table 3). At months 24, 36, 48, and 60, estimated creatinine clearance and GFR results were lower in the tenofovir group compared with the placebo group using all formulas. At month 60, the estimate of creatinine clearance was 5.2 mL/minute lower in the tenofovir group than the placebo group (P = .002), and the estimate of GFR was 3.4 mL/minute/1.73 m2 lower in the tenofovir group using the MDRD formula (P = .003) and 3.3 mL/minute/1.73 m2 lower using the CKD-EPI formula (P = .002). The Thai modification of the MDRD formula gave mean estimates of GFR 10–12 mL/minute/1.73 m2 higher than the MDRD formula, but because the modification multiplies the MDRD result by a constant, did not alter the relationship (test statistic and P values) of the tenofovir and placebo groups.
Table 2.
Estimated Creatinine Clearance of Bangkok Tenofovir Study Participants, by Study Group
| Creatinine Clearance, mL/min, Cockcroft-Gault Equation | GFR, mL/min/1.73 m2, MDRD | GFR, mL/min/1.73 m2, CKD-EPI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo Mean (95% CI) | Tenofovir Mean (95% CI) | P Value | Placebo Mean (95% CI) | Tenofovir Mean (95% CI) | P Value | Placebo Mean (95% CI) | Tenofovir Mean (95% CI) | P Value | |
| Baseline | n = 1196 98.5 (97.0–99.9) |
n = 1194 100.8 (99.3–102.2) |
.03 | n = 1209 95.1 (94.1–96.0) |
n = 1204 95.8 (94.8–96.7) |
.30 | n = 1209 105.4 (104.6–106.2) |
n = 1204 106.0 (105.2–106.9) |
.27 |
| Month 12 | n = 961 97.0 (95.5–98.6) |
n = 929 95.2 (93.5–96.8) |
.11 | n = 962 93.0 (91.9–94.1) |
n = 929 91.4 (90.3–92.4) |
.04 | n = 962 103.0 (102.0–103.9) |
n = 929 101.9 (100.9–102.9) |
.13 |
| Month 24 | n = 880 98.2 (96.5–99.9) |
n = 846 95.5 (93.8–97.3) |
.03 | n = 881 94.0 (92.9–95.2) |
n = 846 91.5 (90.4–92.6) |
.002 | n = 881 103.8 (102.8–104.8) |
n = 846 101.8 (100.7–102.8) |
.004 |
| Month 36 | n = 777 98.2 (96.3–100.1) |
n = 776 93.9 (92.1–95.7) |
.002 | n = 777 93.1 (91.9–94.3) |
n = 776 90.3 (89.1–91.5) |
.001 | n = 777 102.7 (101.6–103.8) |
n = 776 100.1 (99.0–101.2) |
.001 |
| Month 48 | n = 672 97.4 (95.4–99.4) |
n = 665 92.2 (90.2–94.2) |
<.001 | n = 672 92.2 (90.9–93.5) |
n = 665 88.2 (86.9–89.5) |
<.001 | n = 672 101.7 (100.5–102.8) |
n = 665 97.9 (96.6–99.1) |
<.001 |
| Month 60 | n = 511 97.0 (94.7–99.4) |
n = 524 91.8 (89.4–94.1) |
.002 | n = 511 91.9 (90.3–93.5) |
n = 524 88.5 (86.8–90.1) |
.003 | n = 511 100.7 (99.4–102.1) |
n = 524 97.4 (95.9–98.9) |
.002 |
Abbreviations: CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Table 3.
Mean Creatinine Clearance Results Using the Cockcroft-Gault Formula of Bangkok Tenofovir Study Participants at Annual Visits, by Demographic Characteristics, Injecting Risk, and Baseline Creatinine Clearance
| No. (%) and Mean Creatinine Clearance (SD) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | Month 12 | Month 24 | Month 36 | Month 48 | Month 60 | P Valuea |
| Sex | |||||||
| Male | |||||||
| Tenofovir | n = 949 (39.7%) 98.7 (24.0) |
n = 734 (38.8%) 93.5 (23.2) |
n = 666 (38.6%) 93.5 (23.7) |
n = 607 (39.1%) 92.4 (24.0) |
n = 527 (39.4%) 91.2 (25.3) |
n = 414 (40.0%) 90.8 (26.9) |
<.001 |
| Placebo | n = 954 (39.9%) 97.5 (24.2) |
n = 764 (40.4%) 96.4 (24.0) |
n = 699 (40.5%) 97.3 (24.9) |
n = 625 (40.2%) 98.1 (26.2) |
n = 535 (40.0%) 97.2 (25.5) |
n = 407 (39.3%) 96.5 (27.2) |
.35 |
| Female | |||||||
| Tenofovir | n = 245 (10.3%) 108.7 (30.6) |
n = 195 (10.3%) 101.3 (33.4) |
n = 180 (10.4%) 103.0 (32.3) |
n = 169 (10.9%) 99.4 (31.4) |
n = 138 (10.3%) 95.9 (27.8) |
n = 110 (10.6%) 95.3 (28.8) |
<.001 |
| Placebo | n = 242 (10.1%) 102.4 (29.4) |
n = 197 (10.4%) 99.7 (27.2) |
n = 181 (10.5%) 101.7 (29.2) |
n = 152 (9.8%) 98.5 (30.3) |
n = 137 (10.2%) 98.0 (30.4) |
n = 104 (10.1%) 99.1 (26.9) |
.41 |
| Age group | |||||||
| 20–29y | |||||||
| Tenofovir | n = 511 (21.4%) 107.7 (27.1) |
n = 363 (19.2%) 102.7 (27.5) |
n = 328 (19.0%) 104.1 (26.9) |
n = 303 (19.5%) 102.0 (28.0) |
n = 253 (18.9%) 101.5 (26.9) |
n = 198 (19.1%) 101.2 (26.6) |
.07 |
| Placebo | n = 516 (21.6%) 107.1 (27.2) |
n = 388 (20.5%) 106.3 (26.4) |
n = 343 (19.9%) 107.6 (28.5) |
n = 305 (19.6%) 109.0 (29.4) |
n = 263 (19.7%) 107.5 (28.6) |
n = 192 (18.6%) 107.9 (26.4) |
.15 |
| 30–39 y | |||||||
| Tenofovir | n = 453 (19.0%) 100.2 (24.2) |
n = 365 (19.3%) 96.0 (23.9) |
n = 326 (18.9%) 95.2 (24.8) |
n = 288 (18.6%) 94.8 (22.7) |
n = 246 (18.4%) 93.1 (23.9) |
n = 188 (18.2%) 92.7 (25.8) |
<.001 |
| Placebo | n = 439 (18.4%) 96.7 (22.1) |
n = 364 (19.3%) 96.3 (21.5) |
n = 341 (19.8%) 97.9 (21.4) |
n = 293 (18.9%) 96.9 (23.2) |
n = 251 (18.8%) 96.2 (22.9) |
n = 185 (17.9%) 97.9 (27.1) |
.04 |
| 40–59 y | |||||||
| Tenofovir | n = 230 (9.6%) 86.6 (19.3) |
n = 201 (10.6%) 79.9 (18.8) |
n = 192 (11.1%) 81.6 (19.8) |
n = 185 (11.9%) 79.3 (20.3) |
n = 166 (12.4%) 76.7 (19.3) |
n = 138 (13.3%) 76.9 (23.9) |
<.001 |
| Placebo | n = 241 (10.1%) 83.0 (18.3) |
n = 209 (11.1%) 81.1 (17.3) |
n = 196 (11.4%) 82.4 (19.8) |
n = 179 (11.5%) 81.9 (19.0) |
n = 158 (11.8%) 82.4 (20.4) |
n = 134 (13.0%) 80.4 (19.0) |
.04 |
| Injected drugs in the 3 mo before enrollment | |||||||
| Tenofovir | n = 729 (30.6%) 101.0 (26.8) |
n = 567 (30.1%) 94.8 (27.7) |
n = 510 (29.7%) 94.7 (27.6) |
n = 468 (30.2%) 93.2 (27.1) |
n = 404 (30.2%) 91.2 (27.0) |
n = 321 (31.0%) 90.1 (28.4) |
<.001 |
| Placebo | n = 759 (31.9%) 97.7 (25.2) |
n = 614 (32.6%) 96.4 (24.1) |
n = 557 (32.4%) 97.5 (25.3) |
n = 495 (32.0%) 97.4 (26.4) |
n = 419 (31.4%) 96.2 (26.1) |
n = 314 (30.3%) 96.8 (28.5) |
.04 |
| Did not inject drugs in the 3 mo before enrollment | |||||||
| Tenofovir | n = 462 (19.4%) 100.6 (24.2) |
n = 359 (19.1%) 95.9 (22.7) |
n = 333 (19.4%) 96.9 (23.5) |
n = 307 (19.8%) 95.0 (24.0) |
n = 261 (19.5%) 93.8 (24.1) |
n = 203 (19.6%) 94.4 (25.5) |
<.001 |
| Placebo | n = 432 (18.1%) 99.8 (25.9) |
n = 342 (18.2%) 98.3 (26.0) |
n = 319 (18.6%) 99.6 (27.0) |
n = 279 (18.0%) 99.4 (28.1) |
n = 252 (18.9%) 99.4 (27.3) |
n = 197 (19.0%) 97.3 (24.9) |
.58 |
| Creatinine clearance at baseline | |||||||
| 60–79 mL/min | |||||||
| Tenofovir | n = 224 (9.4%) N/Aa |
n = 175 (9.4%) 70.4 (12.6) |
n = 162 (9.4%) 71.9 (13.2) |
n = 158 (10.2%) 70.1 (13.8) |
n = 140 (10.5%) 69.0 (14.0) |
n = 123 (11.9%) 68.0 (15.0) |
.05 |
| Placebo | n = 275 (11.5%) N/A |
n = 227 (12.1%) 74.4 (11.1) |
n = 217 (12.6%) 75.5 (12.0) |
n = 202 (13.0%) 75.3 (13.4) |
n = 169 (12.6%) 75.0 (13.7) |
n = 133 (12.9%) 72.8 (12.4) |
.36 |
| 80–99 mL/min | |||||||
| Tenofovir | n = 451 (18.9%) N/A |
n = 350 (18.7%) 87.0 (13.2) |
n = 320 (18.6%) 86.7 (12.7) |
n = 292 (18.8%) 86.3 (13.9) |
n = 241 (18.0%) 84.2 (14.4) |
n = 190 (18.4%) 85.1 (14.2) |
.01 |
| Placebo | n = 447 (18.7%) N/A |
n = 347 (18.5%) 90.9 (13.4) |
n = 325 (18.9%) 92.6 (15.0) |
n = 277 (17.9%) 92.5 (14.7) |
n = 245 (18.3%) 92.4 (15.2) |
n = 185 (17.9%) 92.8 (16.2) |
.006 |
| ≥100 mL/min | |||||||
| Tenofovir | n = 519 (21.7%) N/A |
n = 397 (21.2%) 113.1 (26.2) |
n = 362 (21.0%) 113.9 (26.5) |
n = 325 (20.9%) 112.1 (25.9) |
n = 284 (21.2%) 110.4 (25.5) |
n = 211 (20.4%) 111.7 (27.9) |
<.001 |
| Tenofovir | n = 474 (19.8%) N/A |
n = 376 (20.1%) 116.6 (24.3) |
n = 337 (19.6%) 118.3 (26.0) |
n = 298 (19.2%) 119.0 (27.7) |
n = 258 (19.3%) 116.7 (27.8) |
n = 193 (18.7%) 117.8 (27.3) |
.74 |
Abbreviations: N/A, not applicable; SD, standard deviation.
Creatinine clearance results at baseline and annual visits are shown in the table; data from baseline and months 1, 2, 3, and all 3-month visits thereafter through month 60 were used in marginal longitudinal linear regression analysis of demographic characteristics and injecting drug use. Because baseline data were used to define creatinine clearance categories, baseline data were excluded from the analysis of this subgroup.
Longitudinal Analyses
In longitudinal analysis through month 60, we found a significant decline in mean creatinine clearance results (Cockcroft-Gault) in the tenofovir group (slope −0.04; P < .001) but not the placebo group (slope 0.02; P = .08), and a significant difference in the slopes of the tenofovir and placebo groups (P < .001; Figure 1). Using the MDRD formula, there was a significant decrease in GFR in the tenofovir (slope −0.04; P < .001) and placebo (slope −0.02; P = .004) groups, but the slopes of the 2 groups were not significantly different (P = .12). Using the CKD-EPI formula, the GFR declined in the tenofovir (slope −0.06; P < .001) and placebo (slope −0.04; P < .001) groups, and there was a significant difference in the slopes of the groups (P = .007). Among tenofovir recipients, we found that the estimated creatinine clearance was, on average, 5.7 mL/minute lower among participants reporting >80% adherence compared with those reporting ≤80% adherence. This difference did not change significantly through month 60 (P = .11). The results were similar for GFR with an average decrease of 2.7 mL/minute/1.73 m2 using the MDRD formula and 3.1 mL/minute/1.73 m2 using the CKD-EPI formula.
Figure 1.
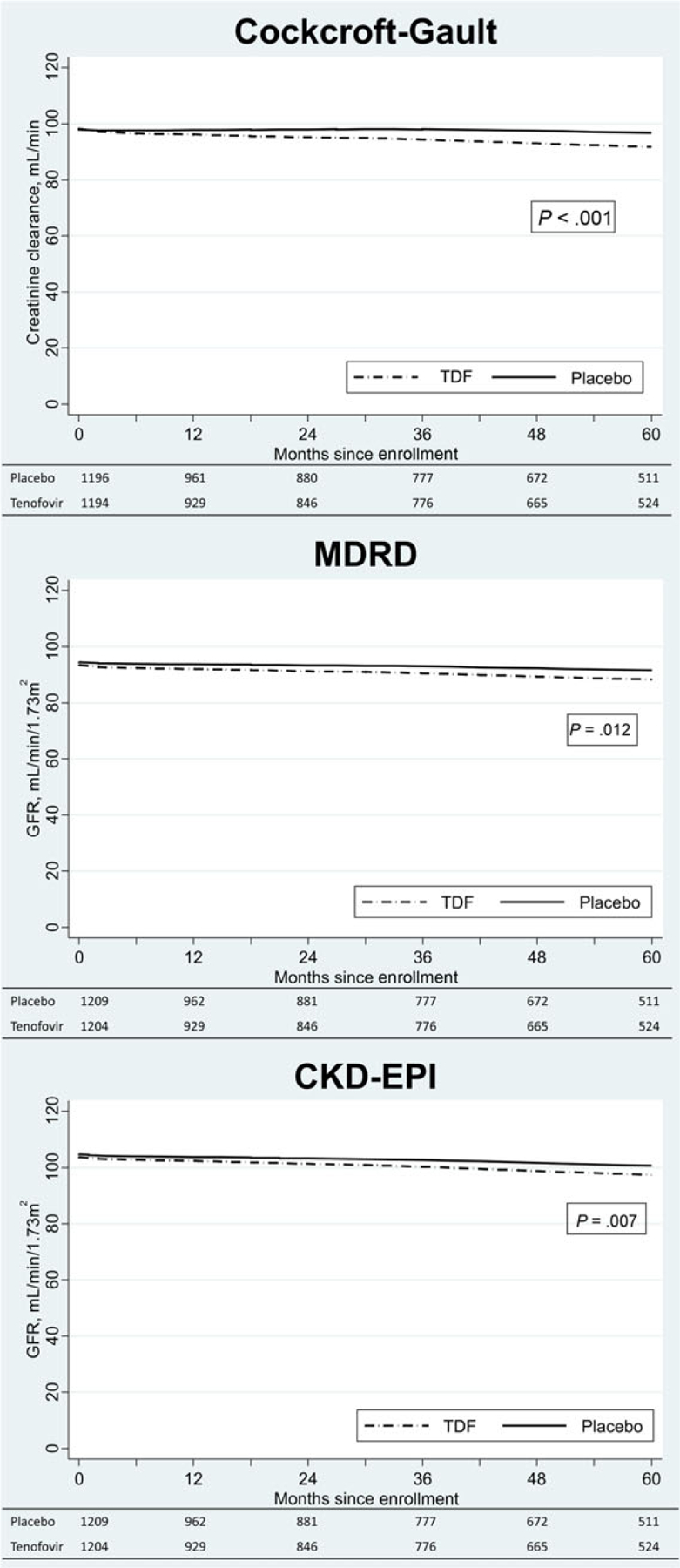
Lowess curves fitted to scatterplots of mean creatinine clearance using the Cockcroft-Gault formula and glomerular filtration rate using the Modification of Diet in Renal Disease and Chronic Kidney Disease Epidemiology Collaboration formulas by study group using all follow-up data from Bangkok Tenofovir Study participants through 60 months. P values for the difference in the slopes of the tenofovir and placebo groups are provided. Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; TDF, tenofovir.
We examined creatinine clearance results in demographic, risk, and baseline creatinine clearance subgroups to determine if the impact of tenofovir on renal function varied by subgroup (Table 3). We used the Cockcroft-Gault formula because it is commonly used to assess renal function. Creatinine clearance decreased 6–14 mL/minute from baseline to month 60 in participants receiving tenofovir in the subgroups, and 1–10 mL/minute lower in the tenofovir subgroups than the placebo subgroups. Among participants receiving tenofovir, the creatinine clearance was lower in men than women (P < .001), but the difference did not change significantly over time (P = .67). In the tenofovir group, creatinine clearance was lower among participants aged ≥30 years than among those aged 20–29 years (P < .001), and the difference increased over time (P = .002); creatinine clearance results among participants who reported injecting drugs during the 3 months before enrollment did not differ significantly from those who did not inject (P = .55). We compared the slopes of mean creatinine clearance results through month 60 of participants with baseline creatinine clearance of 60–79 mL/minute, 80–99 mL/minute, and >100 mL/minute; the slopes did not differ significantly (P = .18). The subgroup-specific changes in creatinine clearance were similar between the tenofovir and placebo groups.
Posttrial Assessment of Creatinine Clearance
Following the announcement of trial results that daily oral tenofovir reduced the risk of HIV infection, participants were offered 1 year of daily tenofovir; 749 (31.0%) elected to take tenofovir. The demographic characteristics of these 749 participants were similar to the entire cohort, and they had been off study drug (ie, placebo or tenofovir) a median of 20 months (IQR, 19–21 months). Their mean creatinine clearance (Cockcroft-Gault) when they enrolled in the Bangkok Tenofovir Study was 99.0 mL/minute; 98.9 mL/minute (95% CI, 96.0–101.7) in those who received tenofovir, and 99.0 mL/minute (95% CI, 96.3–101.8) in those who received placebo (P = .93); however, 2–5 years later when they exited the randomized phase of the study, the mean creatinine clearance result was lower in the tenofovir group (89.7 mL/minute [95% CI, 86.7–92.7]) than in the placebo group (97.9 mL/minute [95% CI, 95.1–100.7]) (P < .001). When these participants returned to receive tenofovir, mean creatinine clearance was, once again, similar between those who had received tenofovir (91.5 mL/minute [95% CI, 88.6–94.4]) and those who had received placebo (94.7 mL/minute [95% CI, 91.9–97.5]) (P = .12).
DISCUSSION
In this large, randomized, placebo-controlled, HIV preexposure prophylaxis trial, daily use of oral tenofovir was not associated with higher rates of grade 2, 3, or 4 creatinine results or renal disease compared with placebo, an observation that is consistent with findings from HIV treatment trials [3, 7, 8] and other preexposure prophylaxis trials [22–24, 30].Similar to findings of HIV clinic–based cohort studies and the iPrEx study, which have shown modest decreases in estimated creatinine clearance associated with use of tenofovir [14, 15, 31, 41], estimates of creatinine clearance and GFR in this study were significantly lower for participants randomized to tenofovir compared with placebo at months 24, 36, 48, and 60. Although the differences were statistically significant, they were small, ranging from 2.7 to 5.2 mL/minute by the Cockcroft-Gault formula, 2.5 to 4.0 mL/minute/1.73 m2 by the MDRD formula, and 2.0 to 3.8 mL/minute/1.73 m2 by the CKD-EPI formula. Based on the analysis of 749 participants who stopped study drug (ie, placebo or tenofovir) for a median of 20 months, the decrease in creatinine clearance among tenofovir recipients was reversible.
Longitudinal analysis showed a significant decline in creatinine clearance, measured using the Cockcroft-Gault formula, in the tenofovir group compared with the placebo group (P < .001); and in GFR, using the CKD-EPI formula (P = .007) but not with the MDRD formula (P = .12). The CKD-EPI equation has been shown to more accurately classify individuals with respect to their risk of mortality and end-stage renal disease than the MDRD formula, particularly people with GFR rates >45 mL/minute/1.73 m2 [42], and may provide a more accurate estimate of GFR in this study population. Among participants taking tenofovir, creatinine clearance was lower in men than women (P < .001) and declined more in older participants than participants aged 20–29 years during follow-up (P = .002), but the differences in the change from baseline to month 60 were small (1–3 mL/minute).
The study has several limitations. We did not measure GFR directly, but used serum creatinine and demographic variables to estimate GFR. The decrease in estimated GFR we describe may be due to tenofovir-associated inhibition of creatinine secretion in the proximal tubule and may not reflect a true decline in GFR [43]. Participants were predominantly men; in addition, Bangkok Tenofovir Study entry criteria required a creatinine clearance, measured with the Cockcroft-Gault formula, of ≥60 mL/minute, limiting our assessment to people with normal baseline renal function. In addition, we did not collect urine for analysis, and cannot directly assess renal tubular function.
Based on its efficacy, safety, and ease of administration, tenofovir is widely used in combination with other antiretroviral medications for the treatment of HIV [1, 2]. Recent evidence that daily oral tenofovir and tenofovir-emtricitabine can prevent or reduce the risk of HIV infection among people at high risk of HIV infection [22–25] defines an important new use for this antiretroviral medication [26–29]. In this analysis of 2413 HIV-uninfected people randomized to receive daily tenofovir or placebo and followed for an average of 4 years, we found small, but significantly lower cross-sectional measures of creatinine clearance and GFR among participants who received tenofovir compared with those who received placebo, and modest differences in the downward trends of creatinine clearance and GFR in longitudinal analysis. Analysis of a subset of participants who stopped tenofovir indicates that the decrease in creatinine clearance was reversible. These results, and the results of other preexposure prophylaxis trials [22–24], suggest that daily oral tenofovir can be used safely as a component of HIV preexposure prophylaxis, but it will be important to include baseline assessments of renal function and routine monitoring of creatinine clearance during follow-up as part of this new HIV prevention strategy.
Acknowledgments.
We thank the study participants, clinic staff, and the members of the Bangkok Tenofovir Study Group. We also thank John Brooks and Robert Harrington for reviewing the manuscript.
Financial support.
This work was supported by the US Centers for Disease Control and Prevention and the Bangkok Metropolitan Administration.
Footnotes
Bangkok Tenofovir Study Group. Principal Investigator: Kachit Choopanya. Advisory Group: Sompob Snidvongs Na Ayudhya, Sithisat Chiamwongpaet, Kraichack Kaewnil, Praphan Kitisin, Malinee Kukavejworakit, Manoj Leethochawalit, Pitinan Natrujirote, Saengchai Simakajorn, Wonchat Subhachaturas. Study Clinic Coordination Team: Lead: Suphak Vanichseni; Members: Boonrawd Prasittipol, Udomsak Sangkum, Pravan Suntharasamai. Bangkok Metropolitan Administration: Rapeepan Anekvorapong, Chanchai Khoomphong, Surin Koocharoenprasit, Parnrudee Manomaipiboon, Siriwat Manotham, Pirapong Saicheua, Piyathida Smutraprapoot, Sravudthi Sonthikaew, La-Ong Srisuwanvilai, Samart Tanariyakul, Montira Thongsari, Wantanee Wattana, Kovit Yongvanitjit. Thailand Ministry of Public Health: Sumet Angwandee, Somyot Kittimunkong. Thailand Ministry of Public Health–US Centers for Disease Control and Prevention Collaboration: Wichuda Aueaksorn, Benjamaporn Chaipung, Nartlada Chantharojwong, Thanyanan Chaowanachan, Thitima Cherdtrakulkiat, Wannee Chonwattana, Rutt Chuachoowong, Marcel Curlin, Pitthaya Disprayoon, Kanjana Kamkong, Chonticha Kittinunvorakoon, Wanna Leelawiwat, Robert Linkins, Michael Martin, Janet McNicholl, Philip Mock, Supawadee Na-Pompet, Tanarak Plipat, Anchala Sa-nguansat, Panurassamee Sittidech, Pairote Tararut, Rungtiva Thongtew, Dararat Worrajittanon, Chariya Utenpitak, Anchalee Warapornmongkholkul, Punneeporn Wasinrapee. US Centers for Disease Control and Prevention: Jennifer Brannon, Monique Brown, Roman Gvetadze, Lisa Harper, Lynn Paxton, Charles Rose; Johns Hopkins University: Craig Hendrix, Mark Marzinke.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 16 April 2014. [Google Scholar]
- 2.Sungkanuparph S, Anekthananon T, Hiransuthikul N, et al. Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents: the recommendations of the Thai AIDS Society (TAS) 2008. J Med Assoc Thai 2008; 91:1925–35. [PubMed] [Google Scholar]
- 3.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 4.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43:595–612. [DOI] [PubMed] [Google Scholar]
- 5.Kahn J, Lagakos S, Wulfsohn M, et al. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA 1999; 282:2305–12. [DOI] [PubMed] [Google Scholar]
- 6.Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol 2000; 11:383–93. [DOI] [PubMed] [Google Scholar]
- 7.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006; 354:251–60. [DOI] [PubMed] [Google Scholar]
- 8.Arribas JR, Pozniak AL, Gallant JE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr 2008; 47:74–8. [DOI] [PubMed] [Google Scholar]
- 9.Izzedine H, Isnard-Bagnis C, Hulot JS, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS 2004; 18:1074–6. [DOI] [PubMed] [Google Scholar]
- 10.Peyriere H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr 2004; 35:269–73. [DOI] [PubMed] [Google Scholar]
- 11.Karras A, Lafaurie M, Furco A, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 2003; 36:1070–3. [DOI] [PubMed] [Google Scholar]
- 12.Rollot F, Nazal EM, Chauvelot-Moachon L, et al. Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: the role of lopinavirritonavir-didanosine. Clin Infect Dis 2003; 37:e174–6. [DOI] [PubMed] [Google Scholar]
- 13.Mauss S, Berger F, Schmutz G. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS 2005; 19:93–5. [DOI] [PubMed] [Google Scholar]
- 14.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis 2005; 40:1194–8. [DOI] [PubMed] [Google Scholar]
- 15.Young B, Buchacz K, Baker RK, et al. Renal function in tenofovir-exposed and tenofovir-unexposed patients receiving highly active anti-retroviral therapy in the HIV Outpatient Study. J Int Assoc Physicians AIDS Care 2007; 6:178–87. [DOI] [PubMed] [Google Scholar]
- 16.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010; 51:496–505. [DOI] [PubMed] [Google Scholar]
- 17.Gallant JE, Winston JA, DeJesus E, et al. The 3-year renal safety of a tenofovir disoproxil fumarate vs. a thymidine analogue-containing regimen in antiretroviral-naive patients. AIDS 2008; 22:2155–63. [DOI] [PubMed] [Google Scholar]
- 18.Fux CA, Simcock M, Wolbers M, et al. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir Ther 2007; 12:1165–73. [PubMed] [Google Scholar]
- 19.Gayet-Ageron A, Ananworanich J, Jupimai T, et al. No change in calculated creatinine clearance after tenofovir initiation among Thai patients. J Antimicrob Chemother 2007; 59:1034–7. [DOI] [PubMed] [Google Scholar]
- 20.Pushpakom SP, Liptrott NJ, Rodriguez-Novoa S, et al. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis 2011; 204:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Novoa S, Labarga P, Soriano V. Pharmacogenetics of tenofovir treatment. Pharmacogenomics 2009; 10:1675–85. [DOI] [PubMed] [Google Scholar]
- 22.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 24.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep 2012; 61:586–9. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep 2011; 60:65–8. [PubMed] [Google Scholar]
- 28.World Health Organization. Guidance on oral pre-exposure prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Geneva, Switzerland: WHO, 2012. Available at: http://www.who.int/hiv/pub/guidance_prep/en/. Accessed 16 April 2014. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Update to interim guidance for preexposure prophylaxis (PrEP) for the prevention of HIV infection: PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep 2013; 62:463–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS 2014; 28:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 33.Martin M, Vanichseni S, Suntharasamai P, et al. Enrollment characteristics and risk behaviors of injection drug users participating in the Bangkok Tenofovir Study, Thailand. PLoS One 2011; 6:e25127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viread (tenofovir disoproxil fumarate) tablets [full prescribing information] 2010. Foster City: Gilead Sciences, 2010. [Google Scholar]
- 35.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145:247–54. [DOI] [PubMed] [Google Scholar]
- 36.Praditpornsilpa K, Avihingsanon A, Chaiwatanarat T, et al. Comparisons between validated estimated glomerular filtration rate equations and isotopic glomerular filtration rate in HIV patients. AIDS 2012; 26:1781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiggins JE. Aging in the glomerulus. J Gerontol A Biol Sci Med Sci 2012; 67:1358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diggle PJ, Liang K, Zeger SL. Analysis of longitudinal data. Oxford: Oxford University Press, 1994. [Google Scholar]
- 40.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Amer Statist Assoc 1979; 74:829–36. [Google Scholar]
- 41.Winston A, Amin J, Mallon P, et al. Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med 2006; 7:105–11. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307:1941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrouenraets SM, Fux CA, Wit FW, et al. Persistent decline in estimated but not measured glomerular filtration rate on tenofovir may reflect tubular rather than glomerular toxicity. AIDS 2011; 25:2149–55. [DOI] [PubMed] [Google Scholar]


