Abstract
An Epstein-Barr virus (EBV) recombinant (MS231) that expresses the first 231 amino acids (aa) of LMP1 and is truncated 155 aa before the carboxyl terminus transformed resting B lymphocytes into lymphoblastoid cell lines (LCLs) only when the infected cells were grown on fibroblast feeder cells (K. M. Kaye et al., J. Virol. 69:675–683, 1995). Higher-titer MS231 virus has now been compared to wild-type (WT) EBV recombinants for the ability to cause resting primary B-lymphocyte transformation. Unexpectedly, MS231 is as potent as WT EBV recombinants in causing infected B lymphocytes to proliferate in culture for up to 5 weeks. When more than one transforming event is initiated in a microwell, the MS231 recombinant supports efficient long-term LCL outgrowth and fibroblast feeder cells are not required. However, with limited virus input, MS231-infected cells differed in their growth from WT virus-infected cells as early as 6 weeks after infection. In contrast to WT virus-infected cells, most MS231-infected cells could not be grown into long-term LCLs. Thus, the LMP1 amino-terminal 231 aa are sufficient for initial growth transformation but the carboxyl-terminal 155 aa are necessary for efficient long-term outgrowth. Despite the absence of the carboxyl-terminal 155 aa, MS231- and WT-transformed LCLs are similar in latent EBV gene expression, in ICAM-1 and CD23 expression, and in NF-κB and c-jun N-terminal kinase activation. MS231 recombinant-infected LCLs, however, require 16- to 64-fold higher cell density than WT-infected LCLs for regrowth after limiting dilution. These data indicate that the LMP1 carboxyl-terminal 155 aa are important for growth at lower cell density and appear to reduce dependence on paracrine growth factors.
Epstein-Barr virus (EBV) is etiologically associated with human B-cell malignancies and can efficiently transform resting human B lymphocytes into lymphoblastoid cell lines (LCLs). In LCLs, EBV expresses 12 genes, including those for six nuclear proteins (EBNAs LP, 1, 2, 3A, 3B, and 3C), three integral membrane proteins (LMP1, -2A, and -2B), two small nuclear RNAs, and a transcript whose function is uncertain (BARF0) (30, 42). Recombinant EBV-based genetic analyses have shown that EBNAs LP, 2, 3A, and 3C and LMP1 are critical for primary B-lymphocyte growth transformation, while EBNA 3B, LMP2A and -2B, EBERs, BARF0, and most of the rest of the EBV genome are dispensable for LCL outgrowth (7, 18, 28, 35, 37, 43, 47, 49).
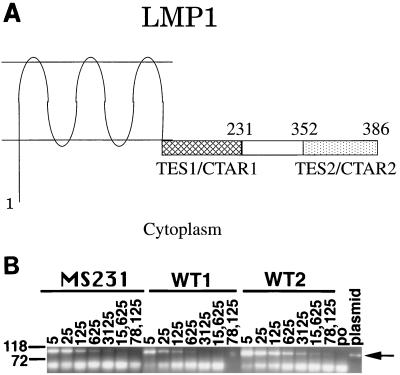
LMP1 is not only essential for primary B-lymphocyte growth transformation but is unique among EBV genes expressed in latent infection in its broad activating effects in B lymphocytes and transforming effects in rodent fibroblasts (54, 55). Genetic and biochemical data indicate that LMP1 is equivalent to a constitutively activated receptor of the tumor necrosis factor receptor (TNFR) family (14, 19, 25, 32, 40). TNFRs are important regulators of lymphocyte differentiation, growth, and survival (2). LMP1 has three components that enable it to mimic activated TNFRs (Fig. 1A). These components are the six hydrophobic transmembrane domains that cause LMP1 to aggregate in the plasma membrane independent of any ligand; a carboxyl-terminal, membrane-proximal, cytoplasmic sequence that is similar to the CD40 and CD30 sequences that interact with TNFR-associated factors (TRAFs); and a carboxyl terminus that interacts with the TNFRI-associated death domain protein (TRADD) (5, 9, 25, 44, 55).
FIG. 1.
(A) Predicted structure of LMP1 in the plasma membrane. Numbers indicate LMP1 amino acids. Transforming effector site 1 (TES1/CTAR1) is indicated by hatched bars, and TES2/CTAR2 is indicated by stippling. Amino acids after 231 are not expressed in MS231 (22, 25). (B) PCR analysis of virus DNA. Primers LMP1MS84B (AGA CCT TCT CTG TCC ACT TG) and LMP1R (CGT TAG AGA AGG AAG AGT AAG GGA) were used to quantitate EBV DNA from viral supernatants. These primers amplify DNA from within the LMP1 gene but upstream of the nonsense linker insertion. Serial fivefold dilutions of virus DNA were made prior to amplification. The reciprocal of each dilution is shown above each lane. Lanes containing amplified MS231, WT1, and WT2 virus DNAs are indicated. The plasmid lane indicates cloned LMP1 DNA used as a template, and the po lane indicates primers only without the template. The 104-bp PCR product is indicated by the arrow. Size markers (base pairs) are indicated to the left. The bands migrating below 72 bp represent primer dimers. DNA was amplified by 40 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 75 s (GeneAmp System 9600; Perkin-Elmer Cetus).
This report characterizes the biologic and biochemical effects of transformation of primary human B lymphocytes with an EBV recombinant (MS231) that has a specifically mutated LMP1 gene (29). The MS231 recombinant is of interest because its LMP1 open reading frame is interrupted by a nonsense codon inserted after the core TRAF binding site and at least 120 codons before the TRADD binding site. Consequently, MS231 expresses an LMP1 that includes the transmembrane domains and the TRAF binding site, both of which are essential for primary B-lymphocyte growth transformation, but lacks the last 155 amino acids, including the TRADD binding site, which is critical for LCL outgrowth (24, 25, 28, 29). Thus, understanding the transforming potency of the MS231 EBV recombinant for primary B lymphocytes is critical to an evaluation of the relative importance of the TRAF engagement site in LMP1’s overall effects.
The MS231 recombinant was obtained by second-site homologous recombination using P3HR-1 EBV to provide most of the genetic background and two bacterial plasmids with cloned EBV DNA. One plasmid marker rescues a transforming virus, and a second plasmid provides a specifically mutated LMP1 (29). The recombinant was recovered by growth of infected primary B lymphocytes on fibroblast feeder cells. Induction of lytic virus infection and lethal irradiation of MS231-infected cells, followed by cocultivation with primary human B lymphocytes on fibroblast feeder cells, resulted in transformation of the primary B lymphocytes. These second-generation MS231-infected LCLs also required fibroblast feeder cells for their expansion (29). We eventually succeeded in obtaining substantial quantities of virus from these MS231-infected LCLs, and this has permitted us to directly compare the effect of the MS231 EBV recombinant on primary B lymphocytes with the effect of otherwise isogenic wild-type (WT) recombinants.
MS231 EBV can transform primary B lymphocytes into LCLs without fibroblast feeder cells.
T-cell-depleted human peripheral blood mononuclear cells were exposed to 0.45-μm-filtered virus supernatants from MS231 or WT EBV recombinant-infected LCLs, and the newly infected lymphocytes were seeded into growth medium at 3.3 × 105/ml in 96-well plates with or without fibroblast feeder layers. Five weeks later, LCL outgrowth was evident in most wells for both MS231 and WT EBV-infected cells, indicating a high titer of free, transforming virus for both MS231 and WT EBV. The fraction of positive wells and the microscopic appearance of the proliferating cells were similar whether the MS231 or WT EBV-infected cells were plated on or off fibroblast feeders. At 7 weeks postinfection, WT- and MS231-infected LCLs were expanded into 48-well plates without fibroblast feeders and most of the cells continued to grow. These results were quite unexpected, since we had previously noted that MS231-infected lymphocytes required fibroblast feeder cells for their growth (29). The possibility that the unexpectedly robust outgrowth of the MS231-infected cells was related to the unusually high titer of virus that had been used in these experiments was considered.
Efficient outgrowth of MS231-infected LCLs requires more than a single transforming event in a microwell.
One hypothesis that could account for the similar growth of the MS231 and WT EBV recombinant-infected lymphoblasts without fibroblast feeder layers is that the initiation of several transforming events in a single microwell results in a paracrine effect that is similar to that of fibroblast feeder layers. To test whether several transforming events per microwell are necessary for efficient outgrowth of MS231-infected cells, increasing dilutions of MS231 or WT virus were used to infect primary human B lymphocytes. The relative amount of viral DNA in the preparations was estimated by endpoint dilution PCR. Viral DNA could be amplified from one WT virus preparation (WT1) at dilutions of up to 1:625, from a second WT virus preparation (WT2) at dilutions of up to 1:78,125, and from the MS231 preparation at dilutions of up to 1:3,125 (Fig. 1B). Based on these results, we estimate that the MS231 virus preparation has five times as much viral DNA as WT1 and 1/25 of the viral DNA of WT2. At 5 weeks after infection of primary human B lymphocytes with dilutions of MS231, WT1, or WT2 virus and culture of the infected cells in microwells without fibroblast feeders, fewer than half of the microwells were positive for LCL outgrowth at the 1:5 and 1:25 dilutions of WT1, at the 1:125 and 1:625 dilutions of MS231, and at the 1:500 and 1:2,500 dilutions of WT2. Thus, the 50% transforming titer of the MS231 virus was similar to that expected for WT EBV when corrected for the relative amount of viral DNA. The MS231-infected cells were also indistinguishable from WT-infected cells in their macroscopic and microscopic appearances at 5 weeks. Most of the cells in the MS231- and WT-infected cell wells were in clumps of at least several hundred cells that were indistinguishable in microscopic appearance from established LCLs.
However, by 6 weeks postinfection, there was an obvious difference in the growth of MS231- and WT-infected cells from the plates of cells infected with dilutions of virus for which fewer than 50% of the wells were positive for cell growth. Only 2 (7%) of 27 MS231-infected LCLs had grown sufficiently to enable transfer to 48-well plates, whereas 12 (32%) of 37 WT-infected LCLs were transferable to 48-well plates. At 8 months postinfection, the difference was even more striking. Only 4 (15%) of the initial 27 MS231-infected cells were still growing in culture, whereas 15 (89%) of the initial 17 representative WT-infected cells were still growing. The 23 of 27 MS231-infected cells and 2 of 17 WT EBV-infected cells that were not still growing had been lost through irreversible cell crises. Thus, cells growing out after infection with dilutions of MS231 EBV which yielded less than 50% positive wells differ substantially from WT EBV-infected cells in their potential for long-term LCL outgrowth.
As expected from the initial results, MS231-infected cells growing on plates infected with concentrations of virus at which most or all of the wells were initially positive for growth were similar to WT-infected cells in their potential for long-term LCL outgrowth. Of 12 MS231 LCLs from a plate that had 80% positive wells at 5 weeks, 11 were still actively growing at 8 months. The more efficient long-term outgrowth of MS231-infected LCLs from plates with a high fraction of positive wells is consistent with the notion that the absence of the carboxy-terminal 155 amino acids can be compensated for by the initial infection of a larger number of cells. The larger number of infected cells may provide a cross-feeding effect that is sufficient to enable outgrowth. Alternatively, the larger number of infected cells may result in the infection of a cell that is more competent for outgrowth.
MS231 and WT EBV are similar in latent infection gene expression.
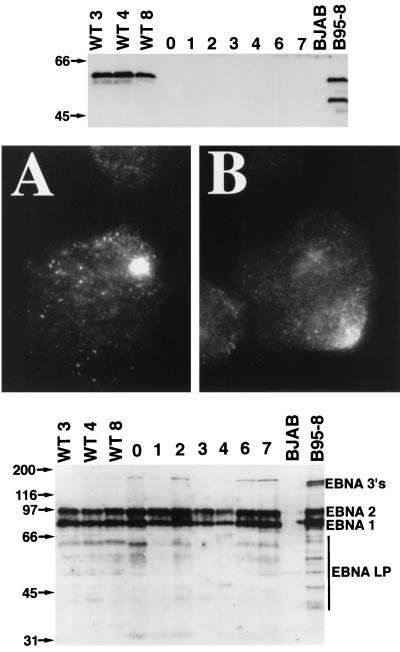
The possibility that the robust MS231 EBV transforming phenotype could be due to altered EBV gene expression was evaluated. First, the LMP1 genotype and protein expression were examined. The LMP1 carboxyl terminus encoding DNA from two different MS231-transformed LCLs was PCR amplified by using primers MS187A and MS187B (29) and directly sequenced. Unique and unambiguous sequences of the LMP1 DNA were obtained, including the site of insertion of the nonsense linker after codon 231. The sequence was identical to the previously published sequence for the MS231 recombinant (29). Further, the MS231-transformed cells did not express WT LMP1, as was readily detected by immunoblotting of lysates of WT-transformed cells using a monoclonal antibody that recognizes an epitope that is C terminal to amino acid 231 (Fig. 2, top). MS231 LMP1 was detected by fluorescence microscopy using a polyclonal rabbit serum directed against the LMP1 N-terminal cytoplasmic domain. MS231 LMP1 was in a tight patch in the plasma membrane of infected LCLs. The plasma membrane distribution and level of antigen detection were indistinguishable from those of WT LCLs examined in parallel (Fig. 2, middle). Finally, a human serum was used to detect the EBNA 1, 2, 3, and LP proteins in lysates of the MS231- and WT-infected LCLs. The EBNA 1, 2, 3, and LP proteins were expressed at similar levels in the MS231- and WT-infected LCLs (Fig. 2, bottom).
FIG. 2.
(Top) Immunoblot analysis of LCLs for LMP1. LMP1 was detected with S12 (36) monoclonal antibody from LCLs infected with WT EBV but not from LCLs infected with MS231 virus, since the S12 recognition sequence is not expressed in MS231. Lanes WT3, WT4, and WT8 contained protein from WT LCLs, and lanes 0, 1, 2, 3, 4, 6, and 7 contained protein from LCLs MS231-0, MS231-1, MS231-2, MS231-3, MS231-4, MS231-6, and MS231-7, respectively. BJAB is an EBV-negative B-lymphoma cell line. B95-8 is a WT EBV-infected marmoset cell line. Proteins from 2.5 × 105 cells were separated by SDS–8% PAGE. Size markers (kilodaltons) are indicated at the left. A truncated form of LMP1 is also expressed in B95-8 due to lytic replication occurring in this cell line (21). S12 monoclonal antibody was detected with sheep anti-mouse immunoglobulin conjugated to horseradish peroxidase (Amersham), followed by a chemiluminescence assay. (Middle) Immunofluorescent staining with rabbit antiserum recognizing the LMP1 amino terminus (22). (A) LCL infected with MS231 virus. (B) LCL infected with WT EBV. Magnification, ×1,000. (Bottom) Immunoblot analysis of LCLs for EBNA proteins. The EBNA 1, 2, 3, and LP proteins were detected with human serum and are indicated on the right. EBNA LP has multiple sizes in each LCL due to variable numbers of a repeat element within the gene. Lanes WT3, WT4, and WT8 contained protein from WT LCLs. Lanes 0, 1, 2, 3, 4, 6, and 7 contained protein from LCLs MS231-0, MS231-1, MS231-2, MS231-3, MS231-4, MS231-6, and MS231-7, respectively. Proteins from 5 × 105 cells were loaded in each lane. EBNA proteins were detected with human serum and protein A conjugated to horseradish peroxidase (Amersham), followed by chemiluminescence assay. Size markers (kilodaltons) are indicated on the left.
MS231 and WT EBV-infected LCLs differ in their dependence on cell concentration for growth.
To further test the importance of paracrine growth factors for MS231-infected cell growth, three MS231- and four WT-infected LCLs were each seeded into at least 32 microwells at increasing dilutions and in medium supplemented with 15, 10, or 5% serum. Three separate experiments were done. At standard cell passage concentrations (1 × 105 to 3 × 105 cells/ml of culture medium), MS231- and WT-infected cells grew in all of the microwells. However, a striking difference in regrowth was evident between MS231 and WT EBV-infected LCLs after plating at lower cell concentrations. In 10% serum, the endpoint for regrowth in >50% of the microwells for the three MS231-infected cell lines was 67,000 cells/ml whereas the endpoints for WT-infected LCL regrowth were 1,100 (three LCLs) and 4,300 (one LCL) cells/ml. Therefore, in 10% serum, MS231-infected LCLs required a 16- to 64-fold higher cell concentration than did WT-infected LCLs for regrowth. Interestingly, the cell density requirement for MS231 regrowth was not significantly altered by plating of cells in medium at various serum concentrations (5 to 15%). Thus, MS231-infected LCLs are considerably more dependent on paracrine effects for their continued growth than are WT-infected LCLs, even after many months in culture.
Phenotypes of MS231 and WT recombinant-infected LCLs.
LMP1 activates NF-κB and c-jun N-terminal kinase, up-regulates the expression of activation markers and adhesion molecules, and functionally activates cell adhesion (12, 17, 19, 31, 34, 39, 54, 56). Despite the lack of expression of the LMP1 carboxyl-terminal 155 amino acids, MS231-infected LCLs grew in clumps, similar to WT-infected LCLs. In order to assess the levels of NF-κB activation in MS231- versus WT-infected LCLs, the level of expression of an NF-κB-regulated surface marker, ICAM-1 (45), was assessed. Surface ICAM-1 expression levels, as measured by fluorescence-activated cell sorter analysis after incubation with excess ICAM-1 monoclonal antibody, were similar on five MS231 recombinant virus-infected LCLs and three WT-infected LCLs (data not shown). Surface CD23 expression levels were also similar on 11 of 14 MS231 recombinant virus-infected LCLs and 10 WT-infected LCLs. However, the CD23 mean fluorescence for three MS231-infected LCLs was one-fourth as much as for the other MS231- or WT-infected LCLs. These results indicate that cellular factors can compensate for any deficiency in ICAM-1 and CD23 upregulation that may result from the absence of the LMP1 C-terminal 155 amino acids.
NF-κB activation in MS231 versus WT recombinant virus-infected LCLs.
NF-κB activation was measured by gel shift using a major histocompatibility complex class I NF-κB site (39) with nuclear extracts from each of four MS231-infected LCLs and four WT recombinant-infected LCLs (Fig. 3A; compare MS231-infected LCL nuclear extracts in lanes 5 to 8 with WT-infected cell nuclear extracts in lanes 1 to 4). Antibodies to p50, p52, and rel were used to identify specific NF-κB complexes. MS231- and WT recombinant-infected LCLs had similar levels of p50/rel heterodimers and p52/rel heterodimers. MS231 recombinant-infected LCL nuclear extracts had somewhat increased p50 homodimer levels. Since p50/p50 homodimers are transcriptionally inactive (1), competition between the increased levels of p50/p50 homodimers and rel heterodimers for NF-κB binding sites could result in reduced NF-κB activation. However, as noted above, MS231- and WT-infected LCLs expressed similar ICAM-1 levels, indicating that overall levels of NF-κB activation are similar in these cells. The p50/p50 homodimer gel-shifted NF-κB probe and all other gel-shifted probe migrating above the p50/p50 homodimer were specifically competed with cold excess NF-κB competitor DNA and were not competed with cold excess mutated NF-κB DNA (data not shown).
FIG. 3.
(A) Analysis of NF-κB activity in LCLs. Gel shift assays (26) were performed with nuclear extracts of WT or MS231 recombinant virus-infected LCLs. Nuclear extracts were prepared by a modified form of the Dignam method (57) and incubated with radiolabeled NF-κB probe. NF-κB probe was prepared by annealing oligonucleotides CCC GGA ATT CTG GGG ATT CCC CAT GGG GAT TCC CCA TGG GGA TTC CCC AGG ATC CCG and CCC GGG ATC CTG GGG AAT CCC CAT GGG GAA TCC CCA TGG GGA ATC CCC AGA ATT CCG. NF-κB subunit compositions in each of the different complexes were determined by supershift assays of a WT LCL (data not shown) and are indicated at the left. The bottom band of the doublet at p52/rel supershifted with p52 antibody. The top part of the p50/rel band and the upper band of the doublet at p52/rel supershifted with p65 antibody. The bottom part of the p50/rel band supershifted with c-rel antibody. The bottom part of the p50/rel band and the bottom band of the doublet at p52/rel supershifted with relB antibody. Rabbit polyclonal antibodies directed against p50, p52 (provided by Ulrich Siebenlist, National Institutes of Health, Bethesda, Md.), relB (C-19), c-rel (C), and p65 (A) (Santa Cruz Biotechnology, Inc.) were used in supershift assays. Lanes WT1, WTa, WTb, and WTc contained nuclear extract from WT LCLs, and lanes B, C, D, and E contained nuclear extract from LCLs MS231-B, MS231-C, MS231-D, and MS231-E. The asterisk denotes free probe. (B) c-jun N-terminal kinase activity in MS231 versus WT recombinant-infected LCLs. c-jun N-terminal kinase was immunoprecipitated from LCLs and incubated with GST-jun in the presence of [γ-32P]ATP. After separation of proteins by SDS-PAGE, c-jun N-terminal kinase (JNK) was detected by immunoblotting (top) and phosphorylated GST-jun was detected by autoradiography (bottom). JNK was detected with rabbit polyclonal antibody JNK1 FL (Santa Cruz Biotechnology, Inc.) at 1 μg/ml and protein A conjugated to horseradish peroxidase (Amersham), followed by chemiluminescence assay. A, B, C, and D indicate LCLs MS231-A, MS231-B, MS231-C, and MS231-D; WTa, WTb, WTc, and WTd indicate WT LCLs. The p46 and p54 forms of c-jun N-terminal kinase are indicated. IVK (in vitro kinase reaction) indicates phosphorylated GST-jun. Extents of phosphorylation were quantitated with a Molecular Dynamics PhosphorImager using ImageQuant software and were similar for all of the lanes.
c-jun N-terminal kinase activation in MS231 versus WT recombinant virus-infected LCLs.
Endogenous c-jun N-terminal kinase activity in MS231- and WT-infected LCLs was evaluated by incubating c-jun N-terminal kinase immunoprecipitates from each LCL with glutathione S-transferase (GST)-jun in the presence of [γ-32P]ATP. Cells (15 × 106) were lysed for 15 min at 4°C in 1 ml of lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40, 3% glycerol, 1 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin per ml, 2 μg of aprotinin per ml, 5 μM NaF, 0.5 μM Na3VO4). Cells were passed five times through a 21-gauge needle and centrifuged at 14,000 rpm (IEC Micromax microcentrifuge) for 10 min at 4°C. Protein concentrations were quantitated with bicinchoninic acid protein assay reagents (Pierce), and the same amount of protein was used for each immunoprecipitate. For immunoprecipitation, 1 μg of rabbit anti-JNK antibody (JNK1 FL) was incubated with cell extract at 4°C for 45 min and then with protein A/G-Sepharose (Santa Cruz) for 2 h at 4°C. The same amount of GST-jun (a fusion of GST with c-Jun amino acids 1 to 79) (19) was added to each immunoprecipitate, beads were washed once with lysis buffer, twice with phosphate-buffered saline, and once with kinase buffer lacking [32P]ATP prior to incubation in kinase buffer (20 mM HEPES [pH 7.4], 20 mM β-glycerol phosphate, 10 mM MgCl2, 10 mM dithiothreitol, 50 μM Na2VO3, 20 μM unlabeled ATP, 2.5 μCi of [γ-32P]ATP per sample). Kinase reactions were carried out at 30°C for 20 min, and reactions were terminated with 2× sodium dodecyl sulfate (SDS) running buffer and boiling. Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE). JNK was detected by immunoblotting, and 32P-labeled GST-jun was detected by autoradiography. Similar amounts of the p46 and p54 JNK forms were present in the immunoprecipitates of each LCL (Fig. 3B, JNK), and levels of JNK activity, as determined by GST-jun phosphorylation (Fig. 3B, IVK), were similar in MS231 (Fig. 3B, lanes 1 to 4)- and WT (Fig. 3B, lanes 5 to 8)-infected LCLs.
These data indicate that the MS231 EBV recombinant is similar to WT EBV recombinants in initiating primary B-lymphocyte proliferation. At 5 weeks after infection, MS231 and WT EBV-infected cells were indistinguishable in their growth and the microscopic appearance of the cells was similar to that of established LCLs. The MS231 recombinant expresses only the LMP1 amino terminus, the hydrophobic transmembrane domains which provide ligand-independent aggregation, and the first 45 amino acids of the carboxyl-terminal cytoplasmic domain which associates with TRAFs and activates downstream effectors. These experiments indicate that the transmembrane domains and TRAF binding domain are the principal LMP1 components that mediate initial B-lymphocyte proliferation. These results are consistent with the previous finding that deletion of sequences encoding the LMP1 TRAF binding site abrogates the ability of EBV to transform primary B lymphocytes (24).
Earlier experiments have investigated the signaling potential of the LMP1 TRAF binding domain. In LCLs, LMP1 associates with TRAF1, -2, -3, and -5 (9, 10). TRAF2 and -5 activate NF-κB and c-jun N-terminal kinase (46). In lymphocytes, the LMP1 TRAF binding domain mediates a substantial component of LMP1-induced NF-κB activation by signaling through NF-κB-inducing kinase (NIK) and the IκBα kinases (IKKα and IKKβ) (48). NF-κB activation from the LMP1 TRAF binding domain is an important regulator of TRAF1, EBI3 (an interleukin-12 [IL-12] p40 homologous cytokine), and epidermal growth factor receptor expression (10). Activation of TRAF1, EBI3, and epidermal growth factor receptor expression in EBV-infected cells is mediated primarily by the LMP1 TRAF binding domain (10, 38). In addition, NF-κB activation from this domain contributes to the LMP1-induced CD40, LFA-3, ICAM-1, and fas up-regulation in B cells (10). Therefore, the LMP1 TRAF binding domain exerts multiple signals capable of affecting cell growth.
The extent of similarity between MS231- and WT-transformed LCLs in NF-κB and c-jun N-terminal kinase activation and surface marker expression is unexpected. The LMP1 TRADD/RIP binding site, which is not expressed in MS231-infected cells, is responsible for at least half of LMP1-induced NF-κB activity and most or all of LMP1-induced c-jun N-terminal kinase activity as assayed in 293 cells (9, 11, 12, 22, 23, 25, 27, 31, 39). Reduced NF-κB and c-jun N-terminal kinase activity and lower activation and adhesion molecule expression were therefore expected. However, deletion of the last 155 LMP1 amino acids, as is characteristic of MS231, has been associated with increased binding of TRAF1 and TRAF2 to the LMP1 TRAF binding domain (9, 44) and this could result in somewhat enhanced signaling. In addition, the correlation of high NF-κB and c-jun N-terminal kinase activity with increased dependence on cell density is compatible with the notion that MS231-infected cells have an increased requirement for paracrine growth factors that indirectly activate NF-κB and c-jun N-terminal kinase, enabling cell growth and survival.
Several autocrine and paracrine growth factor effects have been shown to be important for LCL growth, including tumor necrosis factor beta (TNF-β), IL-1, IL-5, IL-6, IL-10, and lactic acid (3, 6, 13, 15, 16, 41, 51–53). Lymphotoxin alpha and IL-6 are NF-κB regulated (45). These factors may be expressed at lower levels by MS231-infected cells than by WT EBV-infected cells, and higher cell densities might compensate for and mask lower factor secretion. In addition, increased concentration of paracrine factors in high-density MS231 cultures could support the unexpected high levels of NF-κB and c-jun N-terminal kinase activity. For instance, IL-1 and TNF-β both activate NF-κB (45) and IL-1, IL-5, and TNF activate c-jun N-terminal kinase activity (4, 8, 33, 46). Interestingly, the necessary paracrine factors could not be provided by fetal calf serum. Serum concentration did not significantly affect the MS231-transformed cell density requirement for regrowth after limiting dilution.
Despite requiring a higher cell density for MS231-infected cell regrowth after limiting dilution, the MS231 EBV recombinant was remarkably similar to WT recombinants in inducing primary B-lymphocyte proliferation for 5 weeks, even after initiating only one transforming event in a microwell. The simplest explanation for this apparent difference is that uninfected cells in the microwell provide paracrine growth factors to the initial MS231-infected, transformed cell. These experiments are typically set up with 3 × 105 to 10 × 105 uninfected cells per ml. Many of these cells persist for several weeks in culture, and the presence of uninfected cells is important for LCL outgrowth (20). After EBV infection, some nontransformed B cells become activated and secrete immunoglobulin (50). These immunoglobulin-secreting cells likely also secrete growth factors which likely further enhance initial MS231 growth. MS231 infection of more than one cell in a microwell would be expected to result in more infected cells at 5 weeks postinfection than MS231 infection of only one cell in a microwell. Therefore, MS231 infection of multiple cells in a microwell is more likely than infection of one cell to achieve the minimum concentration of 67,000 cells/ml necessary for adequate paracrine growth support by 5 weeks postinfection, at which time the cocultivated, uninfected cells have largely disappeared from the culture.
Acknowledgments
We thank Ulrich Siebenlist (National Institutes of Health) for his generous gift of antibodies. Erin Williams provided assistance with PCR of the LMP1 carboxyl terminus for DNA sequencing. Martin Rowe provided the rabbit antiserum recognizing the LMP1 N terminus. Josephine Harada provided assistance with PCR quantification of DNA.
This work was supported by grants CA67380 (K.M.K.), CA47006 (E.K.), CA62234 (R.L.), and CA73507 (R.L.) from the National Cancer Institute. R.L. is a Scholar of the Leukemia Society of America.
REFERENCES
- 1.Baeuerle P A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 3.Baumann M A, Paul C C. Interleukin-5 is an autocrine growth factor for Epstein-Barr virus-transformed B lymphocytes. Blood. 1992;79:1763–1767. [PubMed] [Google Scholar]
- 4.Brenner D A, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur S R, Cheng G, Baltimore D, Thorley-Lawson D A. Localization of the major NF-kappaB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J Biol Chem. 1997;272:19777–19784. doi: 10.1074/jbc.272.32.19777. [DOI] [PubMed] [Google Scholar]
- 6.Burdin N, Peronne C, Banchereau J, Rousset F. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med. 1993;177:295–304. doi: 10.1084/jem.177.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J I, Wang F, Mannick J, Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci USA. 1989;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot R P, van Dijk T B, Caldenhoven E, Coffer P J, Raaijmakers J A, Lammers J W J, Koenderman L. Activation of 12-O-tetradecanoylphorbol-13-acetate response element- and dyad symmetry element-dependent transcription by interleukin-5 is mediated by Jun N-terminal kinase/stress-activated protein kinase kinases. J Biol Chem. 1997;272:2319–2325. doi: 10.1074/jbc.272.4.2319. [DOI] [PubMed] [Google Scholar]
- 9.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devergne O, McFarland E C, Mosialos G, Izumi K M, Ware C F, Kieff E. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliopoulos A G, Blake S M S, Floettmann J E, Rowe M, Young L S. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos A G, Young L S. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 13.Estrov Z, Kurzuock R, Pocsik E, Pathak S, Kantarjian H M, Zipf T F, Harris D, Talpaz M, Aggarwal B B. Lymphotoxin is an autocrine growth factor for Epstein-Barr virus infected B cell lines. J Exp Med. 1993;177:763–774. doi: 10.1084/jem.177.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floettmann J E, Rowe M. Epstein-Barr virus latent membrane protein-1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C-terminus and requires oligomerisation for NF-kappaB activation. Oncogene. 1997;15:1851–1858. doi: 10.1038/sj.onc.1201359. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons D L, Rowe M, Cope A P, Feldmann M, Brennan F M. Lymphotoxin acts as an autocrine growth factor for Epstein-Barr virus transformed B cells and differentiated Burkitt lymphoma cell lines. Eur J Immunol. 1994;24:1879–1885. doi: 10.1002/eji.1830240825. [DOI] [PubMed] [Google Scholar]
- 16.Gordon J, Guy G, Walker L. Autocrine models of B-lymphocyte growth. II. Interleukin-1 supports the proliferation of transformed lymphoblasts but not the stimulation of resting B cells triggered through their receptors for antigen. Immunology. 1986;57:419–423. [PMC free article] [PubMed] [Google Scholar]
- 17.Hammarskjold M-L, Simurda M C. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through NF-κB activity. J Virol. 1992;66:6496–6501. doi: 10.1128/jvi.66.11.6496-6501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 19.Hatzivassiliou E, Miller W E, Raab-Traub N, Kieff E, Mosialos G. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 20.Henderson E, Miller G, Robinson J, Heston L. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology. 1977;76:152–163. doi: 10.1016/0042-6822(77)90292-6. [DOI] [PubMed] [Google Scholar]
- 21.Hudson G S, Farrell P J, Barrell B G. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J Virol. 1985;53:528–535. doi: 10.1128/jvi.53.2.528-535.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huen D S, Henderson S A, Croom C D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 23.Izumi K M, Cahir-McFarland E, Ting A T, Riley E A, Seed B, Kieff E D. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-kB activation. Mol Cell Biol. 1999;19:5759–5767. doi: 10.1128/mcb.19.8.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izumi K M, Kaye K M, Kieff E D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi K M, Kieff E D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman S R. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by Jκ and PU.1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaye K M, Deverge O, Harada J N, Izumi K M, Yalamanchili R, Kieff E, Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye K M, Izumi K M, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaye K M, Izumi K M, Mosialos G, Kieff E. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B-lymphocyte transformation; fibroblast cocultivation complements a critical function within the terminal 155 residues. J Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 31.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilger E, Kieser A, Baumann M, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause A, Holtmann H, Eickemeier S, Winzen R, Szamel M, Resch K, Saklatvala J, Kracht M. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J Biol Chem. 1998;273:23681–23689. doi: 10.1074/jbc.273.37.23681. [DOI] [PubMed] [Google Scholar]
- 34.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 35.Longnecker R, Miller C L, Miao X-Q, Tomkinson B, Kieff E. The last seven transmembrane and carboxy-terminal cytoplasmic domains of Epstein-Barr virus latent membrane protein 2 (LMP2) are dispensable for lymphocyte infection and growth transformation in vitro. J Virol. 1993;67:2006–2013. doi: 10.1128/jvi.67.4.2006-2013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann K P, Staunton D, Thorley-Lawson D A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985;55:710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mannick J B, Cohen J I, Birkenbach M, Marchini A, Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991;65:6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller W E, Mosialos G, Kieff E, Raab-Traub N. Epstein-Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-κB activation. J Virol. 1997;71:586–594. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell T, Sugden B. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 41.Pike S E, Markey S P, Ijames C, Jones K D, Tosato G. The role of lactic acid in autocrine B-cell growth stimulation. Proc Natl Acad Sci USA. 1991;88:11081–11085. doi: 10.1073/pnas.88.24.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 43.Robertson E S, Tomkinson B, Kieff E. An Epstein-Barr virus with a 58-kilobase-pair deletion that includes BARF0 transforms B lymphocytes in vitro. J Virol. 1994;68:1449–1458. doi: 10.1128/jvi.68.3.1449-1458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP-1’s association with TRAF1, TRAF2, and TRAF3. J Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 46.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci USA. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sylla B S, Hung S C, Davidson D M, Hatzivassiliou E, Malinin N L, Wallach D, Gilmore T D, Kieff E, Mosialos G. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-kappaB through a pathway that includes the NF-kappaB-inducing kinase and the IkappaB kinases IKKalpha and IKKbeta. Proc Natl Acad Sci USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomkinson B, Robertson E, Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tosato G, Blaese R M, Yarchoan R. Relationship between immunoglobulin production and immortalization by Epstein Barr virus. J Immunol. 1985;135:959–964. [PubMed] [Google Scholar]
- 51.Tosato G, Tanner J, Jones K D, Revel M, Pike S E. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990;64:3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tosato G, Teruya-Feldstein J, Setsuda J, Pike S E, Jones K D, Jaffe E S. Post-transplant lymphoproliferative disease (PTLD): lymphokine production and PTLD. Springer Semin Immunopathol. 1998;20:405–423. doi: 10.1007/BF00838052. [DOI] [PubMed] [Google Scholar]
- 53.Wakasugi H, Rimsky L, Mahe Y, Kamel A M, Fradelizi D, Tursz T, Bertoglio J. Epstein-Barr virus-containing B-cell line produces an interleukin 1 that it uses as a growth factor. Proc Natl Acad Sci USA. 1987;84:804–808. doi: 10.1073/pnas.84.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 55.Wang D, Liebowitz D, Wang F, Gregory C, Rickinson A, Larson R, Springer T, Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yalamanchili R, Tong X, Grossman S, Johannsen E, Mosialos G, Kieff E. Genetic and biochemical evidence that EBNA 2 interaction with a 63-kDa cellular GTG-binding protein is essential for B lymphocyte growth transformation by EBV. Virology. 1994;204:634–641. doi: 10.1006/viro.1994.1578. [DOI] [PubMed] [Google Scholar]