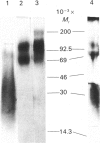
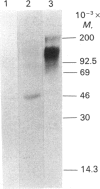
Abstract
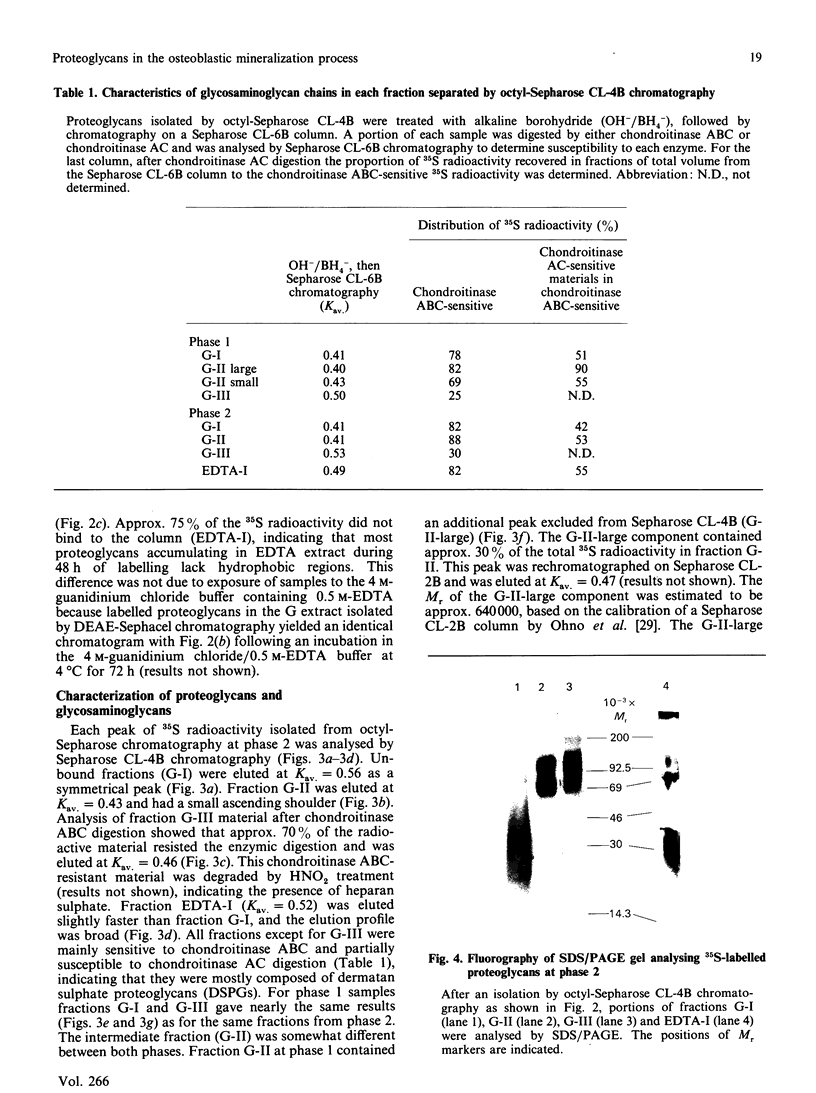
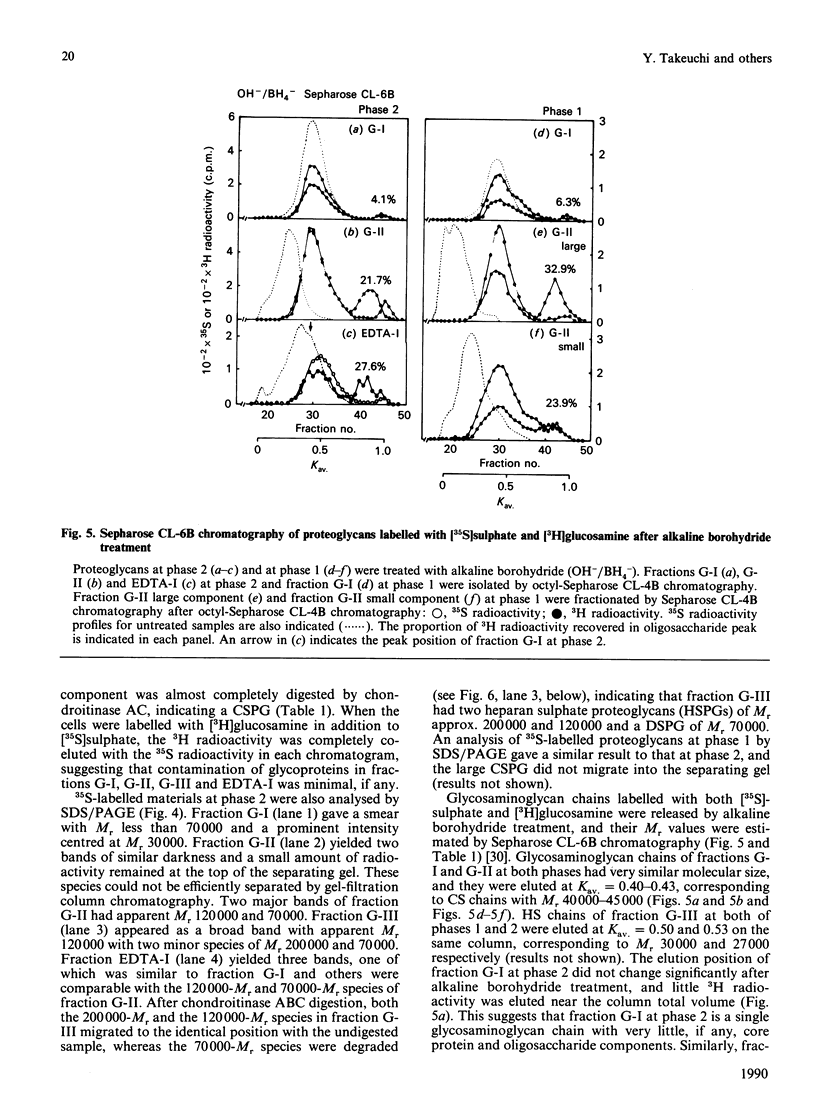
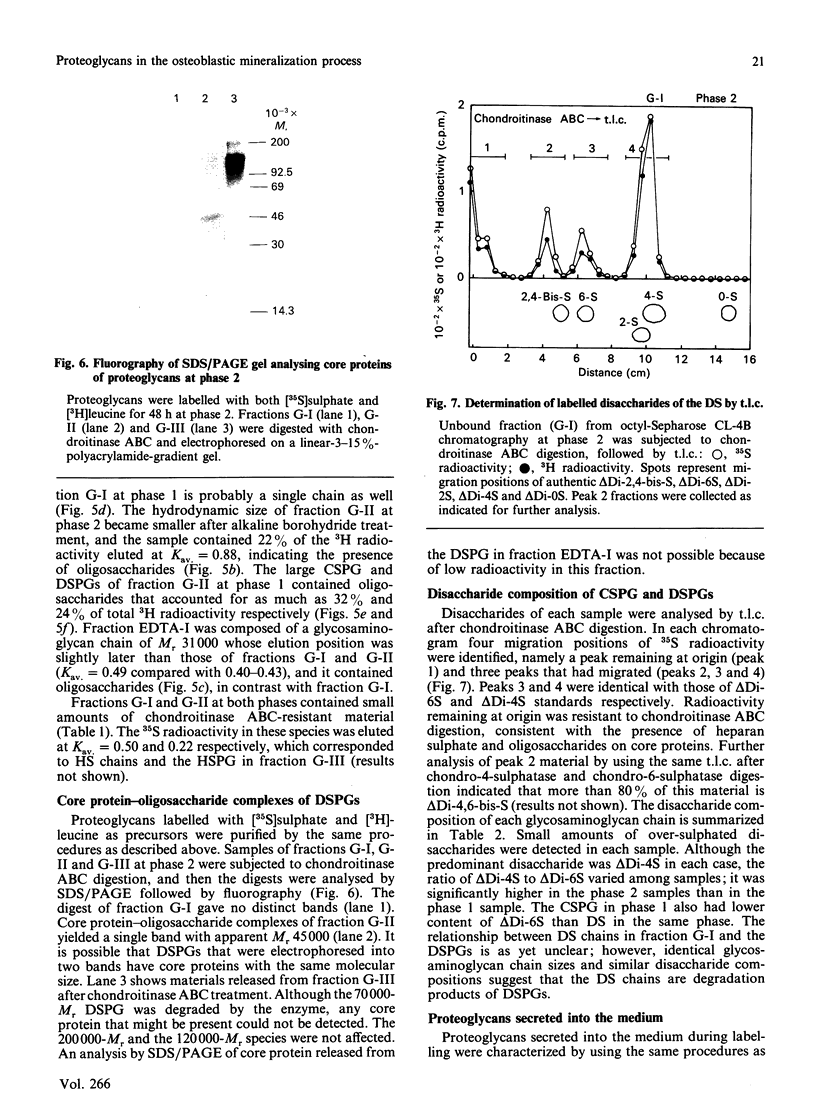
Proteoglycans in mineralized (0.5 M-EDTA/4 M-guanidinium chloride-extractable) and non-mineralized (4 M-guanidinium chloride-extractable) matrices synthesized by a mouse osteoblastic-cell line MC3T3-E1 were characterized at different phases of mineralization in vitro. Cell cultures were labelled with [35S]sulphate and either [3H]glucosamine or 3H-labelled amino acids. At the mineralization phase a large majority of proteoglycans were extracted with 4 M-guanidinium chloride (G extract), and at least five species of labelled proteoglycans were identified; dermatan sulphate proteoglycans (DSPG), apparent Mr approx. 120,000 and 70,000), heparan sulphate proteoglycans (HSPG, apparent Mr approx. 200,000 and 120,000) and DS chains with very little core protein. DSPGs weakly bound to an octyl-Sepharose CL-4B column and HSPGs bound more tightly, whereas DS chains did not bind to the column. Amounts of labelled proteoglycans extracted with 0.5 M-EDTA/4 M-guanidinium chloride (EDTA extract) were much less than those in G extract. Although the predominant species in the EDTA extract were comparable with the DS or DSPGs in the G extract, none of them bound to octyl-Sepharose CL-4B, indicating their lack of hydrophobicity. At the nonmineralizing phase a large chondroitin sulphate proteoglycan (Mr greater than 600,000) was found in the matrix in addition to the five proteoglycan species similar to those at the mineralization phase. Although DS chains at the early phase were similar in size to those at the mineralization phase, the ratio of 2-acetamido-2-deoxy-3-O-(beta-D-gluco-4-enepyranosyluronic acid)-4-O-sulpho-D-galactose to 2-acetamido-2-deoxy-3-O-(beta-D-gluculo-4-enepyranosyluronic acid)-6-O-sulpho-D-galactose was less than that at the mineralization phase. These results agree with those of previous studies performed in vivo and suggest that alteration in the synthesis of proteoglycans is involved in the mineralization process. They also suggest that at the osteoblastic mineralization front proteoglycans undergo partial degradation and lose their hydrophobicity.
Full text
PDF

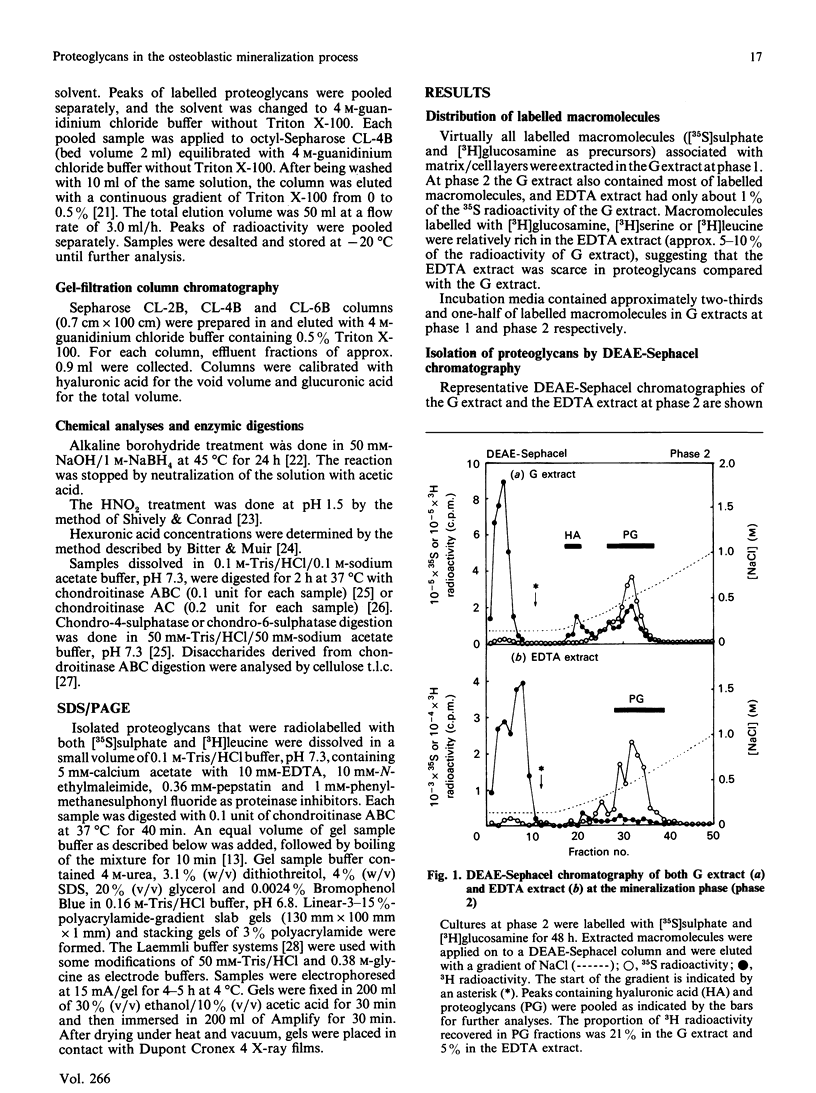
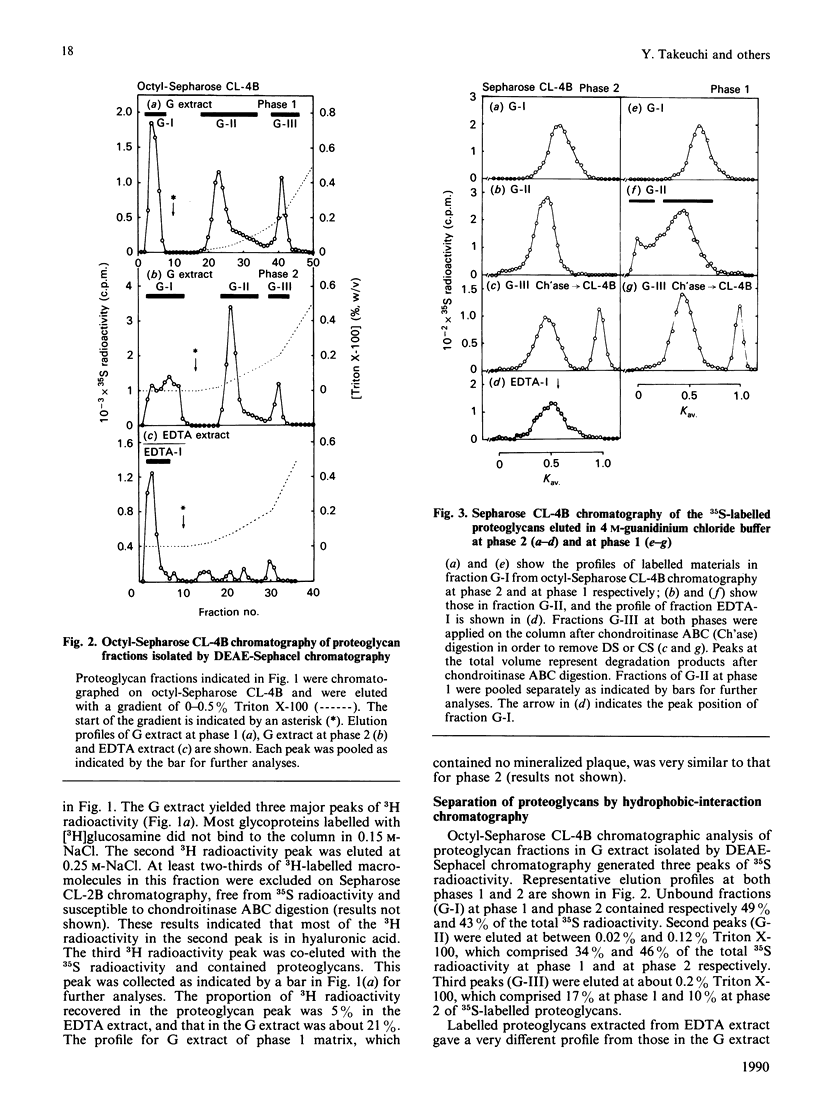



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Beresford J. N., Fedarko N. S., Fisher L. W., Midura R. J., Yanagishita M., Termine J. D., Robey P. G. Analysis of the proteoglycans synthesized by human bone cells in vitro. J Biol Chem. 1987 Dec 15;262(35):17164–17172. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Cheifetz S., Andres J. L., Massagué J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J Biol Chem. 1988 Nov 15;263(32):16984–16991. [PubMed] [Google Scholar]
- Chen C. C., Boskey A. L. Mechanisms of proteoglycan inhibition of hydroxyapatite growth. Calcif Tissue Int. 1985 Jul;37(4):395–400. doi: 10.1007/BF02553709. [DOI] [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Broekhuyse H. Proteoglycans synthesized by cultured mouse osteoblasts. J Biol Chem. 1987 Apr 15;262(11):5345–5351. [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Dejter S. W., Jr, Whitson S. W., Yanagishita M., Kimura J. H., Hascall V. C., Kleinman H. K., Hassell J. R., Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983 May 25;258(10):6588–6594. [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Characterization of proteoglycans from the calcified matrix of bovine bone. Biochem J. 1984 Nov 15;224(1):59–66. doi: 10.1042/bj2240059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén A., Heinegård D., Reiland S., Olsson S. E. Proteoglycans and calcification of cartilage in the femoral head epiphysis of the immature rat. J Bone Joint Surg Am. 1982 Apr;64(4):558–566. [PubMed] [Google Scholar]
- Habuchi H., Kimata K., Suzuki S. Changes in proteoglycan composition during development of rat skin. The occurrence in fetal skin of a chondroitin sulfate proteoglycan with high turnover rate. J Biol Chem. 1986 Jan 25;261(3):1031–1040. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G. K., Heersche J. N., Aubin J. E. Isolation of three species of proteoglycan synthesized by cloned bone cells. Biochemistry. 1983 Feb 15;22(4):831–837. doi: 10.1021/bi00273a019. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Matsumoto T., Morita K., Kurokawa K., Ogata E. Inhibition of in vitro mineralization by aluminum in a clonal osteoblastlike cell line, MC3T3-E1. Calcif Tissue Int. 1986 Nov;39(5):319–323. doi: 10.1007/BF02555198. [DOI] [PubMed] [Google Scholar]
- Kimata K., Oike Y., Tani K., Shinomura T., Yamagata M., Uritani M., Suzuki S. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J Biol Chem. 1986 Oct 15;261(29):13517–13525. [PubMed] [Google Scholar]
- Kurihara N., Ikeda K., Hakeda Y., Tsunoi M., Maeda N., Kumegawa M. Effect of 1,25-dihydroxyvitamin D3 on alkaline phosphatase activity and collagen synthesis in osteoblastic cells, clone MC3T3-E1. Biochem Biophys Res Commun. 1984 Mar 15;119(2):767–771. doi: 10.1016/s0006-291x(84)80316-2. [DOI] [PubMed] [Google Scholar]
- Kurihara N., Ishizuka S., Kiyoki M., Haketa Y., Ikeda K., Kumegawa M. Effects of 1,25-dihydroxyvitamin D3 on osteoblastic MC3T3-E1 cells. Endocrinology. 1986 Mar;118(3):940–947. doi: 10.1210/endo-118-3-940. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Kimura J. H., Hascall V. C. Biosynthesis of hyaluronic acid in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1982 Mar 10;257(5):2236–2245. [PubMed] [Google Scholar]
- Nakatani Y., Tsunoi M., Hakeda Y., Kurihara N., Fujita K., Kumegawa M. Effects of parathyroid hormone on cAMP production and alkaline phosphatase activity in osteoblastic clone MC3T3-E1 cells. Biochem Biophys Res Commun. 1984 Sep 28;123(3):894–898. doi: 10.1016/s0006-291x(84)80219-3. [DOI] [PubMed] [Google Scholar]
- Ohno H., Blackwell J., Jamieson A. M., Carrino D. A., Caplan A. I. Calibration of the relative molecular mass of proteoglycan subunit by column chromatography on Sepharose CL-2B. Biochem J. 1986 Apr 15;235(2):553–557. doi: 10.1042/bj2350553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Rahemtulla F., Butler W. T. Metabolism of rat bone proteoglycans in vivo. Biochem J. 1983 Dec 15;216(3):589–596. doi: 10.1042/bj2160589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Hascall V. C., Hascall G. K. Changes in proteoglycan types during matrix-induced cartilage and bone development. J Biol Chem. 1978 Apr 10;253(7):2429–2436. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-collagen interactions. Ciba Found Symp. 1986;124:104–124. doi: 10.1002/9780470513385.ch7. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Seyedin S. M. The high molecular weight receptor to transforming growth factor-beta contains glycosaminoglycan chains. J Biol Chem. 1988 Jun 15;263(17):8366–8370. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Sudo H., Kodama H. A., Amagai Y., Yamamoto S., Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983 Jan;96(1):191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Matsumoto T., Ogata E., Shishiba Y. 1,25-Dihydroxyvitamin D3 inhibits synthesis and enhances degradation of proteoglycans in osteoblastic cells. J Biol Chem. 1989 Nov 5;264(31):18407–18413. [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Christner P. J., Conn K. M., Nylen M. U. Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem. 1980 Oct 25;255(20):9760–9768. [PubMed] [Google Scholar]
- Tian M. Y., Yanagishita M., Hascall V. C., Reddi A. H. Biosynthesis and fate of proteoglycans in cartilage and bone during development and mineralization. Arch Biochem Biophys. 1986 May 15;247(1):221–232. doi: 10.1016/0003-9861(86)90551-5. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Fisher L. W. Comparisons of antibody reactivity and enzyme sensitivity between small proteoglycans from bovine tendon, bone, and cartilage. J Biol Chem. 1986 Aug 25;261(24):11334–11340. [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Characterization of low buoyant density dermatan sulfate proteoglycans synthesized by rat ovarian granulosa cells in culture. J Biol Chem. 1983 Nov 10;258(21):12847–12856. [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Metabolism of proteoglycans in rat ovarian granulosa cell culture. Multiple intracellular degradative pathways and the effect of chloroquine. J Biol Chem. 1984 Aug 25;259(16):10270–10283. [PubMed] [Google Scholar]
- Yanagishita M., Midura R. J., Hascall V. C. Proteoglycans: isolation and purification from tissue cultures. Methods Enzymol. 1987;138:279–289. doi: 10.1016/0076-6879(87)38023-1. [DOI] [PubMed] [Google Scholar]