A 22-year-old man presented with 7 days of progressive nausea, vomiting, diarrhea, and subjective fevers. He also reported shortness of breath, pleuritic chest pain, and a dry cough. Three days previously, his primary care physician had prescribed amoxicillin. His medical history was significant for mild to moderate anxiety and childhood asthma. In the emergency department, the patient denied smoking cigarettes but admitted to smoking marijuana.
The patient presented with temperature 101.1°F, heart rate 103 beats/min, blood pressure 113/71 mm Hg, respiratory rate 18 breaths/min, and SpO2 89% on room air. Leukocyte count 16.1 K/μL (95.3% neutrophils), C-reactive protein 38.9 mg/L, and erythrocyte sedimentation rate 87 mm/h were noted on laboratory testing. The chest x-ray (CXR) showed bibasilar consolidative opacities and bilateral hilar adenopathy. An extensive work-up for infectious disease was unrevealing, and antibiotic treatment did not resolve symptoms. On further questioning, the patient admitted to using both nicotine- and tetrahydrocannabinol (THC)-containing e-cigarette, or vaping, products multiple times per day. This history supported the diagnosis of e-cigarette, or vaping, product use-associated lung injury (EVALI).
What further investigation might this patient need? What are important management considerations?
Clinical Pearls
1. With no specific clinical or diagnostic test for EVALI, hospitalists must maintain a high level of suspicion for such injury and assess for other disorders that present similarly.
Infection must remain high on the list of differentials, and appropriate diagnostic testing is warranted. At a minimum, such testing should include sputum cultures, a respiratory viral pathogen panel, and influenza testing (results of which were all negative in this patient). Additional infectious and noninfectious testing should be based on clinical indication (1). This patient also had negative results on serologic testing for Mycoplasma, Chlamydia pneumonia, Histoplasma, Blastomyces, and HIV; urinary Legionella antigen; and quantiferon assay for tuberculosis.
2. Gastrointestinal and constitutional symptoms are common in cases of EVALI.
Nearly all patients with EVALI have respiratory symptoms, including shortness of breath, pleuritic chest pain, or cough. The vast majority also have such constitutional symptoms as fever, chills, and weight loss. More than three quarters have gastrointestinal symptoms, such as abdominal pain, nausea, vomiting, and diarrhea (1, 2). Some patients with EVALI present with predominantly gastrointestinal symptoms, and abdominal computed tomography (CT) may incidentally pick up basilar lung infiltrates.
3. Radiographic evaluation is indicated when EVALI is suspected.
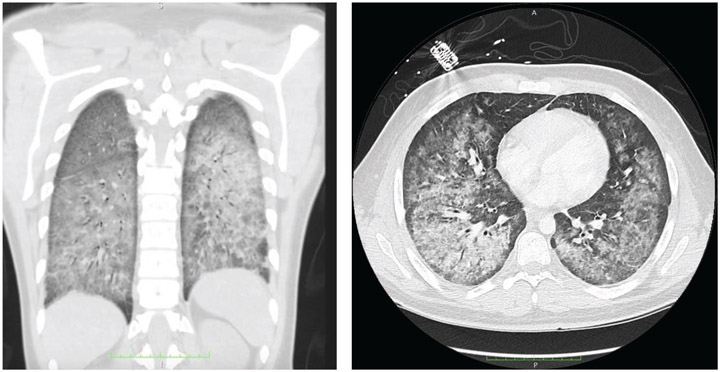
Although a range of imaging findings has been reported in patients with EVALI, most have shown pulmonary infiltrates on CXR and opacities on chest CT—bilateral ground-glass opacities that spare the subpleural lung are common (2, 3) (Figure). Occasionally, abnormal CT findings have been described in the setting of normal CXR findings. Thus, CT may be useful when the CXR is not consistent with clinical findings or to assist in management of severe or worsening disease, when complications are suspected, or when other causes are being considered (1).
Figure.
Coronal (left) and axial (right) views on computed tomography of the lungs of a patient with e-cigarette, or vaping, product use-associated lung injury.
Bilateral ground-glass opacities with subpleural sparing are seen.
4. When EVALI is considered, it is important to inquire about use of e-cigarette, or vaping, products.
Beyond the substances used, important questions to ask include the source of the product, the product's delivery system (e.g., vape pen), duration and frequency of use, and time of last use (1). Products containing THC acquired through informal sources (such as friends, family, illicit dealers, or “off the street”) may be contributing to the ongoing nationwide outbreak of EVALI (1, 4). A urine toxicology screen, including for THC, may help in assessing exposure if a reliable history is unavailable.
5. Patients with EVALI often require supportive care, and treatment with corticosteroids may be beneficial for some patients.
Patients with EVALI should be admitted to the hospital if they have respiratory distress, significant comorbidities, or hypoxia (the Centers for Disease Control and Prevention [CDC] recommends admitting patients with oxygen saturations less than 95% on room air) (2). Nationwide, around half of all patients with EVALI are admitted to the intensive care unit and approximately one quarter require intubation and ventilation. Some clinicians have documented clinical improvements after corticosteroid treatment (1, 2); however, definitive guidance on this treatment is lacking. Pulmonology consultation may be helpful in managing these patients.
Resolution
After several days of treatment with antibiotics and corticosteroids (40 mg IV solumedrol every 8 hours), the patient's fever improved and he no longer required oxygen. He was discharged after 7 days in the hospital with a 12-day taper of prednisolone.
Summary
EVALI is an emerging syndrome, with 2172 cases and 42 deaths reported to the CDC as of 13 November 2019. It is important that hospitalists remain vigilant for this syndrome and question patients about e-cigarette, or vaping, product use. Most patients with EVALI report using THC-containing products acquired from informal sources (4). Vitamin E acetate has been detected in both THC-containing products used by patients with EVALI and in bronchoalveolar lavage fluid from these patients (1). However, the clinical significance of these compounds and their role in the disease course are uncertain and warrant further investigation.
CDC recommends against use of e-cigarette, or vaping, products that contain THC or those obtained from informal sources. In addition, because the specific compounds or ingredients that cause the lung injury are not yet fully known, CDC continues to recommend that persons consider refraining from use of all e-cigarette, or vaping, products while the outbreak investigation continues.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry. The case presents a hypothetical relationship.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-3574.
Contributor Information
Isaac Ghinai, Illinois Department of Public Health, Chicago, Illinois; Epidemic Intelligence Service, Center for Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, Atlanta, Georgia.
Jennifer E. Layden, Illinois Department of Public Health, Chicago, Illinois.
References
- 1.Siegel DA, Jatlaoui TC, Koumans EH, et al. ; Lung Injury Response Clinical Working Group. Update: interim guidance for health care providers evaluating and caring for patients with suspected E-cigarette, or vaping, product use associated lung injury - United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:919–927. doi: 10.15585/mmwr.mm6841e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin - preliminary report. N Engl J Med. 2019. doi: 10.1056/NEJMoa1911614 [DOI] [PubMed] [Google Scholar]
- 3.Henry TS, Kligerman SJ, Raptis CA, et al. Imaging findings of vaping-associated lung injury. AJR Am J Roentgenol. 2019:1–8. doi: 10.2214/AJR.19.22251 [DOI] [PubMed] [Google Scholar]
- 4.Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of E-cigarette, or vaping, product use-associated lung injury - 10 states, August-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1040–1041. doi: 10.15585/mmwr.mm6845e2 [DOI] [PMC free article] [PubMed] [Google Scholar]