Abstract
Hereditary hematopoietic malignancies (HHMs) are inherited syndromes that confer the risk of blood cancer development. With the rapid acceleration of next-generation sequencing (NGS) into commercial biotechnology markets, HHMs are increasingly recognized by genetic counselors and clinicians. In 2020, it was demonstrated that most diagnostic test offerings for HHMs were insufficient for accurate diagnosis, failing to sequence the full spectrum of genetic events known to cause HHMs. We hypothesized the number of genes on commercially available HHM assay increased from 2020 to 2022, consistent with a more comprehensive sequencing approach. Here, we analyzed assays from eight commercial laboratories to determine the HHM-related genes sequenced by these assays. We compared these assays with panels from 2020 to determine trends in sequencing quality. Most HHM diagnostic assays did not change and remain insensitive for the detection of all HHM-related variants. Most (75%) HHM assays do not sequence CHEK2, the gene most frequently mutated in HHMs, and 25% of HHM assays does not sequence DDX41, the second most frequent HHM driver. The quality of HHM diagnostic assays stagnated despite the discovery of novel HHM-related genes and prior work demonstrating heterogeneity in the quality of HHM testing. Most commercially available HHM tests remain insufficient.
Keywords: cancer risk, clinical cancer genetics, germline genetics, hereditary hematopoietic malignancies, hereditary leukemia, inherited leukemia, next-generation sequencing
Genetic counselors have traditionally been the health care providers responsible for the ordering of genetic tests, but the number of other health care providers who now order and interpret genetic tests has increased exponentially as the availability of genetic testing has increased over the past decade (George et al., 2016; Rahman, 2014; Valencia et al., 2017). These other health care providers, with little to no genetics training, often rely on the laboratory themselves to provide the most up-to-date, scientifically sound assays (Farmer et al., 2021; Ramos & Weissman, 2018).
Hereditary hematopoietic malignancies (HHMs) are hereditary blood cancer syndromes driven by germline pathogenic/likely pathogenic (P/LP) variants. Although research regarding HHMs is relatively new, all HHMs to date have followed Mendelian inheritance patterns (Roloff, Drazer, et al., 2021). The scientific basis and clinical recognition of HHMs have increased with the rapid uptake of next-generation sequencing (NGS) in research and clinical settings. The process of confirming germline variants in HHMs requires multiple diagnostic steps, as peripheral blood represents tumor tissue that carries both germline and somatic alterations. Although germline variants can be incidentally detected via tumor-only sequencing panels (Drazer et al., 2018), the gold standard for identifying a germline variant in a patient with an active hematopoietic malignancy is sequencing DNA from cultured skin fibroblasts (Kraft & Godley, 2020).
The accurate diagnosis of an HHM also requires NGS panels that sequence the full spectrum of genes involved in HHMs and sequencing techniques that are capable of detecting the various types of variants that drive HHMs (i.e., single nucleotide variants, insertions/deletions, and structural events such as copy number changes). The number of HHM-related genes, however, has rapidly increased as the genetic basis of HHMs continues to be elucidated (Tawana et al., 2022). This requires HHM diagnostic assays to be updated frequently in response to advances in scientific knowledge. One unique problem in the HHM field, however, is that the quality of HHM diagnostic assays is highly variable (Roloff, Godley, et al., 2021). Most HHM assays used in 2020, for example, did not sequence all research-identified HHM-related genes. These omissions increase the risk of false-negative test results. Specific to HHM patients, this omission leaves them vulnerable to a treatment-related second malignancy, such as a donor-derived leukemia. A donor-derived leukemia occurs when stem cells from a matched related donor, who unknowingly carries an HHM-related genetic mutation, are provided to an affected recipient. Other concerns, shared with solid-tumor hereditary cancers, include missed opportunities for additional cancer screenings (some HHMs also carry an increased risk for solid tumors) and reduce opportunities for cascade testing of family members (who may benefit from genetic counseling and personalized cancer screening). In addition, other HHM assays in 2020 accepted DNA from peripheral blood mononuclear cells, as a permissible tissue source in HHM patients. Sequencing DNA from peripheral blood in patients with active hematopoietic malignancies increases the risk for false-positive HHM test results, as NGS assays may detect both somatic variants in residual tumor tissue and/or clonal hematopoiesis-related variants (Churpek et al., 2015; DiFilippo et al., 2020; Drazer et al., 2018, 2022; McReynolds et al., 2019).
It is unclear how the landscape of HHM diagnostic testing has changed since we performed our original analysis of commercial testing practices in 2020 (Roloff, Godley, et al., 2021). We identified all commercial diagnostic companies available in the United States that offered NGS panels intended for HHM diagnosis in January 2022 to investigate which laboratories have updated their assays. We analyzed the spectrum of HHM-related genes sequenced by each commercial panel and compared these genes with prior offerings from the same company. Here, we demonstrate that the quality of commercial HHM test offerings has largely stagnated since 2020, despite growing recognition of these hereditary blood syndromes and prior work demonstrating the low quality of HHM diagnostic assays.
We identified HHM assays offered by commercial laboratories that we analyzed in our previous report. In addition, we queried the US National Center for Biotechnology Information’s Genetic Testing Registry to identify all newly offered HHM test offerings since our original analysis. The updated analysis was performed in January 2022, 1 year after the print publication and 16 months after the e-publication of our prior study (Roloff, Godley, et al., 2021). We focused our analysis on NGS panels for the diagnosis of hereditary myelodysplastic syndrome and hereditary leukemia.
We evaluated all genes on HHM-related panels via a binary matrix approach. We calculated the proportion of panels that sequenced each HHM gene of interest and queried the Online Mendelian Inheritance in Man catalog to confirm the association of each HHM-related gene and a relevant HHM phenotype (Table S1). Our analysis also included HHM-related genes that were most commonly mutated in a recent series of unselected patients with acute myeloid leukemia (Table S1; Yang et al., 2022). The most frequently sequenced genes across HHM panels for 2020 and 2022 are plotted in Figure 1, defining a core set of HHM genes that were analyzed by the majority of commercial assays. We also plotted the proportion of HHM panels that sequenced a gene of interest in 2020 and 2022 to identify trends in gene-specific sequencing during this time period (Figure 2, Figure S1).
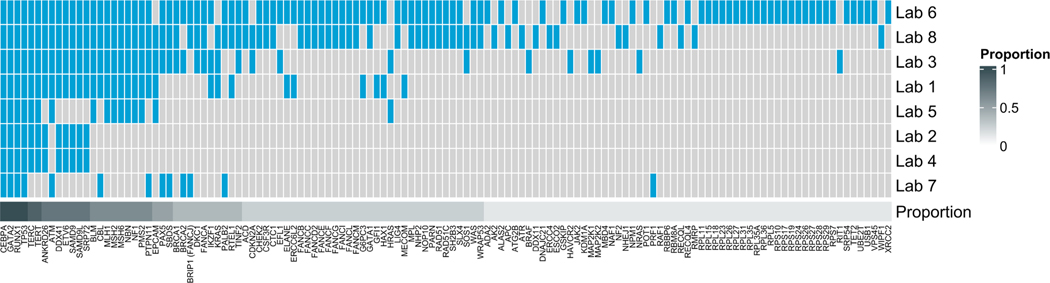
FIGURE 1.
Comprehensive analysis of hereditary hematopoietic malignancy diagnostic panels from eight commercial offerings. The heatmap depicts data via a binary matrix whereby any single gene included on one commercial panel is cross-referenced for inclusion on other assays analyzed. Genes are organized from left to right by decreasing proportion across all panels.
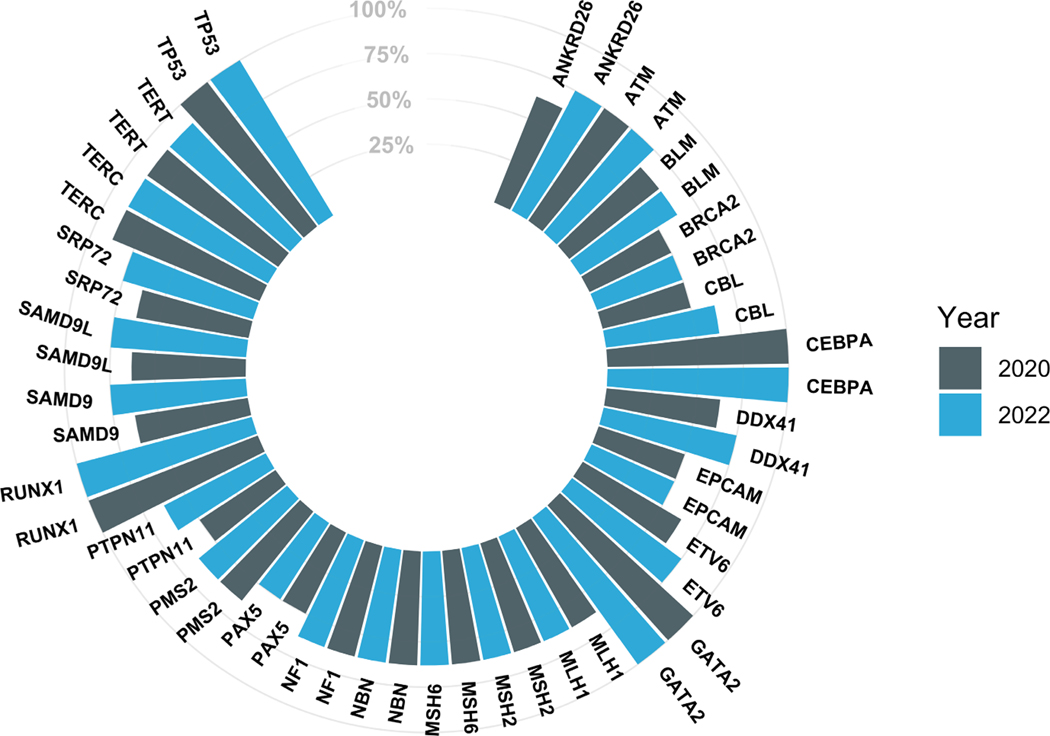
FIGURE 2.
Analysis of commercial assay gene composition over time. This plot demonstrates percent composition of commercial panel inclusion for genes implicated in hereditary hematopoietic malignancies (HHMs). To emphasize high-yield HHM genes, only those included on at least half (4/8) of panels are displayed. Genes are depicted in pairs, demonstrating longitudinal change by the year analyzed.
We analyzed HHM panels from eight companies (Figure 1). Only four genes (CEBPA, GATA2, RUNX1, TP53) were sequenced by all eight panels. An additional 25 genes were sequenced on 50% or more of the panels. Only 25% of HHM panels sequenced CHEK2. Variants in CHEK2 have been known to be associated with lymphoid malignancies since the 2000s, and CHEK2 variants were the most common germline drivers observed in a recent series of patients with myeloid malignancies (Cybulski et al., 2004; Rudd et al., 2006; Yang et al., 2022). CHEK2 was also mutated in the germline of a patient who developed a therapy-related myeloid neoplasm after receiving radiation monotherapy (Patel et al., 2021) as well as in two patients who carried pathogenic/likely pathogenic variants that they shared with their matched related donors during stem cell transplant (Feurstein et al., 2022). Given the important and evolving role of germline CHEK2 variants in both lymphoid and myeloid blood cancers, we anticipate that sequencing of CHEK2 will increase during the upcoming years.
Only 75% of HHM panels sequenced DDX41, which was previously considered the gene that is most commonly altered in HHMs (Polprasert et al., 2015). The discordance between the noted prevalence of variants in HHM-associated genes in Yang et al. (2022) and their inclusion on commercial HHM panels is depicted in Figure S2. Several important HHM genes such as BRCA1 and BRCA2, which are known to be enriched for germline variants within the therapy-related myeloid neoplasm population (Churpek et al., 2016; Martin et al., 2009; McNerney et al., 2017; Schulz et al., 2012), appeared on only a minority of panels (Figure 1). Similarly, CSF3R, FANCA, and MBD4 were sequenced only by a minority of panels despite known roles in HHMs (Roloff, Godley, et al., 2021). By contrast, EPCAM, which is not implicated in the etiology or pathogenesis of any known HHM, was present on half of all panels.
Trends in the analysis of the 25 most-analyzed HHM-related genes from 2020 to January 2022 are shown in Figure 2. Most HHM assays that previously omitted critical HHM genes made no changes to their panels over this time period. This stagnancy persisted despite multiple associations between candidate genes and HHM phenotypes in public databases (Figure S2). Only one laboratory made significant changes to their panel by adding eight HHM-related genes (ANKRD26, CBL, DDX41, ETV6, PTPN11, SAMD9, SAMD9L, SRP72; Figure S1). As in our original study, the criteria used to include and/or exclude genes from HHM-sequencing assays were largely not published on patient and/or provider-facing resources. No major changes in sequencing methodology had occurred since the time of our initial publication. Similarly, we once again observed that many laboratories did not accept germline tissue specimens, such as cultured skin fibroblasts, that are necessary to perform germline sequencing in patients with active blood cancers (Roloff, Godley, et al., 2021).
The accurate diagnosis of an HHM guides patient care in several clinically meaningful ways. First, the identification of an HHM variant informs genetic counseling for the affected patient, while also streamlining cascade genetic testing for both affected and unaffected family members (University of Chicago Hematopoietic Malignancies Cancer Risk Team, 2016). Additionally, the identification of an HHM P/LP variant refines the stem cell transplant process by allowing physicians to avoid using unaffected related variant carriers as stem cell donors. This approach reduces the likelihood of donor-derived malignancies while also avoiding the currently unknown risks associated with the use of hematopoietic growth factors for stem cell mobilization in HHM P/LP variant carriers (Kobayashi et al., 2017). Finally, the identification of an HHM-related variants may also guide therapeutic decision-making. Recent evidence, for example, supports the utilization of lenalidomide for patients with myeloid malignancies driven by P/LP germline DDX41 variants even in the absence of the recurrent del(5q) abnormality (Abou Dalle et al., 2020; Negoro et al., 2016).
Here, we provide further evidence that the majority of HHM diagnostic assays remain inadequate for the accuracy of diagnosis of these syndromes. Stagnation in the quality of HHM diagnostic assays has occurred despite the discovery of novel HHM-related genes, the publication of society-level guidelines including recommendations on HHM testing (Arber et al., 2022; Döhner et al., 2022), as well as the publication of our initial study in January 2021. Four genes (CEBPA, GATA2, RUNX1, and TP53) were uniformly sequenced, likely because the role of these genes in HHMs is well-characterized. However, P/LP germline variants in these genes are now known to be relatively infrequent as compared to variants in genes such as CHEK2 and DDX41 (Feurstein et al., 2022; Yang et al., 2022). This demonstrates that contemporary HHM diagnostic panels must be updated to reflect developments in the field. In order to reduce the risk of false-negative diagnostic reports, the quality of HHM diagnostic testing must be improved so that the most common drivers of HHMs, such as CHEK2 and DDX41, as well as highly penetrant, but less common, HHMs, such as ETV6, GATA2, and RUNX1, are universally sequenced and analyzed.
Supplementary Material
What is known about this topic
Most commercially available tests assessing inherited blood cancer risk are not sufficiently comprehensive. Stewardship of commercial testing has become a responsibility largely assumed by genetic counselors.
What this paper adds to the topic
This study systematically appraises the evolution of diagnostic tests for hereditary hematopoietic malignancies over time.
ACKNOWLEDGEMENTS
This work was supported by the Edward P. Evans Foundation Young Investigator Award, the Cancer Research Foundation Young Investigator Award, and the National Institutes of Health (NIH) Paul Calabresi K12 Program in Oncology (M.W.D.). G.W.R. is supported by the T32 Basic Medical Research Training in Oncology and the Shui-Chin Lee Fellowship Endowment Fund at the University of Chicago.
Footnotes
HUMAN STUDIES AND INFORMED CONSENT
No human studies were performed for the work presented in this manuscript.
ANIMAL STUDIES
No non-human studies were performed for the work presented in this manuscript.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
M.W.D. serves on the Scientific Advisory Board for Argenx. The remaining authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abou Dalle I, Kantarjian H, Bannon SA, Kanagal-Shamanna R, Routbort M, Patel KP, Hu S, Bhalla K, Garcia-Manero G, & DiNardo C. (2020). Successful lenalidomide treatment in high risk myelodysplastic syndrome with germline DDX41 mutation. American Journal of Hematology, 95(2), 227–229. 10.1002/ajh.25610 [DOI] [PubMed] [Google Scholar]
- Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo C, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, … Tefferi A. (2022). International consensus classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood, 140(11), 1200–1228. 10.1182/blood.2022015850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM, Huo D, Weiner H, Bannerjee M, Godley LA, Le Beau MM, Pritchard CC, Walsh T, King MC, Olopade OI, & Larson RA (2016). Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer, 122(2), 304–311. 10.1002/cncr.29615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churpek JE, Pyrtel K, Kanchi KL, Shao J, Koboldt D, Miller CA, Shen D, Fulton R, O’Laughlin M, Fronick C, Pusic I, Uy GL, Braunstein EM, Levis M, Ross J, Elliott K, Heath S, Jiang A, Westervelt P, … Graubert TA (2015). Genomic analysis of germ line and somatic variants in familial myelodysplasia/acute myeloid leukemia. Blood, 126(22), 2484–2490. 10.1182/blood-2015-04-641100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski C, Górski B, Huzarski T, Masojć B, Mierzejewski M, Debniak T, Teodorczyk U, Byrski T, Gronwald J, Matyjasik J, Zlowocka E, Lenner M, Grabowska E, Nej K, Castaneda J, Medrek K, Szymańska A, Szymańska J, Kurzawski G, … Lubiński J. (2004). CHEK2 is a multiorgan cancer susceptibility gene. American Journal of Human Genetics, 75(6), 1131–1135. 10.1086/426403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFilippo EC, Coltro G, Carr RM, Mangaonkar AA, Binder M, Khan SP, Rodriguez V, Gangat N, Wolanskyj A, Pruthi RK, Chen D, He R, Viswanatha DS, Lasho T, Finke C, Tefferi A, Pardanani A, & Patnaik MM (2020). Spectrum of abnormalities and clonal transformation in germline RUNX1 familial platelet disorder and a genomic comparative analysis with somatic RUNX1 mutations in MDS/MPN overlap neoplasms. Leukemia, 34(9), 2519–2524. 10.1038/s41375-020-0752-x [DOI] [PubMed] [Google Scholar]
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian RP, Larson RA, Levine RL, Miyazaki Y, Niederwieser D, Ossenkoppele G, Röllig C, Sierra J, Stein EM, Tallman MS, … Löwenberg B. (2022). Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood, 140(12), 1345–1377. 10.1182/blood.2022016867 [DOI] [PubMed] [Google Scholar]
- Drazer MW, Homan CC, Yu K, Cavalcante de Andrade Silva, M., McNeely KE, Pozsgai MJ, Acevedo-Mendez MG, Segal JP, Wang P, Feng J, King-Smith SL, Kim E, Korotev S, Lawrence DM, Schreiber AW, Hahn CN, Scott HS, Sood R, NISC Comparative Sequencing Program, … Godley LA (2022). Clonal hematopoiesis in patients with ANKRD26 or ETV6 germline mutations. Blood Advances, 6(15), 4357–4359. 10.1182/bloodadvances.2022007211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazer MW, Kadri S, Sukhanova M, Patil SA, West AH, Feurstein S, Calderon DA, Jones MF, Weipert CM, Daugherty CK, Ceballos-López AA, Raca G, Lingen MW, Li Z, Segal JP, Churpek JE, & Godley LA (2018). Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Advances, 2(2), 146–150. 10.1182/bloodadvances.2017013037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MB, Bonadies DC, Pederson HJ, Mraz KA, Whatley JW, Darnes DR, Denton JJ, De Rosa D, Heatherly A, Kenney J, Lane K, Paul D, Pelletier RC, Shannon K, Williams D, & Matloff ET (2021). Challenges and errors in genetic testing: The fifth case series. Cancer Journal, 27(6), 417–422. 10.1097/PPO.0000000000000553 [DOI] [PubMed] [Google Scholar]
- Feurstein SK, Trottier AM, Estrada-Merly N, Pozsgai MJ, McNeely KE, Drazer MW, Ruhle B, Sadera K, Koppayi AL, Scott BL, Oran B, Nishihori T, Agrawal V, Saad A, Lindsley RC, Nakamura R, Kim S, Hu Z, Sobecks R, … Godley LA (2022). Germline predisposition variants occur in myelodysplastic syndrome patients of all ages. Blood, 140, 2533–2548. 10.1182/blood.2022015790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, Cloke V, Slade I, Kemp Z, Gore M, Strydom A, Banerjee S, Hanson H, & Rahman N. (2016). Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Scientific Reports, 6, 29506. 10.1038/srep29506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Kobayashi A, Osawa Y, Nagao S, Takano K, Okada Y, Tachi N, Teramoto M, Kawamura T, Horiuchi T, Kato S, Maekawa T, Yamamura T, Watanabe J, Harada Y, Harada H, Sato K, & Kimura F. (2017). Donor cell leukemia arising from preleukemic clones with a novel germline DDX41 mutation after allogenic hematopoietic stem cell transplantation. Leukemia, 31(4), 1020–1022. 10.1038/leu.2017.44 [DOI] [PubMed] [Google Scholar]
- Kraft IL, & Godley LA (2020). Identifying potential germline variants from sequencing hematopoietic malignancies. Blood, 136(22), 2498–2506. 10.1182/blood.2020006910 [DOI] [PubMed] [Google Scholar]
- Martin MG, Jacoby M, Shao J, Deych E, Graubert T, & Walter MJ (2009). BRCA1 and BRCA2 nucleotide variants in young women with therapy related acute myeloid leukemia. Blood, 114(22), 1102. 10.1182/blood.V114.22.1102.1102 [DOI] [Google Scholar]
- McNerney ME, Godley LA, & Le Beau MM (2017). Therapy-related myeloid neoplasms: When genetics and environment collide. Nature Reviews. Cancer, 17(9), 513–527. 10.1038/nrc.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds LJ, Zhang Y, Yang Y, Tang J, Mulé M, Hsu AP, Townsley DM, West RR, Zhu J, Hickstein DD, Holland SM, Calvo KR, & Hourigan CS (2019). Rapid progression to AML in a patient with germline GATA2 mutation and acquired NRAS Q61K mutation. Leukemia Research Reports, 12, 100176. 10.1016/j.lrr.2019.100176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro E, Radivoyevitch T, Polprasert C, Adema V, Hosono N, Makishima H, Przychodzen B, Hirsch C, Clemente MJ, Nazha A, Santini V, McGraw K, List AF, Sole F, Sekeres MA, & Maciejewski JP (2016). Molecular predictors of response in patients with myeloid neoplasms treated with lenalidomide. Leukemia, 30(12), 2405–2409. 10.1038/leu.2016.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AA, Rojek AE, Drazer MW, Weiner H, Godley LA, Le Beau MM, & Larson RA (2021). Therapy-related myeloid neoplasms in 109 patients after radiation monotherapy. Blood Advances, 5(20), 4140–4148. 10.1182/bloodadvances.2021004964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polprasert C, Schulze I, Sekeres MA, Makishima H, Przychodzen B, Hosono N, Singh J, Padgett RA, Gu X, Phillips JG, Clemente M, Parker Y, Lindner D, Dienes B, Jankowsky E, Saunthararajah Y, Du Y, Oakley K, Nguyen N, … Maciejewski JP (2015). Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell, 27(5), 658–670. 10.1016/j.ccell.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N. (2014). Mainstreaming genetic testing of cancer predisposition genes. Clinical Medicine (London, England), 14(4), 436–439. 10.7861/clinmedicine.14-4-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E, & Weissman SM (2018). The dawn of consumer-directed testing. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 178(1), 89–97. 10.1002/ajmg.c.31603 [DOI] [PubMed] [Google Scholar]
- Roloff GW, Drazer MW, & Godley LA (2021). Inherited susceptibility to hematopoietic malignancies in the era of precision oncology. JCO Precision Oncology, 5, 107–122. [DOI] [PubMed] [Google Scholar]
- Roloff GW, Godley LA, & Drazer MW (2021). Assessment of technical heterogeneity among diagnostic tests to detect germline risk variants for hematopoietic malignancies. Genetics in Medicine, 23(1), 211–214. 10.1038/s41436-020-0934-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd MF, Sellick GS, Webb EL, Catovsky D, & Houlston RS (2006). Variants in the ATM-BRCA2-CHEK2 axis predispose to chronic lymphocytic leukemia. Blood, 108(2), 638–644. 10.1182/blood-2005-12-5022 [DOI] [PubMed] [Google Scholar]
- Schulz E, Valentin A, Ulz P, Beham-Schmid C, Lind K, Rupp V, Lackner H, Wölfler A, Zebisch A, Olipitz W, Geigl J, Berghold A, Speicher MR, & Sill H. (2012). Germline mutations in the DNA damage response genes BRCA1, BRCA2, BARD1 and TP53 in patients with therapy related myeloid neoplasms. Journal of Medical Genetics, 49(7), 422–428. 10.1136/jmedgenet-2011-100674 [DOI] [PubMed] [Google Scholar]
- Tawana K, Brown AL, & Churpek JE (2022). Integrating germline variant assessment into routine clinical practice for myelodysplastic syndrome and acute myeloid leukaemia: Current strategies and challenges. British Journal of Haematology, 196(6), 1293–1310. 10.1111/bjh.17855 [DOI] [PubMed] [Google Scholar]
- University of Chicago Hematopoietic Malignancies Cancer Risk Team. (2016). How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood, 128(14), 1800–1813. 10.1182/blood-2016-05-670240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, & Aziz H. (2017). The role of genetic testing in patients with breast cancer: A review. JAMA Surgery, 152(6), 589–594. 10.1001/jamasurg.2017.0552 [DOI] [PubMed] [Google Scholar]
- Yang F, Long N, Anekpuritanang T, Bottomly D, Savage JC, Lee T, Solis-Ruiz J, Borate U, Wilmot B, Tognon C, Bock AM, Pollyea DA, Radhakrishnan S, Radhakrishnan S, Patel P, Collins RH, Tantravahi S, Deininger MW, Fan G, … Agarwal A. (2022). Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult patients with AML. Blood, 139(8), 1208–1221. 10.1182/blood.2021011354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.