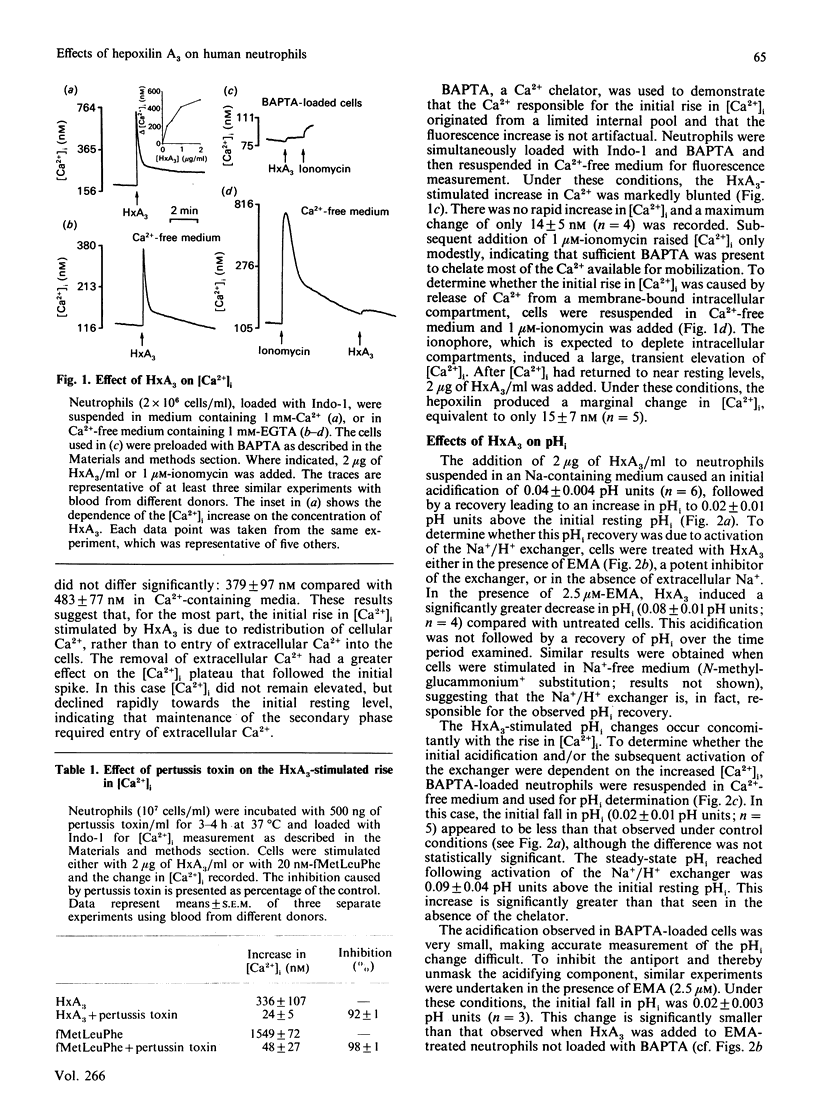
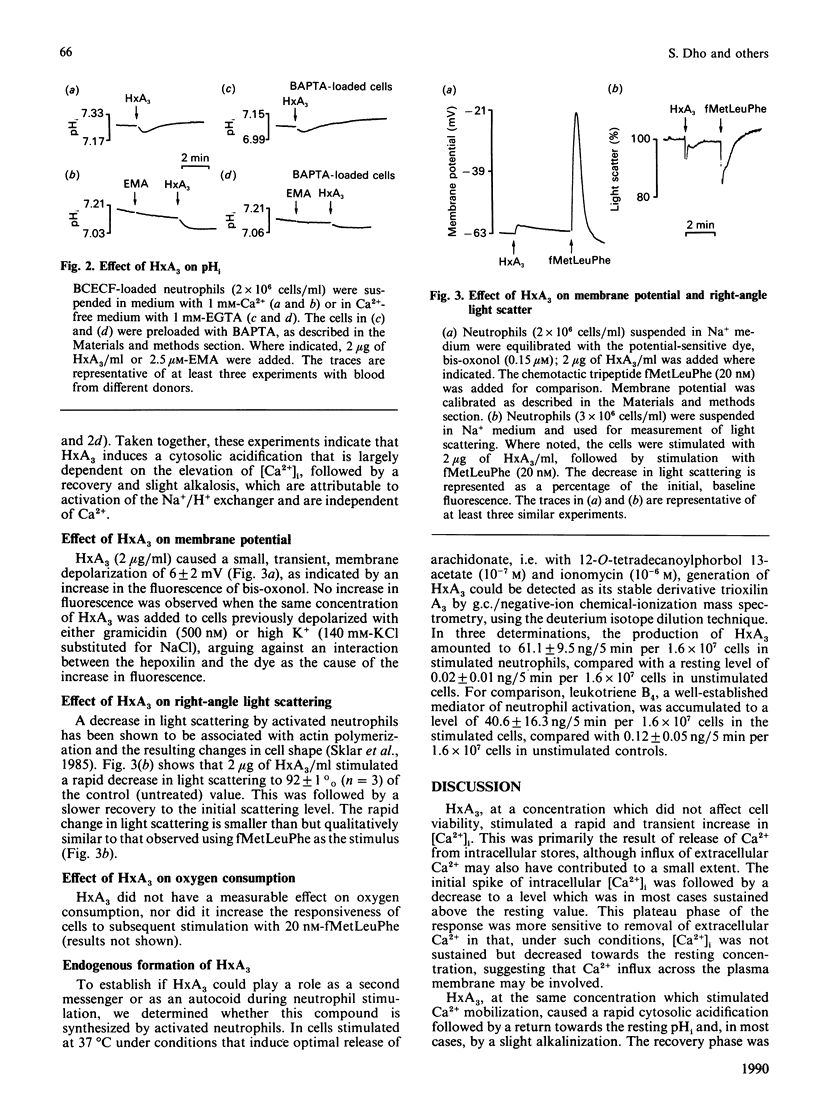
Abstract
The effects of hepoxilin A3 (HxA3), a 12-lipoxygenase metabolite of arachidonic acid, on cytosolic calcium ([Ca2+]i), intracellular pH (pHi), transmembrane potential and right-angle light scattering in human neutrophils were investigated. A rapid, transient elevation of [Ca2+]i was observed with HxA3 which was dependent on the concentration used. The effect of HxA3 on [Ca2+]i was blocked by pertussis toxin, suggesting involvement of receptors coupled to GTP-binding proteins. Experiments in Ca2(+)-free medium and using intracellular Ca2+ chelators indicated that HxA3 mobilized Ca2+ from intracellular stores. At similar concentrations, HxA3 altered pHi, producing an initial acidification followed by an alkalinization. The initial acidification was decreased in cells loaded with a Ca2+ chelator. In the presence of N-ethyl-N-(1-methylethyl)amino amiloride, an inhibitor of the Na+/H+ antiport, HxA3 induced a greater acidification but failed to elicit the recovery phase, suggesting that the latter is due to activation of the antiport. HxA3 also depolarized the membrane potential, although this effect was small. A decrease in right-angle light scattering, qualitatively similar to that observed with chemotactic peptides, was seen with HxA3, indicating that the 12-lipoxygenase metabolite can induce shape changes in neutrophils. At the concentrations used for the above effects, HxA3 was unable to generate a respiratory burst. These findings suggest that hepoxilins, which are formed by stimulated neutrophils, may have a role as messengers in neutrophil activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Curnutte J. T., Robinson J. M., Berde C. B., Karnovsky M. J., Karnovsky M. L. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984 Jun 25;259(12):7870–7877. [PubMed] [Google Scholar]
- Beaumier L., Faucher N., Naccache P. H. Arachidonic acid-induced release of calcium in permeabilized human neutrophils. FEBS Lett. 1987 Sep 14;221(2):289–292. doi: 10.1016/0014-5793(87)80942-0. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Inhibition of the neutrophil oxidative response to a chemotactic peptide by inhibitors of arachidonic acid oxygenation. Biochem Biophys Res Commun. 1979 Sep 27;90(2):481–487. doi: 10.1016/0006-291x(79)91260-9. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Stimulation of arachidonic acid metabolism in the polymorphonuclear leukocyte by an N-formylated peptide. Comparison with ionophore A23187. J Biol Chem. 1980 Nov 10;255(21):10223–10226. [PubMed] [Google Scholar]
- Bryant R. W., Bailey J. M. Isolation of a new lipoxygenase metabolite of arachidonic acid, 8. 11, 12-trihydroxy-5,9,14-eicosatrienoic acid from human platelets. Prostaglandins. 1979 Jan;17(1):9–18. doi: 10.1016/0090-6980(79)90071-6. [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Simon T. C., Bailey J. M. Role of glutathione peroxidase and hexose monophosphate shunt in the platelet lipoxygenase pathway. J Biol Chem. 1982 Dec 25;257(24):14937–14943. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Wu P. H., Su W. G., Corey E. J., Pace-Asciak C. R. Actions of arachidonic acid and hepoxilin A3 on mammalian hippocampal CA1 neurons. Brain Res. 1989 Sep 11;497(1):171–176. doi: 10.1016/0006-8993(89)90984-0. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Gumbay R. S., Doherty D. E., LaBrecque J. F., Henson J. E., Henson P. M., Worthen G. S. Enhancement of pulmonary inflammation by PGE2: evidence for a vasodilator effect. J Appl Physiol (1985) 1988 Feb;64(2):728–741. doi: 10.1152/jappl.1988.64.2.728. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Amiloride-sensitive Na+/H+ exchange in human neutrophils: mechanism of activation by chemotactic factors. Biochem Biophys Res Commun. 1984 Jul 31;122(2):755–762. doi: 10.1016/s0006-291x(84)80098-4. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am J Physiol. 1986 Jul;251(1 Pt 1):C55–C65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Kerry P. J., Poyser N. L., Walker I. C., Wilson N. H. The identification of trihydroxyeicosatrienoic acids as products from the incubation of arachidonic acid with washed blood platelets. Prostaglandins. 1978 Oct;16(4):583–589. doi: 10.1016/0090-6980(78)90188-0. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Dayer J. M., Wollheim C. B., Pozzan T. Effect of leukotriene B4, prostaglandin E2 and arachidonic acid on cytosolic-free calcium in human neutrophils. FEBS Lett. 1984 Jan 23;166(1):44–48. doi: 10.1016/0014-5793(84)80041-1. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Pozzan T. Role of cytosolic free calcium and phospholipase C in leukotriene-B4-stimulated secretion in human neutrophils. Comparison with the chemotactic peptide formyl-methionyl-leucyl-phenylalanine. Eur J Biochem. 1987 Jan 2;162(1):161–168. doi: 10.1111/j.1432-1033.1987.tb10556.x. [DOI] [PubMed] [Google Scholar]
- Maclouf J., de Laclos B. F., Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6042–6046. doi: 10.1073/pnas.79.19.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi T., Ives H. E. Intracellular Ca2+ requirement for activation of the Na+/H+ exchanger in vascular smooth muscle cells. J Biol Chem. 1988 Jun 25;263(18):8790–8795. [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Volpi M., Wolpert L. M., Sha'afi R. I. Specific modulation of the intracellular pH of rabbit neutrophils by chemotactic factors. Biochem Biophys Res Commun. 1980 May 30;94(2):508–514. doi: 10.1016/0006-291x(80)91260-7. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., McColl S. R., Caon A. C., Borgeat P. Arachidonic acid-induced mobilization of calcium in human neutrophils: evidence for a multicomponent mechanism of action. Br J Pharmacol. 1989 Jun;97(2):461–468. doi: 10.1111/j.1476-5381.1989.tb11973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. doi: 10.1016/0006-291x(79)91678-4. [DOI] [PubMed] [Google Scholar]
- Nasmith P. E., Grinstein S. Cytosolic calcium, oxygen consumption and the intracellular pH of stimulated neutrophils. Biosci Rep. 1988 Feb;8(1):65–76. doi: 10.1007/BF01128973. [DOI] [PubMed] [Google Scholar]
- Nasmith P. E., Grinstein S. Phorbol ester-induced changes in cytoplasmic Ca2+ in human neutrophils. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1987 Oct 5;262(28):13558–13566. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol. 1979 May;95(2):433–444. [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak C. R. Arachidonic acid epoxides. Demonstration through [18O]oxygen studies of an intramolecular transfer of the terminal hydroxyl group of (12S)-hydroperoxyeicosa-5,8,10,14-tetraenoic acid to form hydroxyepoxides. J Biol Chem. 1984 Jul 10;259(13):8332–8337. [PubMed] [Google Scholar]
- Pace-Asciak C. R. Formation and metabolism of hepoxilin A3 by the rat brain. Biochem Biophys Res Commun. 1988 Feb 29;151(1):493–498. doi: 10.1016/0006-291x(88)90620-1. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Granström E., Samuelsson B. Arachidonic acid epoxides. Isolation and structure of two hydroxy epoxide intermediates in the formation of 8,11,12- and 10,11,12-trihydroxyeicosatrienoic acids. J Biol Chem. 1983 Jun 10;258(11):6835–6840. [PubMed] [Google Scholar]
- Pace-Asciak C. R., Martin J. M., Corey E. J., Su W. G. Endogenous release of hepoxilin A3 from isolated perifused pancreatic islets of Langerhans. Biochem Biophys Res Commun. 1985 Apr 30;128(2):942–946. doi: 10.1016/0006-291x(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R., Martin J. M., Lee S. P. Appearance of prostaglandins, thromboxane B2, and hepoxilin A3 in the circulation of the normal and diabetic (BB) rat after arachidonic acid administration--correlation with plasma insulin. Biochem Cell Biol. 1988 Aug;66(8):901–909. doi: 10.1139/o88-102. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R. Prostaglandins and related products profiled by GC/MS with negative-ion chemical-ionization detection. Clin Invest Med. 1987 May;10(3):117–120. [PubMed] [Google Scholar]
- Palmblad J., Malmsten C. L., Udén A. M., Rådmark O., Engstedt L., Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981 Sep;58(3):658–661. [PubMed] [Google Scholar]
- Piomelli D., Shapiro E., Zipkin R., Schwartz J. H., Feinmark S. J. Formation and action of 8-hydroxy-11,12-epoxy-5,9,14-icosatrienoic acid in Aplysia: a possible second messenger in neurons. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1721–1725. doi: 10.1073/pnas.86.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Montecucco C., Hesketh T. R., Tsien R. Y. Lymphocyte membrane potential assessed with fluorescent probes. Biochim Biophys Acta. 1980;595(1):15–30. doi: 10.1016/0005-2736(80)90243-6. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Sink L. E., Freer R. J. On the relationship between formylmethionyl-leucyl-phenylalanine stimulation of arachidonyl phosphatidylinositol turnover and lysosomal enzyme secretion by rabbit neutrophils. Mol Pharmacol. 1981 Jan;19(1):31–37. [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F. Activation of the neutrophil. Prog Allergy. 1988;42:1–64. doi: 10.1159/000318681. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Omann G. M., Painter R. G. Relationship of actin polymerization and depolymerization to light scattering in human neutrophils: dependence on receptor occupancy and intracellular Ca++. J Cell Biol. 1985 Sep;101(3):1161–1166. doi: 10.1083/jcb.101.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Sam L. M., Justen J. M., Leach K. L., Epps D. E. Human polymorphonuclear neutrophil activation with arachidonic acid. Br J Pharmacol. 1987 Jul;91(3):641–649. doi: 10.1111/j.1476-5381.1987.tb11258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Sun F. F., Bowman B. J., Iden S. S., Smith H. W., McGuire J. C. Effect of 6,9-deepoxy-6,9-(phenylimino)-delta 6,8-prostaglandin 1(1) (U-60,257), an inhibitor of leukotriene synthesis, on human neutrophil function. Biochem Biophys Res Commun. 1982 Dec 15;109(3):943–949. doi: 10.1016/0006-291x(82)92031-9. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Satoh M., Takeshige K., Cragoe E. J., Jr, Minakami S. Cytoplasmic pH change induced by leukotriene B4 in human neutrophils. Biochim Biophys Acta. 1988 Jun 8;970(1):31–38. doi: 10.1016/0167-4889(88)90219-4. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Takeshige K., Minakami S. Superoxide production of human polymorphonuclear leukocytes stimulated by leukotriene B4. Biochim Biophys Acta. 1984 Apr 16;803(4):271–277. doi: 10.1016/0167-4889(84)90117-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Tao W., Molski T. F., Naccache P. H., Sha'afi R. I. Leukotriene B4 mobilizes calcium without the breakdown of polyphosphoinositides and the production of phosphatidic acid in rabbit neutrophils. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5966–5969. doi: 10.1073/pnas.81.19.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin R., Shefcyk J., White J. R., Tao W., Volpi M., Molski T. F., Naccache P. H., Feinstein M. B., Sha'afi R. I. Effects of chemotactic factors and other agents on the amounts of actin and a 65,000-mol-wt protein associated with the cytoskeleton of rabbit and human neutrophils. J Cell Biol. 1985 Jul;101(1):182–188. doi: 10.1083/jcb.101.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]


