Abstract
The ORF1 sequence was determined for Camberwell virus, a genogroup 2 Norwalk-like virus, completing the full genome of 7,555 nucleotides. ORF1 cDNA was cloned into a simian virus 40-based expression vector, and the viral proteins synthesized following transfection into COS cells were analyzed. By using antisera directed against the helicase, protease, or polymerase regions, eight polypeptides ranging in size from 19 to 117 kDa were detected by radioimmunoprecipitation. The cleavage sites determining the amino and carboxy termini of the 3C-like protease were identified at E1008/A and E1189/G, respectively.
Norwalk-like viruses (NLVs), also known as the small round-structured viruses, constitute a genus within the family Caliciviridae (25). They cause viral gastroenteritis in humans and have not been grown in cell culture (17). The genomes of Southampton (19, 20), Norwalk (10, 16), and Lordsdale (6) NLVs have been completely sequenced. Extensive comparisons of partial sequences for a number of NLVs have shown that the viruses may be divided into at least two genogroups (4, 15, 32, 35). Genogroup 1 includes Southampton and Norwalk viruses, while genogroup 2 includes Lordsdale and Camberwell viruses (4).
The NLV genome is single-stranded, positive-sense RNA of about 7.5 kb organized into three open reading frames (ORFs). The RNA is polyadenylated at the 3′ end. ORF1 encodes a polyprotein of approximately 190 kDa and contains motifs for a 2C-like helicase, a 3C-like protease, and a 3D-like RNA polymerase. ORF2 encodes a capsid protein of about 60 kDa, and ORF3 encodes a small basic protein of 20 to 30 kDa with unknown function (4, 6, 16, 19, 28).
The inability to propagate the NLVs in cell culture has so far restricted the study of the processing of the ORF1 polypeptide to in vitro transcription and translation (21) and synthesis in bacterial cells (22). These experiments have been done with Southampton, a genogroup 1 virus. By using in vitro transcription and translation, cleavage of the ORF1 polyprotein at two Q/G sites was demonstrated. These sites defined the putative helicase protein (21). More recently, three additional cleavage sites, one at E/A and two at E/G, were identified by N-terminal analyses of polypeptides synthesized in Escherichia coli (22). Additional information on polypeptide cleavage is available for two animal caliciviruses, rabbit hemorrhagic disease virus (RHDV) and feline calicivirus (FCV). For RHDV, cleavage sites were identified at E/G and E/T (1, 33, 34), whereas E/A, E/S, E/T, and E/G are utilized in FCV (30, 31). In the experiments described below, the proteolytic processing in mammalian cells of the NLV ORF1 polypeptide was examined for Camberwell virus (genogroup 2). The proteins produced were identified by immunoprecipitation with region-specific antisera, and the results were compared with those reported for Southampton virus and in vitro translation or expression in E. coli.
Completion of the ORF1 sequence.
The sequence of the 3′ half of the Camberwell virus genome (approximately 3.5 kb) incorporating part of ORF1 and the complete ORF2, ORF3, and 3′ untranslated region has been reported by our laboratory (4, 28). Now the complete genome sequence of 7,555 nucleotides (nt) [excluding the poly(A) tail] has been determined (accession no. AF145896). The procedures of RNA extraction and reverse transcription-PCR were described previously (4, 5). Superscript II RNase H− reverse transcriptase (GIBCO-BRL) and AmpliTaq DNA polymerase (Perkin-Elmer) were used. PCR fragments of 500 to 750 bp were sequenced without cloning. Eight overlapping PCR fragments extending towards the 5′ terminus were obtained by using 3′ primers based on the known Camberwell virus sequence and 5′ primers based on the published ORF1 sequences of Southampton, Norwalk, and Lordsdale viruses. The 5′ untranslated region was determined by the rapid amplification of cDNA end method (9).
Extension by RACE towards the 5′ end revealed only four nucleotides preceding the first AUG codon in ORF1. The four nucleotides (GUGA) were identical to those determined for Southampton, Norwalk, and Lordsdale viruses (6, 10, 20). ORF1 of Camberwell virus consists of 5,100 nt (including the stop codon) and encodes a polyprotein of 189 kDa. The motifs for a putative 2C-like helicase, a 3C-like protease, and a 3D-like RNA polymerase are present. The complete genome of Camberwell virus, when compared pairwise with those of Southampton, Norwalk, and Lordsdale viruses, showed 59.7, 59.3, and 93.2% identities, respectively, at the nucleotide level. For ORF1 only, the corresponding identities were 60.0, 59.9, and 93.3%. The close relationships between Camberwell and Lordsdale viruses in ORF2 (93.3%) and ORF3 (90.7%) have been described previously (4, 28). A comparison of the amino acid sequences encoded by ORF1 of these two genogroup 2 viruses showed an identity of 97.8%, with 37 of 1,699 amino acid differences. Of these changes, 20 of 37 were conservative; 12 changes (32.4%) were contained in the N-terminal 257 amino acids (15.1% of ORF1), and the remainder were scattered throughout the polyprotein.
A repeated sequence was observed in the Camberwell virus genome corresponding to nt 1 to 22 at the 5′ end and at nt 5081 to 5102 overlapping the 5′ terminus of ORF2 (capsid gene). There was a 19 of 22 nucleotide match between these sequences. This conservation of a repeated sequence also occurs in Southampton, Norwalk, and Lordsdale viruses, as well as FCV and RHDV, although for the latter the repeated sequence is found at the beginning of the capsid gene in ORF1. For both FCV and RHDV, the repeated sequence is located at the 5′ terminus of the subgenomic RNA encoding the capsid protein (3, 23). The function of the repeated sequences in the NLVs is still unknown, but comparison with RHDV and FCV suggests a possible role in the synthesis of the subgenomic RNA for the human viruses (12).
Analysis of viral proteins in transfected mammalian cells.
The vector pCMV5 (a gift from Anthony Mason) was used to express the ORF1 cDNA of Camberwell virus. The vector contains the cytomegalovirus immediate-early promoter upstream of a multiple-cloning site, followed by the simian virus 40 polyadenylation signal and origin of replication. ORF1 cDNA of Camberwell virus (nt 5 to 5383) was inserted into the vector between the EcoRI and XbaI sites. This plasmid was designated pCMC1 (abbreviated name, C1) and was introduced into COS-M6 cells by transfection with FuGENE 6 reagent (Roche). After 24 h, the cells were incubated in medium deficient in methionine and cysteine for 1 h. The medium was removed and replaced with medium containing 100 μCi of Trans35S-label (ICN) per ml and incubated for 4 h. The same labeling procedure was used in all subsequent experiments with other constructs. The cells were washed with phosphate-buffered saline and cell lysates were immunoprecipitated as described previously (26) with one of three region-specific antisera. These antisera were raised in rabbits against glutathione S-transferase fusion proteins obtained by using the pGEX vectors in E. coli (29). The proteins contained parts of either the putative helicase, protease, or polymerase regions (Fig. 1). The corresponding antisera were later designated anti-Hel, anti-Pro/Pol, and anti-Pol.
FIG. 1.
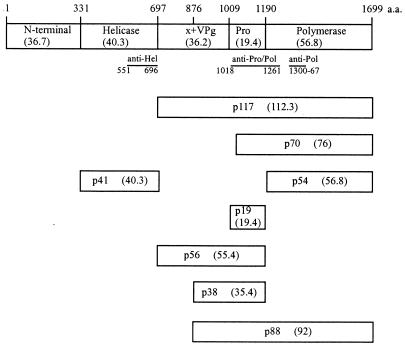
Schematic representation of the Camberwell virus ORF1 polyprotein, containing 1,699 amino acids. Cleavage products are shown as boxes. The molecular masses in kilodaltons determined by SDS-PAGE are indicated as “p” values, whereas those calculated from the deduced amino acid (a.a.) sequence are shown in parentheses. The solid lines below the ORF1 polyprotein represent the fusion proteins used in the production of antisera. Probable cleavage sites are Q330/G and Q696/G (21) and E875/G (22); sites confirmed in these experiments are E1008/A and E1189/G (Fig. 3). x, a predicted protein of 20 kDa.
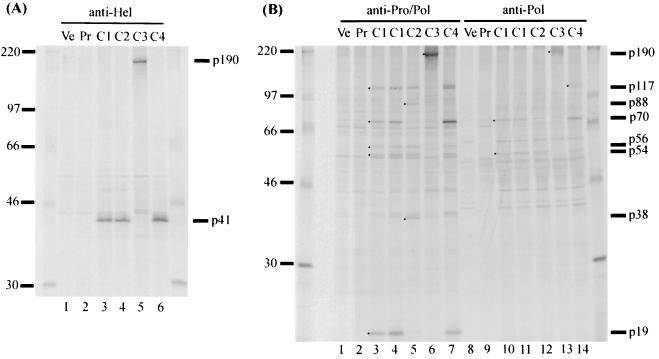
Six polypeptides, p41, p117, p70, p54, p56, and p19, were detected by radioimmunoprecipitation (RIP) of the transfected cell lysate. The anti-Hel antiserum precipitated the protein p41 (Fig. 2A, lane 3), corresponding to the 41-kDa helicase protein of Southampton virus, which was detected following in vitro transcription and translation (21). The probable cleavage sites for Camberwell virus were Q330/G and Q696/G (Fig. 1). The anti-Pro/Pol antiserum precipitated p117 (Fig. 2B, lane 3), corresponding to the 113-kDa product of Southampton virus. It matched the calculated size of a C-terminal product generated by cleavage at Q696/G (Fig. 1). Protein p117 was not precipitated to a detectable level by anti-Pol in the experiment shown in Fig. 2B (lane 10) but was observed on other occasions (see Fig. 4, lane 11).
FIG. 2.
SDS-PAGE analysis of proteins immunoprecipitated from transfected COS cells by the anti-Hel (A) and anti-Pro/Pol or anti-Pol (B) antisera. Lanes Ve, lysates of COS cells transfected with the vector pCMV5 lacking an insert; lanes Pr, lysates of cells transfected with pCMC1 and precipitated with corresponding preimmune sera; lanes C1 to C4, lysates of cells transfected with the pCMC constructs listed in Fig. 3. C1 samples are shown in duplicate lanes in Fig. 2B. The 14C-labeled molecular mass markers (in kilodaltons) are shown on the left. The viral proteins detected are indicated on the right and by dots in the lanes where appropriate.
FIG. 4.
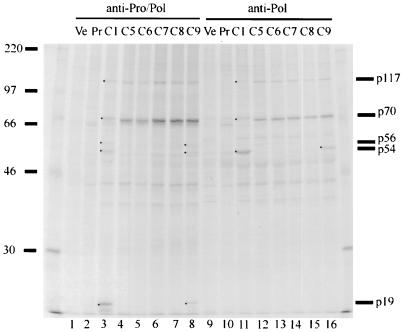
SDS-PAGE analysis of proteins immunoprecipitated from transfected cells by the anti-Pro/Pol or anti-Pol antiserum. Lanes Ve, lysates of COS cells transfected with the vector pCMV5 lacking an insert; lanes Pr, lysates of cells transfected with pCMC1 and precipitated with corresponding preimmune sera; lanes C1 and C5 to C9, lysates of cells transfected with constructs listed in Fig. 3. The 14C-labeled molecular mass markers (in kilodaltons) are shown on the left. The viral proteins detected are indicated on the right and by dots in the lanes where appropriate.
Proteins p70 and p54 were precipitated by both anti-Pro/Pol and anti-Pol (Fig. 2B, lanes 3 and 10) antisera, suggesting that these products are also located in the C-terminal region of the ORF1 polyprotein (Fig. 1). They matched the calculated sizes of proteins generated by cleavage at the N and C termini of the putative protease protein (see below).
Two other products, p56 and p19, were precipitated by anti-Pro/Pol antiserum only (Fig. 2B, lanes 3 and 10). Based on their sizes and precipitation pattern, the proposed locations of the two polypeptides in the ORF1 polyprotein are shown in Fig. 1. The protease motif H1038…E1062…GDC1147 (18) is contained in p19, which is also similar in size to the protease of RHDV (15 kDa) (33). The C residue in the conserved GDC1147 motif is thought to be involved in peptide bond cleavage (7). Thus, to confirm that a virus-encoded protease was required for the observed proteolytic processing, C1147 and the neighboring C1149 were mutated to A in construct pCMC3 (Fig. 3). A single product of 190 kDa corresponding to the size of the complete ORF1 polyprotein was observed following RIPs using either anti-Hel, anti-Pro/Pol, or anti-Pol antiserum with pCMC3-transfected cell lysate (Fig. 2A, lane 5; Fig. 2B, lanes 6 and 13). Therefore, mutagenesis of the protease motif abolished proteolytic activity, showing that the viral 3C-like protease was responsible for the processing of the polyprotein.
FIG. 3.
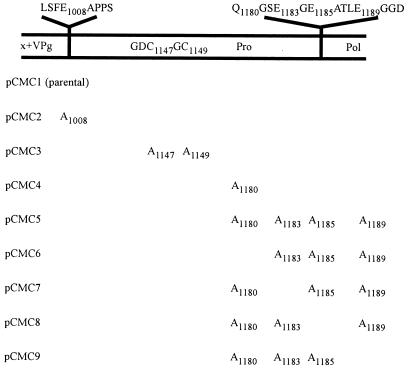
Constructs containing the cDNA of Camberwell virus ORF1 mutagenized in the protease-encoding region. Mutations were introduced by overlap PCR (14) and confirmed by sequencing. Amino acid sequences flanking the cleavage sites and the protease motif are shown. The amino acid(s) mutated to alanine for each construct is listed. Lysates of cells transfected with the various constructs are designated by the letter C and corresponding number in Fig. 2 and 4.
Mutation at E1008/A.
To identify the cleavage sites defining p19, the deduced amino acid sequences of Southampton, Norwalk, Lordsdale, and Camberwell viruses were compared. A conserved sequence, FE1008/APP (Camberwell sequence numbering), was identified in all four viruses; this site was chosen as possibly defining the N terminus of p19 since E/A was known to be a cleavage site in FCV (30). Downstream, the sequence TQ1180/GSE was conserved in Lordsdale and Camberwell viruses; cleavage at Q/G was known to define the Southampton virus helicase (21) and, presumably, Camberwell virus helicase (Q330/G and Q696/G) from the results shown in Fig. 2A (lane 3). The region defined by these sites corresponded to a protein of 172 amino acids (18.5 kDa). Overlap PCR (14) was used to introduce mutations at the proposed cleavage sites.
Residue E1008 was mutated to A in construct pCMC2 (Fig. 3). RIPs using the anti-Pro/Pol antiserum with cells transfected by pCMC2 showed a loss of p70 and p19 and the addition of p88 and p38 (Fig. 2B, lane 5). We propose that the latter two proteins arose by cleavage at a site upstream of the blocked E1008/A site, possibly at KTE875/GKK (Fig. 1). This site is conserved in Southampton, Norwalk, and Lordsdale viruses, and cleavage at this location in the Southampton virus ORF1 polyprotein synthesized in E. coli was demonstrated (22). We also suggest that the C terminus of p38 is generated by cleavage at the C terminus of the protease. In support of these proposals, there were good matches between the observed (in gels) and calculated (deduced from amino acid sequence) sizes of the two proteins (Fig. 1). It is likely that cleavage at E875/G occurs at a lower rate than cleavage at E1008/A since p88 and p38 were not observed in cells transfected with pCMC1 (Fig. 2B, lanes 3 and 5).
RIPs using anti-Pro/Pol with cells transfected by pCMC2 also showed a loss of p70 (Fig. 2B, lane 5). A similar result was obtained when anti-Pol (Fig. 2B, lane 12) was used, although the lack of p70 was less clear due to a closely migrating host cell band. Together, the RIP results obtained with anti-Pro/Pol and anti-Pol implicated E1008/A as the cleavage site, defining the N terminus of p19 and p70.
Mutation at Q1180/G and adjoining sites.
Cleavage at Q1180/G, proposed to define the C terminus of p19, was tested by mutagenesis of Q1180 to A in pCMC4 (Fig. 3). The proteins immunoprecipitated by anti-Pro/Pol and anti-Pol antisera from lysates of cells transfected by pCMC4 or pCMC1 were identical (Fig. 2B, lanes 3 and 7 and 10 and 14). This indicated either that Q1180/G was not the cleavage site or that other potential cleavage sites in the vicinity were utilized as a result of the blocked Q1180/G. The amino acid sequences of this region in Southampton, Norwalk, Lordsdale, and Camberwell viruses were compared for conserved sites. Three potential cleavage locations downstream of Q1180G were identified at E1183/G, E1185/A, and E1189/G; E/G and E/A were known cleavage sites in RHDV and FCV, respectively (1, 30, 33). Cleavage at any one of these sites would produce a protein the size of p19. In order to identify the true cleavage site, a series of constructs was made (Fig. 3). In pCMC5, all four cleavage sites were mutagenized, and then each of the potential cleavage sites was restored individually in pCMC6 to pCMC9. Proteins p56, p54, and p19 were not immunoprecipitated by anti-Pro/Pol from lysates of cells transfected with pCMC5, pCMC6, pCMC7, or pCMC8, whereas a strong band corresponding to p70 was detected. These results were consistent with no cleavage in the vicinity of amino acids 1180 to 1185 (Fig. 4, lanes 3 to 7). However, p56, p54, and p19 were present in cells transfected with pCMC9 (Fig. 4, lane 8), indicating that the restored cleavage site E1189/G in pCMC9 is utilized in the processing of the ORF1 polyprotein. Similarly, when immunoprecipitated by anti-Pol, lysates of cells transfected with pCMC5, pCMC6, pCMC7, and pCMC8 lacked p54 and a strong band of p70 was detected (Fig. 4, lanes 11 to 15). The protein p54 was, however, immunoprecipitated from lysates of cells transfected with pCMC9 (Fig. 4, lane 16). Together, these results showed that cleavage at the site E1189/G generated the C terminus of the protease. The faster migration of p70 from cells transfected with pCMC5 to pCMC9 (Fig. 4, lanes 4 to 8 and 12 to 16) was presumably due to alanine substitution for three or more amino acids. Although the cleavage site was restored in pCMC9, lysates of cells transfected with this construct still showed a strong retention of p70 (Fig. 4, lanes 8 and 16). This suggested that cleavage at E1189/G was suboptimal, due to the changed sequence (three alanine substitutions) immediately upstream.
The results presented above demonstrate that cleavage occurs at a minimum of five sites in the Camberwell virus ORF1 polyprotein when synthesized in mammalian cells (Fig. 1). They extend the results obtained for the genogroup 1 virus, Southampton, by using in vitro-coupled transcription and translation (Q/G sites identified) and expression in E. coli (E/G and E/A sites identified) (21, 22). The proteins detected in COS cells include p41, p19, and p54.
The protein p41 of Camberwell virus corresponds to the putative helicase p41 of Southampton virus and is presumably released by cleavage at Q330/G and Q696/G. For Southampton virus, these sites were identified by using site-directed mutagenesis and in vitro transcription and translation. The protease is p19; the two cleavage sites defining p19 were identified by N-terminal analysis of bacterially synthesized protein for Southampton virus (22) and are now confirmed by Camberwell virus ORF1 expression in COS cells and site-specific mutagenesis. Cleavage occurred at E1008/A and E1189/G in our experiments, whereas none was detected for Southampton virus by using coupled transcription and translation. Presumably, host cell factors not available in the cell-free system are required for the proteolysis. Mutagenesis of the cysteines in the ..GDC1147GC1149.. motif in p19 abolished proteolytic activity (C3 in Fig. 2), confirming the assignment of p19 as the protease. The putative polymerase protein p54 was detected as a cleavage product and also as part of the proteins p117, p70, and p88 (expressed from pCMC2). These intermediate products and others such as p56 (containing the protease) may act in regulating the replication cycle (22), as proposed for picornaviruses (2, 11, 24, 36).
Some additional products of cleavage of the ORF1 polyprotein are predicted, although suitable antisera for their identification were not available in this study and they were not detected in direct analyses of nonimmunoprecipitated cell lysates (data not shown). First, the predicted N-terminal protein encoded by Camberwell virus has a size calculated from the deduced amino acid sequence of 36.7 kDa. Mainly because of the differences in sequence towards the N terminus, it is smaller than the corresponding Southampton p48 detected after coupled transcription and translation. This is of interest because the analogous 39-kDa protein of RHDV is cleaved into picornavirus 2A-like (16-kDa) and 2B-like (23-kDa) proteins (34), and 2A-like proteins are variable in size and sequence (27). No processing of the Southampton p48 was detected in vitro (21). It is possible that the Camberwell and Southampton N-terminal proteins are further processed in mammalian cells, but this remains to be investigated. Second, a protein of 20 kDa (Fig. 1, labeled “X”) corresponding to amino acids 697 to 875 is predicted. The detection of proteins p38 and p88 following mutagenesis at the E1008/A site were consistent with cleavage at E875/G. This latter site was identified for Southampton virus by N-terminal analysis of bacterially synthesized protein (22). The protein occupying this region is hypothesized to be the picornavirus 3A-like protein (22, 34). Third, the likely VPg lies between E875/G and E1008/A based on the similarities of its size and position to RHDV (23, 34), FCV (13), and the primate calicivirus Pan-1 (8). The conserved motifs of VPg, KGK(N/T)K and EY(E/D)E, are also found here (8).
In summary, we determined the full genomic sequence of Camberwell virus and demonstrated its close similarity to Lordsdale virus. Processing of the NLV ORF1 polyprotein in mammalian (COS) cells produced polypeptides ranging in size from 19 to 117 kDa. In particular, we have shown that cleavage in mammalian cells at E/A and E/G forms p19, a 3C-like protease.
Nucleotide sequence accession number.
The GenBank accession number for the sequence of the Camberwell virus genome is AF145896.
Acknowledgments
This work was supported by a project grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Alonso J M M, Casais R, Boga J A, Parra F. Processing of rabbit hemorrhagic disease virus polyprotein. J Virol. 1996;70:1261–1265. doi: 10.1128/jvi.70.2.1261-1265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 3.Carter M J, Milton I D, Meanger J, Bennett M, Gaskell R M, Turner P C. The complete nucleotide sequence of a feline calicivirus. Virology. 1992;190:443–448. doi: 10.1016/0042-6822(92)91231-i. [DOI] [PubMed] [Google Scholar]
- 4.Cauchi M R, Doultree J C, Marshall J A, Wright P J. Molecular characterization of Camberwell virus and sequence variation in ORF3 of small round-structured (Norwalk-like) viruses. J Med Virol. 1996;49:70–76. doi: 10.1002/(SICI)1096-9071(199605)49:1<70::AID-JMV12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.De Leon R, Matsui S M, Baric R S, Herrmann J E, Blacklow N R, Greenberg H B, Sobsey M D. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol. 1992;30:3151–3157. doi: 10.1128/jcm.30.12.3151-3157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingle K E, Lambden P R, Caul E O, Clarke I N. Human enteric Caliciviridae—the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J Gen Virol. 1995;76:2349–2355. doi: 10.1099/0022-1317-76-9-2349. [DOI] [PubMed] [Google Scholar]
- 7.Dougherty W G, Semler B L. Expression of virus-encoded proteinases: functional and structural similarities with cellular enzymes. Microbiol Rev. 1993;57:781–822. doi: 10.1128/mr.57.4.781-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunham D M, Jiang X, Berke T, Smith A W, Matson D O. Genomic mapping of a calicivirus VPg. Arch Virol. 1998;143:2421–2430. doi: 10.1007/s007050050471. [DOI] [PubMed] [Google Scholar]
- 9.Frohman M A, Dush M K, Martin G R. Rapid production of full length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy M E, Estes M K. Completion of the Norwalk virus genome sequence. Virus Genes. 1996;12:287–290. doi: 10.1007/BF00284649. [DOI] [PubMed] [Google Scholar]
- 11.Harris K S, Reddigari S R, Nicklin M J, Hammerle T, Wimmer E. Purification and characterization of poliovirus polypeptide 3CD, a proteinase and a precursor for RNA polymerase. J Virol. 1992;66:7481–7489. doi: 10.1128/jvi.66.12.7481-7489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert T P, Brierley I, Brown T D K. Detection of the ORF3 polypeptide of feline calicivirus in infected cells and evidence for its expression from a single, functionally bicistronic, subgenomic mRNA. J Gen Virol. 1996;77:123–127. doi: 10.1099/0022-1317-77-1-123. [DOI] [PubMed] [Google Scholar]
- 13.Herbert T P, Brierley I, Brown T D K. Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J Virol. 1997;78:1033–1040. doi: 10.1099/0022-1317-78-5-1033. [DOI] [PubMed] [Google Scholar]
- 14.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Matson D O, Cubitt W D, Estes M K. Genetic and antigenic diversity of human caliciviruses (HuCVs) using RT-PCR and new EIAs. Arch Virol Suppl. 1996;12:251–262. doi: 10.1007/978-3-7091-6553-9_27. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Wang M, Wang K N, Estes M K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 17.Kapikian A Z. Norwalk and Norwalk-like viruses. In: Kapikian A Z, editor. Viral infections of the gastrointestinal tract. 2nd ed. New York, N.Y: Marcel Dekker; 1994. pp. 471–518. [Google Scholar]
- 18.Koonin E V, Dolja V V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 19.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 20.Lambden P R, Liu B, Clarke I N. A conserved sequence motif at the 5′ terminus of the Southampton virus genome is characteristic of the Caliciviridae. Virus Genes. 1995;10:149–152. doi: 10.1007/BF01702595. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Clarke I N, Lambden P R. Polyprotein processing in Southampton virus: identification of 3C-like protease cleavage sites by in vitro mutagenesis. J Virol. 1996;70:2605–2610. doi: 10.1128/jvi.70.4.2605-2610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B L, Viljoen G J, Clarke I N, Lambden P R. Identification of further proteolytic cleavage sites in the Southampton calicivirus polyprotein by expression of the viral protease in E. coli. J Gen Virol. 1999;80:291–296. doi: 10.1099/0022-1317-80-2-291. [DOI] [PubMed] [Google Scholar]
- 23.Meyers G, Wirblich C, Thiel H J. Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology. 1991;184:677–686. doi: 10.1016/0042-6822(91)90437-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotch S J, Palant O. Poliovirus protein 3AB forms a complex with and stimulates the activity of the viral RNA polymerase, 3Dpol. J Virol. 1995;69:7169–7179. doi: 10.1128/jvi.69.11.7169-7179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pringle C R. Virus taxonomy—San Diego 1998. Arch Virol. 1998;143:1449–1459. doi: 10.1007/s007050050389. [DOI] [PubMed] [Google Scholar]
- 26.Pryor M J, Wright P J. The effects of site directed mutagenesis on the dimerisation and secretion of the NS1 protein specified by dengue virus. Virology. 1993;194:769–780. doi: 10.1006/viro.1993.1318. [DOI] [PubMed] [Google Scholar]
- 27.Ryan M D, Flint M. Virus-encoded proteinases of the picornavirus super-group. J Gen Virol. 1997;78:699–723. doi: 10.1099/0022-1317-78-4-699. [DOI] [PubMed] [Google Scholar]
- 28.Seah E L, Gunesekere I C, Marshall J A, Wright P J. Variation in ORF3 of genogroup 2 Norwalk-like viruses. Arch Virol. 1999;144:1007–1014. doi: 10.1007/s007050050563. [DOI] [PubMed] [Google Scholar]
- 29.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Sosnovtsev S V, Sosnovtseva S A, Green K Y. Cleavage of the feline calicivirus capsid precursor is mediated by a virus-encoded proteinase. J Virol. 1998;72:3051–3059. doi: 10.1128/jvi.72.4.3051-3059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosnovtseva S A, Sosnovtsev S V, Green K Y. Mapping of the feline calicivirus proteinase responsible for autocatalytic processing of the nonstructural polyprotein and identification of a stable proteinase-polymerase precursor. J Virol. 1999;73:6626–6633. doi: 10.1128/jvi.73.8.6626-6633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J X, Jiang X, Madore H P, Gray J, Desselberger U, Ando T, Seto Y, Oishi I, Lew J F, Green K Y, Estes M. Sequence diversity of small, round-structured viruses in the Norwalk virus group. J Virol. 1994;68:5982–5990. doi: 10.1128/jvi.68.9.5982-5990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirblich C, Sibilia M, Boniotti M B, Rossi C, Thiel H J, Meyers G. 3C-like protease of rabbit hemorrhagic disease virus: identification of cleavage sites in the ORF1 polyprotein and analysis of cleavage specificity. J Virol. 1995;69:7159–7168. doi: 10.1128/jvi.69.11.7159-7168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wirblich C, Thiel H J, Meyers G. Genetic map of the calicivirus rabbit hemorrhagic disease virus as deduced from in vitro translation studies. J Virol. 1996;70:7974–7983. doi: 10.1128/jvi.70.11.7974-7983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright P J, Gunesekere I C, Doultree J C, Marshall J A. Small round-structured (Norwalk-like) viruses and classical human caliciviruses in southeastern Australia, 1980–1996. J Med Virol. 1998;55:312–320. [PubMed] [Google Scholar]
- 36.Xiang W, Harris K S, Alexander L, Wimmer E. Interaction between the 5′-terminal cloverleaf and 3AB/3CDpro of poliovirus is essential for RNA replication. J Virol. 1995;69:3658–3667. doi: 10.1128/jvi.69.6.3658-3667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]