Abstract
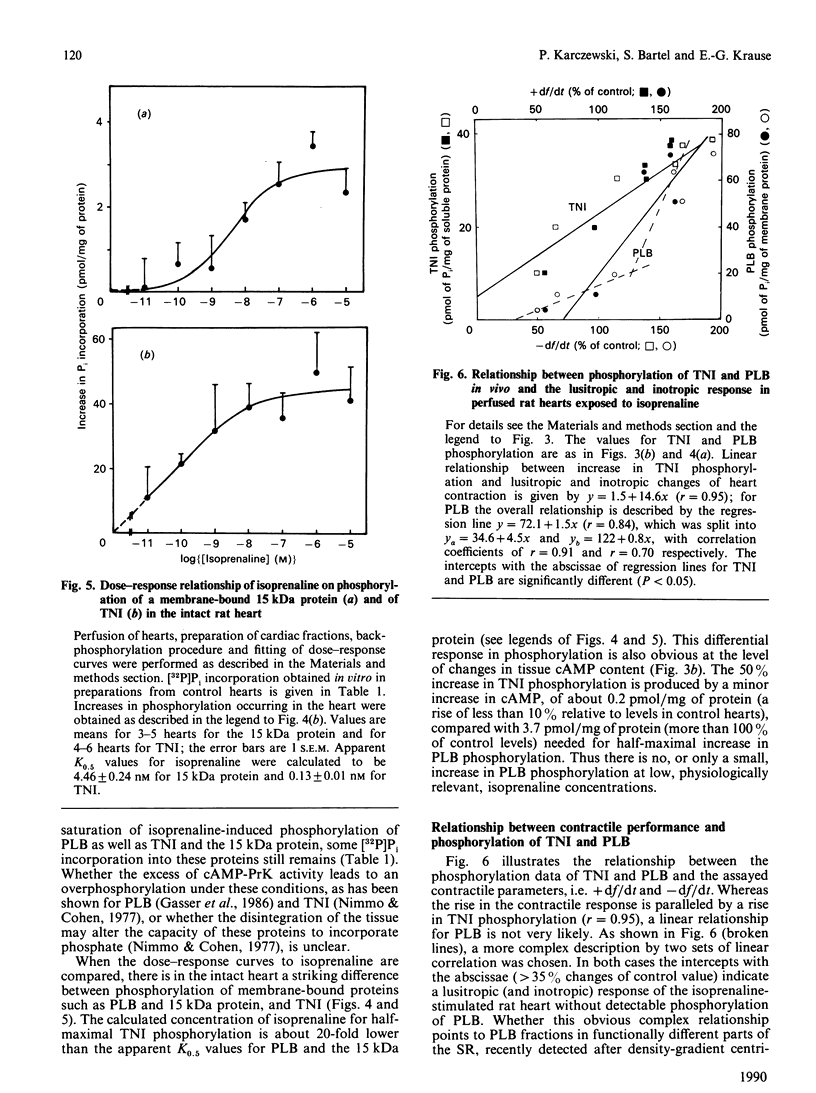
Phosphorylation of phospholamban (PLB), a membrane-bound 15 kDa protein and troponin I (TNI) was studied in isolated perfused rat hearts by using the back-phosphorylation technique with [32P]ATP catalysed by an excess of exogenous catalytic subunit of cyclic AMP (cAMP)-dependent protein kinase, followed by protein separation. This standardized method allows the quantitative detection of protein phosphorylation specifically stimulated by cAMP. In control hearts the extent of specific phosphorylation was equivalent to 3.3 nmol of PLB and 11.0 mumol of TNI per g of cardiac tissue. In hearts freeze-clamped 30 s after exposure to isoprenaline (10 pM-10 microM), there was a dose-dependent decrease in phosphate incorporation in vitro, indicating a phosphorylation of the respective proteins in vivo. A differential sensitivity of TNI and PLB phosphorylation towards the beta-adrenergic agonist and the subsequent increase in tissue cAMP was found, favouring TNI phosphorylation. K0.5 values for isoprenaline were 2.94 +/- 0.04 nM and 4.46 +/- 0.24 nM for PLB and the 15 kDa protein, but 0.13 +/- 0.01 nM for TNI phosphorylation in the intact tissue. At an isoprenaline-induced increase in cAMP less than 3 pmol/mg of protein there was no or only a small increase in PLB phosphorylation, whereas TNI phosphorylation was nearly maximal. By plotting phosphorylation data against changes in contractile parameters a strong correlation was obtained for TNI (r = 0.95), assuming a linear relationship. For PLB a complex relationship is likely to exist. Our data (i) indicate a functional compartmentalization of the cAMP signal cascade and (ii) confirm that phosphorylation of TNI rather than of PLB is related to changes in mechanical myocardial responses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunton L. L., Hayes J. S., Mayer S. E. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res. 1981;14:391–397. [PubMed] [Google Scholar]
- Ceconi C., Curello S., Cargnoni A., Ferrari R., Albertini A., Visioli O. The role of glutathione status in the protection against ischaemic and reperfusion damage: effects of N-acetyl cysteine. J Mol Cell Cardiol. 1988 Jan;20(1):5–13. doi: 10.1016/s0022-2828(88)80174-3. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Correlation between contraction and phosphorylation of the inhibitory subunit of troponin in perfused rat heart. FEBS Lett. 1975 Jan 15;50(1):57–60. doi: 10.1016/0014-5793(75)81040-4. [DOI] [PubMed] [Google Scholar]
- England P. J., Shahid M. Effects of forskolin on contractile responses and protein phosphorylation in the isolated perfused rat heart. Biochem J. 1987 Sep 15;246(3):687–695. doi: 10.1042/bj2460687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forn J., Greengard P. Depolarizing agents and cyclic nucleotides regulate the phosphorylation of specific neuronal proteins in rat cerebral cortex slices. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5195–5199. doi: 10.1073/pnas.75.10.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii J., Ueno A., Kitano K., Tanaka S., Kadoma M., Tada M. Complete complementary DNA-derived amino acid sequence of canine cardiac phospholamban. J Clin Invest. 1987 Jan;79(1):301–304. doi: 10.1172/JCI112799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey J. L., Kranias E. G., Solaro R. J. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem J. 1988 Feb 1;249(3):709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser J. T., Chiesi M. P., Carafoli E. Concerted phosphorylation of the 26-kilodalton phospholamban oligomer and of the low molecular weight phospholamban subunits. Biochemistry. 1986 Nov 18;25(23):7615–7623. doi: 10.1021/bi00371a052. [DOI] [PubMed] [Google Scholar]
- Gasser J., Paganetti P., Carafoli E., Chiesi M. Heterogeneous distribution of calmodulin- and cAMP-dependent regulation of Ca2+ uptake in cardiac sarcoplasmic reticulum subfractions. Eur J Biochem. 1988 Oct 1;176(3):535–541. doi: 10.1111/j.1432-1033.1988.tb14311.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Tada M., Kirchberger M. A. Control of calcium transport in the myocardium by the cyclic AMP-Protein kinase system. Adv Cyclic Nucleotide Res. 1975;5:453–472. [PubMed] [Google Scholar]
- Kovacs R. J., Nelson M. T., Simmerman H. K., Jones L. R. Phospholamban forms Ca2+-selective channels in lipid bilayers. J Biol Chem. 1988 Dec 5;263(34):18364–18368. [PubMed] [Google Scholar]
- Kranias E. G., Mandel F., Wang T., Schwartz A. Mechanism of the stimulation of calcium ion dependent adenosine triphosphatase of cardiac sarcoplasmic reticulum by adenosine 3',5'-monophosphate dependent protein kinase. Biochemistry. 1980 Nov 11;19(23):5434–5439. doi: 10.1021/bi00564a044. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Lindemann J. P., Watanabe A. M. Phosphorylation of phospholamban in intact myocardium. Role of Ca2+-calmodulin-dependent mechanisms. J Biol Chem. 1985 Apr 10;260(7):4516–4525. [PubMed] [Google Scholar]
- Manalan A. S., Jones L. R. Characterization of the intrinsic cAMP-dependent protein kinase activity and endogenous substrates in highly purified cardiac sarcolemmal vesicles. J Biol Chem. 1982 Sep 10;257(17):10052–10062. [PubMed] [Google Scholar]
- Manning D. R., DiSalvo J., Stull J. T. Protein phosphorylation: quantitative analysis in vivo and in intact cell systems. Mol Cell Endocrinol. 1980 Jul;19(1):1–19. doi: 10.1016/0303-7207(80)90026-x. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Guidotti A. Simultaneous isolation of adenosine 3',5'-cyclic monophosphate (cAMP) and guanosine 3',5'-cyclic monophosphate (cGMP) in small tissue samples. Anal Biochem. 1974 May;59(1):63–68. doi: 10.1016/0003-2697(74)90009-8. [DOI] [PubMed] [Google Scholar]
- Moir A. J., Solaro R. J., Perry S. V. The site of phosphorylation of troponin I in the perfused rabbit heart. The effect of adrenaline. Biochem J. 1980 Feb 1;185(2):505–513. doi: 10.1042/bj1850505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mope L., McClellan G. B., Winegrad S. Calcium sensitivity of the contractile system and phosphorylation of troponin in hyperpermeable cardiac cells. J Gen Physiol. 1980 Mar;75(3):271–282. doi: 10.1085/jgp.75.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesian M. A., Nishikawa M., Adelstein R. S. Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. Stimulation of cardiac sarcoplasmic reticulum calcium uptake. J Biol Chem. 1984 Jul 10;259(13):8029–8032. [PubMed] [Google Scholar]
- Mundiña de Weilenmann C., Vittone L., de Cingolani G., Mattiazzi A. Dissociation between contraction and relaxation: the possible role of phospholamban phosphorylation. Basic Res Cardiol. 1987 Nov-Dec;82(6):507–516. doi: 10.1007/BF01907220. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Presti C. F., Jones L. R., Lindemann J. P. Isoproterenol-induced phosphorylation of a 15-kilodalton sarcolemmal protein in intact myocardium. J Biol Chem. 1985 Mar 25;260(6):3860–3867. [PubMed] [Google Scholar]
- Presti C. F., Scott B. T., Jones L. R. Identification of an endogenous protein kinase C activity and its intrinsic 15-kilodalton substrate in purified canine cardiac sarcolemmal vesicles. J Biol Chem. 1985 Nov 5;260(25):13879–13889. [PubMed] [Google Scholar]
- Rapundalo S. T., Solaro R. J., Kranias E. G. Inotropic responses to isoproterenol and phosphodiesterase inhibitors in intact guinea pig hearts: comparison of cyclic AMP levels and phosphorylation of sarcoplasmic reticulum and myofibrillar proteins. Circ Res. 1989 Jan;64(1):104–111. doi: 10.1161/01.res.64.1.104. [DOI] [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Reich J. G., Wangermann G., Falck M., Rohde K. A general strategy for parameter estimation from isosteric and allosteric-kinetic data and binding measurements. Eur J Biochem. 1972 Apr 11;26(3):368–379. doi: 10.1111/j.1432-1033.1972.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Resink T. J., Gevers W. Dephosphorylation of myofibrillar proteins in actomyosin preparations and in isolated perfused rat hearts after beta-agonist withdrawal. J Mol Cell Cardiol. 1982 Jun;14(6):329–337. doi: 10.1016/0022-2828(82)90248-6. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Tada M., Yamada M., Ohmori F., Kuzuya T., Inui M., Abe H. Transient state kinetic studies of Ca2+-dependent ATPase and calcium transport by cardiac sarcoplasmic reticulum. Effect of cyclic AMP-dependent protein kinase-catalyzed phosphorylation of phospholamban. J Biol Chem. 1980 Mar 10;255(5):1985–1992. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]



