Abstract
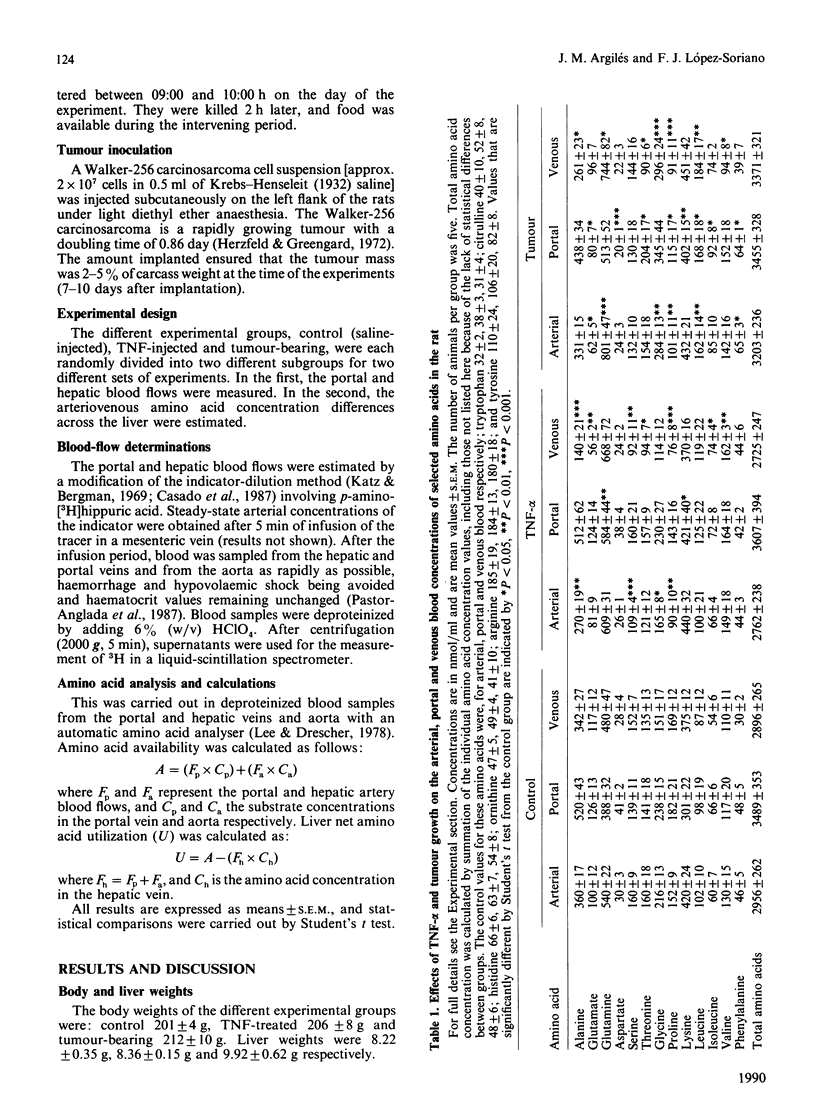
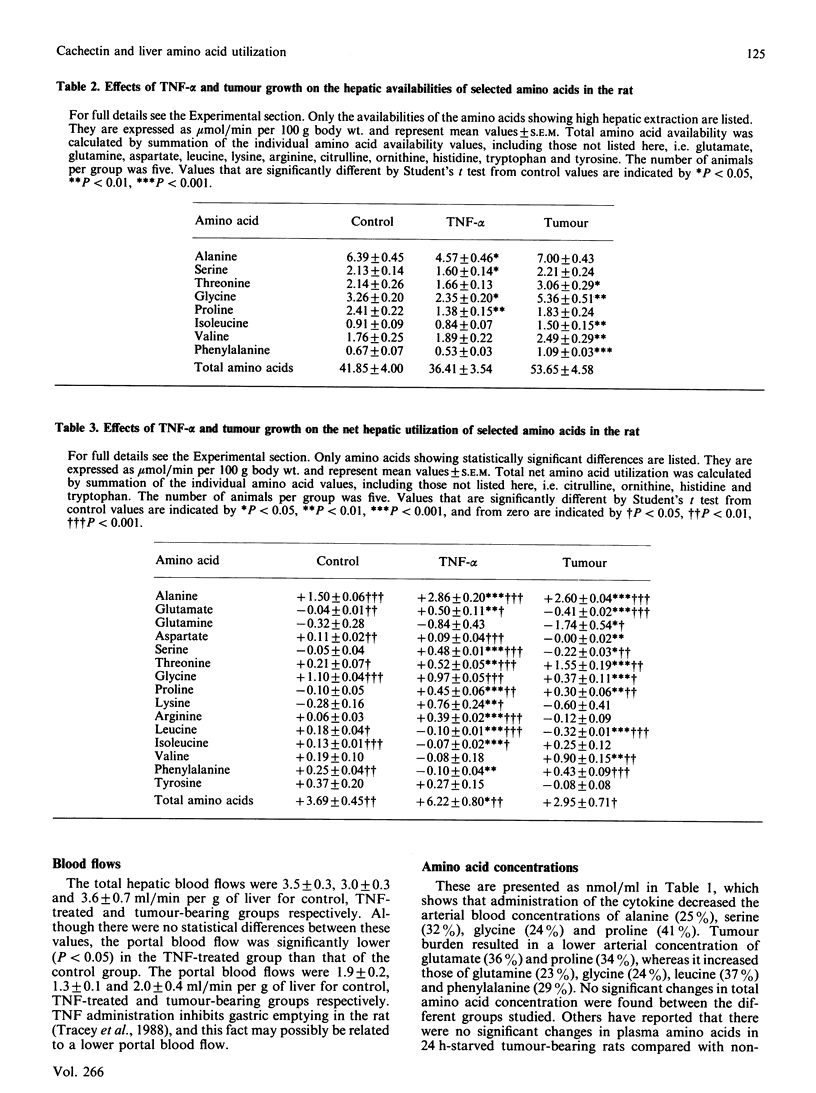
The effects of acute administration of tumour necrosis factor-alpha (cachectin) (TNF-alpha) or of malignant tumour growth (Walker-256 carcinosarcoma) on hepatic availability and uptake of individual amino acids were compared. The results show that, in spite of lowering the hepatic availability of alanine, aspartate, serine, glycine and proline, the cytokine increased both the total amino acid hepatic uptake and the individual uptakes of alanine, glutamate, serine, threonine, proline, lysine and arginine, while decreasing those of leucine, isoleucine and phenylalanine. Tumour burden resulted in an increase in the hepatic availability of glutamine, threonine, glycine, lysine, leucine, isoleucine, valine and phenylalanine. Total liver amino acid uptake was unaffected, whereas the individual uptakes of alanine, threonine and proline were increased and those of glutamate, glutamine, serine and leucine were decreased. When effects of the cytokine are compared with those induced by tumour growth, there are similar increases in net utilization for alanine, proline and leucine, and a 3-fold difference in the increase observed for threonine. Unmatched effects are seen for glutamate, glutamine, aspartate, glycine, lysine, arginine, valine, phenylalanine and serine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit J. M., Gorschboth C. M., Brennan M. F. Basal amino acid concentrations and the response to incremental glucose infusion in tumor bearing rats. Cancer Res. 1985 Dec;45(12 Pt 1):6296–6300. [PubMed] [Google Scholar]
- Argilés J. M., Azcón-Bieto J. The metabolic environment of cancer. Mol Cell Biochem. 1988 May;81(1):3–17. doi: 10.1007/BF00225648. [DOI] [PubMed] [Google Scholar]
- Argilés J. M., López-Soriano F. J., Wiggins D., Williamson D. H. Comparative effects of tumour necrosis factor-alpha (cachectin), interleukin-1-beta and tumour growth on amino acid metabolism in the rat in vivo. Absorption and tissue uptake of alpha-amino[1-14C]isobutyrate. Biochem J. 1989 Jul 15;261(2):357–362. doi: 10.1042/bj2610357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Casado J., Pastor-Anglada M., Remesar X. Hepatic uptake of amino acids at mid-lactation in the rat. Biochem J. 1987 Jul 1;245(1):297–300. doi: 10.1042/bj2450297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Evans R. D., Williamson D. H. Tissue-specific effects of rapid tumour growth on lipid metabolism in the rat during lactation and on litter removal. Biochem J. 1988 May 15;252(1):65–72. doi: 10.1042/bj2520065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. D., Williamson D. H. Tumour necrosis factor alpha (cachectin) mimics some of the effects of tumour growth on the disposal of a [14C]lipid load in virgin, lactating and litter-removed rats. Biochem J. 1988 Dec 15;256(3):1055–1058. doi: 10.1042/bj2561055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. The dedifferentiated pattern of enzymes in livers of tumor-bearing rats. Cancer Res. 1972 Sep;32(9):1826–1832. doi: 10.2172/4649739. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Simultaneous measurements of hepatic and portal venous blood flow in the sheep and dog. Am J Physiol. 1969 Apr;216(4):946–952. doi: 10.1152/ajplegacy.1969.216.4.946. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Murase T., Ogawa H., Ishibashi S., Mori N., Takaku F., Shibata S. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J Biochem. 1987 Feb;101(2):331–338. doi: 10.1093/oxfordjournals.jbchem.a121917. [DOI] [PubMed] [Google Scholar]
- Lawson D. H., Richmond A., Nixon D. W., Rudman D. Metabolic approaches to cancer cachexia. Annu Rev Nutr. 1982;2:277–301. doi: 10.1146/annurev.nu.02.070182.001425. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Drescher D. G. Fluorometric amino-acid analysis with o-phthaldialdehyde (OPA). Int J Biochem. 1978;9(7):457–467. doi: 10.1016/0020-711x(78)90075-7. [DOI] [PubMed] [Google Scholar]
- Mahony S. M., Beck S. A., Tisdale M. J. Comparison of weight loss induced by recombinant tumour necrosis factor with that produced by a cachexia-inducing tumour. Br J Cancer. 1988 Apr;57(4):385–389. doi: 10.1038/bjc.1988.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M., Remesar X., Bourdel G. Alanine uptake by liver at midpregnancy in rats. Am J Physiol. 1987 Mar;252(3 Pt 1):E408–E413. doi: 10.1152/ajpendo.1987.252.3.E408. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. R., Mizel S. B., Pekala P. H. Regulation of lipoprotein lipase synthesis and 3T3-L1 adipocyte metabolism by recombinant interleukin 1. Biochim Biophys Acta. 1986 Dec 19;889(3):374–381. doi: 10.1016/0167-4889(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Roh M. S., Moldawer L. L., Ekman L. G., Dinarello C. A., Bistrian B. R., Jeevanandam M., Brennan M. F. Stimulatory effect of interleukin-1 upon hepatic metabolism. Metabolism. 1986 May;35(5):419–424. doi: 10.1016/0026-0495(86)90131-9. [DOI] [PubMed] [Google Scholar]
- Saez S. Adrenal function in cancer: relation to the evolution. Eur J Cancer. 1971 Oct;7(5):381–387. doi: 10.1016/0014-2964(71)90035-1. [DOI] [PubMed] [Google Scholar]
- Shapot V. S. On the multiform relationships between the tumor and the host. Adv Cancer Res. 1979;30:89–150. doi: 10.1016/s0065-230x(08)60895-7. [DOI] [PubMed] [Google Scholar]
- Stovroff M. C., Fraker D. L., Swedenborg J. A., Norton J. A. Cachectin/tumor necrosis factor: a possible mediator of cancer anorexia in the rat. Cancer Res. 1988 Aug 15;48(16):4567–4572. [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Donner D. B., Starnes H. F., Jr, Brennan M. F. Modulation of endogenous hormone action by recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8619–8622. doi: 10.1073/pnas.84.23.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Alcock N., Calvano S., Brennan M. F. Hormonal and metabolic response to recombinant human tumor necrosis factor in rat: in vitro and in vivo. Am J Physiol. 1988 Aug;255(2 Pt 1):E206–E212. doi: 10.1152/ajpendo.1988.255.2.E206. [DOI] [PubMed] [Google Scholar]


