Abstract
HBB [2-(α-hydroxybenzyl)-benzimidazole] selectively inhibits RNA synthesis of most enteroviruses. However, isolation of HBB-dependent variants is possible. Sequence analysis and characterization of recombinant viruses revealed that HBB dependence maps to the nonstructural protein 2C. A single point mutation at position C4782U is sufficient to establish the HBB-dependent phenotype in our echovirus 9 model.
The antiviral agent 2-(α-hydroxybenzyl)-benzimidazole (HBB) was first described in 1958 (10) and was discovered to specifically inhibit the multiplication of many members of the genus Enterovirinae within the picornavirus family (8). Viruses belonging to other families were found to be HBB insusceptible (8). Studies on the mechanism of action of HBB demonstrated that the substance, at concentrations nontoxic to cells, inhibits the synthesis of viral RNA, whereas cellular RNA synthesis remains unaffected (6–8). In the course of this work not only HBB-resistant but also HBB-dependent virus mutants were discovered (5). In the present communication, we show that HBB dependence maps to the nonstructural protein 2C.
Isolation and characterization of an HBB-dependent echovirus 9 variant.
Echovirus 9 Barty MP5 (E9B MP5) (24) was cultivated under selective pressure of 50 μM, 150 μM, and 220 μM concentrations of HBB. To ensure that further experiments were performed with virus stocks as homogeneous as possible, the selected dependent virus stock was plaque purified with 220 μM HBB, and single virus plaques were harvested and propagated after 4 days.
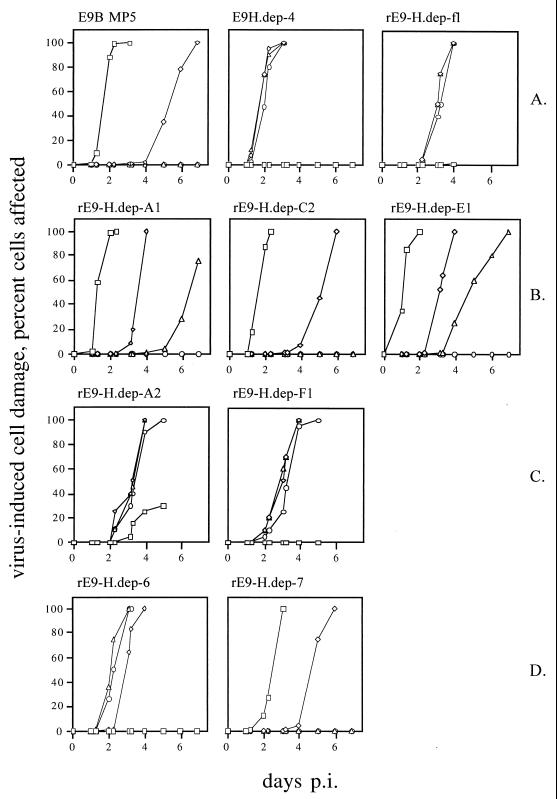
Determination of 50% tissue culture infective doses (TCID50 assays) in the presence or absence of HBB gives a first indication of the influence of HBB on replication efficiency. Ten plaque-purified isolates selected in the presence of 220 μM HBB were investigated (Table 1). Isolate 4 (E9H.dep-4) was chosen for further experiments, since its TCID50 assays revealed the highest Δ value (Table 1). In order to compare the influence of HBB on replication of E9H.dep-4 and the original isolate, E9B MP5, 10 TCID50 of the respective virus was incubated under increasing concentrations of HBB, and the amount of virus-induced cell damage was determined at the indicated time points. These sensitivity assays clearly exhibit the HBB-dependent phenotype of E9H.dep-4 (Fig. 1A).
TABLE 1.
Effect of 200 μM HBB on the replication efficiency of echovirus 9 isolates
| No. of virus isolate | TCID50a
|
Δ valueb | |
|---|---|---|---|
| Without HBB | With HBB | ||
| 1 | −5.8 | −7.2 | 1.4 |
| 2 | −1.8 | −3.6 | 1.8 |
| 3 | −2.8 | −5.5 | 2.7 |
| 4 | −2.7 | −6.0 | 3.3 |
| 5 | −4.3 | −6.6 | 2.3 |
| 6 | −3.8 | −6.8 | 3.0 |
| 7 | −1.5 | −1.5 | 0.0 |
| 8 | −2.7 | −5.9 | 3.2 |
| 9 | −5.5 | −7.6 | 2.1 |
| 10 | −4.5 | −6.4 | 1.9 |
The TCID50 value corresponds with the dilution of the investigated virus stock containing enough particles to infect and lyse cells in 50% of the wells of a microtiter plate. Given is the log10 of the value.
Δ is given by the formula TCID50 (without HBB) − TCID50 (with HBB).
FIG. 1.
HBB sensitivity assays of the echovirus 9 strain Barty, the HBB-dependent variant E9H.dep-4, and recombinant viruses. (A) Echovirus 9 Barty MP5 is sensitive to HBB, and the HBB-dependent variant E9H.dep-4 and the recombinant virus rE9-H.dep-f1 exhibit comparable dependence on the antiviral substance. The chimeras exhibit the sensitive (B) or dependent (C) character, comparable to the progenitors in panel A. (D) HBB sensitivity assays of viruses derived from RNA generated by site-directed mutagenesis. The nomenclature is the same as in Fig. 2. The indicated viruses were cultivated in the presence of 0 μM (square), 73 μM (rhomb), 146 μM (triangle), or 220 μM (circle) HBB. The extent of virus-induced cell damage reflecting viral multiplication was estimated by microscopic examination and is given as a percentage. p.i., postinfection.
Cloning and sequencing of E9H.dep-4.
Isolate E9H.dep-4 was cloned and sequenced (pE9H.dep-40.8). Comparison to the wild-type sequence (24) revealed 10 point mutations in the genome of the HBB-dependent variant, seven of which give rise to amino acid mutations (Table 2).
TABLE 2.
Nucleotide exchanges of HBB-dependent clones as compared to the echovirus 9 wild type
| Exchange no. | Affected gene | Amino acid exchange | Nucleotide position | Sequence ofa:
|
|
|---|---|---|---|---|---|
| Wild type | Dependent variant | ||||
| I | VP2 | Silent | 1066 | ccC | ccU |
| II | VP2 | Silent | 1471 | gcG | gcA |
| 1 | VP2 | Ser→Pro | 1667 | Uca | Cca |
| 2 | VP3 | Glu→Ala | 1812 | gAg | gCg |
| III | VP3 | Silent | 2248 | auC | auU |
| 3 | 2A | Phe→Tyr | 3537 | uUu | uAu |
| 4 | 2A | Gly→Asp | 3738 | gGu | gAu |
| 5 | 2B | Ile→Val | 4016 | Aua | Gua |
| 6 | 2C | Ala→Val | 4782 | gCa | gUa |
| 7 | 2C | Phe→Leu | 4970 | Uuc | Cuc |
Exchanged nucleotides are indicated by larger capital letters.
Proceeding from clone pE9H.dep-40.8 and the infectious E9 Barty clone pE9B described earlier (23), a full-length clone containing the complete dependent genome was constructed (pE9-H.dep-f1) and transcribed in vitro. The infectivity of the resulting RNA was shown in transfection experiments. In sensitivity assays, the resulting viruses (rE9-H.dep-f1) revealed a level of dependence on HBB which was quantitatively comparable to that of the original isolate, E9H.dep-4 (Fig. 1A). Since the virus dilution was done freshly for every experiment, the 24-h delay for the onset of cytopathic effect is in the range of experimental variation.
Determination of the influence of the point mutations on HBB dependence.
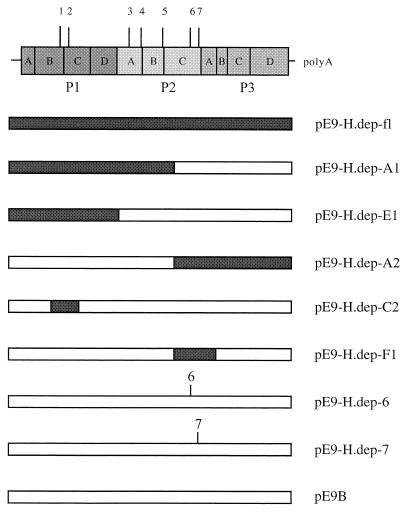
In further experiments, we analyzed which of the seven amino acid mutations gives rise to the HBB-dependent character of the variant. For that purpose, genome regions of the infectious E9 Barty clone pE9B were replaced by corresponding restriction fragments of clone pE9H.dep-40.8 containing series of the point mutations detected in the dependent genome (Fig. 2).
FIG. 2.
Survey of the genomes of chimeric echovirus 9 Barty strains. Name and position of the mutations of the dependent variant are given in the schematic genome above. Genome fragments derived from the dependent variant are represented by filled bars. Fragments obtained from the sensitive wild-type echovirus 9 are depicted by open bars.
Recombinant viral RNA carrying mutations 1 and 2 (pE9-H.dep-C2 and pE9-H.dep-E1) and 1 to 5 (pE9-H.dep-A1) proved to be infectious only in the absence of HBB (data not shown). In contrast, the RNA of clones pE9-H.dep-A2 and pE9-H.dep-F1, both containing mutations 6 and 7, codes for infective virus particles only in the presence of HBB. Sensitivity assays using recombinant viruses purified from the supernatant of the transfection experiments support these results (Fig. 1B and C). Mutations 1 to 5 do not alter the sensitive phenotype of clone pE9B (rE9-H.dep-A1, -C2, and -E1), but the introduction of mutations 6 and 7 together leads to a dependent variant (rE9-H.dep-A2 and -F1).
Introduction of mutation 6 or 7 alone by exchanging appropriate restriction fragments was not possible, since both mutations are located in close proximity. Hence, they were introduced to pE9B-ic by site-directed mutagenesis. In each case, three independent clones were tested in transfection experiments and were discovered to be infectious depending on HBB concentration. RNA containing mutation 6 gives rise to infectious virus only in the presence of HBB, whereas mutation 7 in the genetic background of a sensitive phenotype causes no alteration (data not shown). Subsequently, one construct was analyzed in each sensitivity assay (Fig. 1D), and the results of the transfection experiments were confirmed.
Thus, constructs carrying the mutation C4782T reveal the same dependent phenotype as the original isolate E9H.dep-4. The six other point mutations detected in the genome of E9H.dep-4 that lead to an amino acid exchange are not able to alter the growth behavior of the virus with regard to dependence of HBB.
The genomic regions coding for the 2C proteins of the three plaque isolates (numbers 3, 6, and 8) which were also shown to be HBB dependent (Table 1) were amplified by reverse transcription-PCR and were sequenced. Both mutations C4782U and U4970C are present in the three HBB-dependent isolates (data not shown). It is noteworthy that all plaque isolates investigated in this study were purified from the same virus stock and propagated under the selective pressure of HBB. Hence, the mutations C4782U and U4970C may be early events and all variants are the offspring of the same progenitor.
Discussion.
The data presented suggest that HBB interacts directly or indirectly with the nonstructural protein 2C, a highly conserved gene product within the picornavirus family (1). Comparable results were obtained with guanidine, another substance that specifically inhibits enterovirus replication in cell culture (18). Mutations leading to guanidine-resistant or -dependent poliovirus variants are located within the 2C gene (2, 11, 15–17, 21). The majority of these amino acid exchanges are clustered at positions 179 and 187, respectively; however, the region between amino acids 225 and 235 is also a hot spot for mutations (21). This finding leads us to propose that the two viral inhibitors, HBB and guanidine, may affect similar 2C functions. However, certain differences should be noted; e.g., the antiviral spectrum of both substances is not identical. As shown previously, guanidine is only a weak inhibitor of echovirus 9 replication, in contrast to its strong effect on polioviruses. Under the selective pressure of a 2 mM concentration of guanidine, the virus becomes resistant, but we were not able to select a completely dependent variant of echovirus 9, even after seven passages. Furthermore, whereas mutation C4782T manifests HBB as well as guanidine dependence, variant E9G.dep-12, one of the typical, slightly guanidine-dependent isolates, remains sensitive to HBB to a significant degree (Fig. 3).
FIG. 3.
Sensitivity assays of the recombinant rE9-H.dep-6 and a guanidine-dependent variant, E9G.dep-12. The indicated viruses were cultivated either in the presence of 73 μM (rhomb), 146 μM (triangle), or 220 μM (circle) HBB or under 1 mM (cross) or 2 mM (star) guanidine. The virus control (square) was grown without an antiviral drug. Results of sensitivity assays of rE9-H.dep-6 in the presence of HBB are given in Fig. 1. The extent of virus-induced cell damage reflecting viral multiplication was estimated by microscopic examination and is given as a percentage. p.i., postinfection.
The conclusion that the mutation crucial for the dependence on HBB targets the 2C protein is plausible, considering the significance of the protein for RNA synthesis. It is shown that poliovirus 2C plays a significant role in viral RNA replication (3, 13, 14) and possibly also in virion assembly (12) or encapsidation (22). This explains the observation that removal of HBB from the medium during the period exhibiting the highest RNA polymerase activity abolishes the production of infectious particles of HBB-dependent virus variants in less than 15 min (4).
Among others, a nucleoside triphosphate-binding motif composed of three conserved regions (A, B, and C) typical for nucleoside triphosphate-binding proteins has been identified within 2C of enteroviruses (9). Functional activity of regions A and B (i.e., ATP and GTP binding and splitting) could be demonstrated (14, 19, 20). Region C is suggested to act as a helicase; however, the experimental evidence for this function is still lacking. The Ala-to-Val mutation at position 229 (mutation 6) of the HBB-dependent variant is located in close proximity to the C motif. Whether the proposed helicase activity of the protein, if it exists at all, is influenced by HBB remains to be clarified.
Acknowledgments
We thank Elke Feldmann and Eva Heimes for skillful technical assistance and Herbert Pfister for critical examination of the manuscript.
This project is supported by the Deutsche Forschungsgmeinschaft (NE 586/2-2). B.N.-S. maintained a grant from the Lise-Meitner-Stiftung, and H.Z. was supported by the Förderverein zur Bekämpfung von Viruskrankheiten e.V. (DVV).
REFERENCES
- 1.Argos P, Kamer G, Nicklin M J H, Wimmer E. Similarity in the gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984;12:7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltera R F, Jr, Tershak D R. Guanidine-resistant mutants of poliovirus have distinct mutations in peptide 2C. J Virol. 1989;63:4441–4444. doi: 10.1128/jvi.63.10.4441-4444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton D J, Flanegan J B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol. 1997;71:8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggers H J. Benzimidazoles. Selective inhibitors of picornavirus replication in cell culture and in the organism. In: Came P E, Caliguiri L A, editors. Chemotherapy of viral infections. Berlin, Germany: Springer-Verlag; 1982. pp. 377–417. [Google Scholar]
- 5.Eggers H J, Tamm I. Drug dependence of enteroviruses: variants of coxsackie A9 and echo 13 viruses that require 2-(α-hydroxybenzyl)-benzimidazole for growth. Virology. 1963;20:62–74. [Google Scholar]
- 6.Eggers H J, Tamm I. Inhibition of enterovirus ribonucleic acid synthesis by 2-(α-hydroxybenzyl)-benzimidazole. Nature. 1963;197:1327–1328. [Google Scholar]
- 7.Eggers H J, Tamm I. On the mechanism of selective inhibition of enterovirus multiplication by 2-(α-hydroxybenzyl)-benzimidazole. Virology. 1962;18:426–438. [Google Scholar]
- 8.Eggers H J, Tamm I. Spectrum and characteristics of the virus inhibitory action of 2-(α-hydroxybenzyl)-benzimidazole. J Exp Med. 1961;113:657–682. doi: 10.1084/jem.113.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbalenya A E, Koonin E V, Wolf Y I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990;262:145–148. doi: 10.1016/0014-5793(90)80175-i. [DOI] [PubMed] [Google Scholar]
- 10.Hollinshead A C, Smith P K. Effects of certain purines and related compounds on virus propagation. J Pharmacol Exp Ther. 1958;123:54–62. [PubMed] [Google Scholar]
- 11.Kirkegaard K, Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986;47:433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J P, Baltimore D. An intragenic revertant of a poliovirus 2C mutant has an uncoating defect. J Virol. 1990;64:1102–1107. doi: 10.1128/jvi.64.3.1102-1107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J P, Baltimore D. Isolation of poliovirus 2C mutants defective in viral RNA synthesis. J Virol. 1988;62:4016–4021. doi: 10.1128/jvi.62.11.4016-4021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirzayan C, Wimmer E. Genetic analysis of an NTP-binding motif in poliovirus polypeptide 2C. Virology. 1992;189:547–555. doi: 10.1016/0042-6822(92)90578-d. [DOI] [PubMed] [Google Scholar]
- 15.Pincus S E, Diamond D C, Emini E A, Wimmer E. Guanidine-selected mutants of poliovirus: mapping of point mutations to polypeptide 2C. J Virol. 1986;57:638–646. doi: 10.1128/jvi.57.2.638-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pincus S E, Rohl H, Wimmer E. Guanidine-dependent mutants of poliovirus: identification of three classes with different growth requirements. Virology. 1987;157:83–88. doi: 10.1016/0042-6822(87)90316-3. [DOI] [PubMed] [Google Scholar]
- 17.Pincus S E, Wimmer E. Production of guanidine-resistant and -dependent poliovirus mutants from cloned cDNA: mutations in polypeptide 2C are directly responsible for altered guanidine sensitivity. J Virol. 1986;60:793–796. doi: 10.1128/jvi.60.2.793-796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rightsel W A, Dice J R, McAlpine R J, Timm E A, McLean I W, Jr, Dixon G L, Schnabel F M., Jr Antiviral effect of guanidine. Science. 1961;134:558–559. doi: 10.1126/science.134.3478.558. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez P L, Carrasco L. Poliovirus protein 2C has ATPase and GTPase activities. J Biol Chem. 1993;268:8105–8110. [PubMed] [Google Scholar]
- 20.Teterina N L, Kean K M, Gorbalenya A E, Agol V I, Girard M. Analysis of the functional significance of amino acid residues in the putative NTP-binding pattern of the poliovirus 2C protein. J Gen Virol. 1992;73:1977–1986. doi: 10.1099/0022-1317-73-8-1977. [DOI] [PubMed] [Google Scholar]
- 21.Tolskaya E A, Romanova L I, Kolesnikova M S, Gmyl A P, Gorbalenya A E, Agol V I. Genetic studies on the poliovirus 2C protein, an NTPase. A plausible mechanism of the guanidine effect on the 2C function and evidence for the importance of 2C oligomerization. J Mol Biol. 1994;236:1310–1323. doi: 10.1016/0022-2836(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 22.Vance L M, Moscufo N, Chow M, Heinz B A. Poliovirus 2C region functions during encapsidation of viral RNA. J Virol. 1997;71:8759–8765. doi: 10.1128/jvi.71.11.8759-8765.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann H, Eggers H J, Nelsen-Salz B. Cell attachment and mouse virulence of echovirus 9 correlate with an RGD motif in the capsid protein VP1. Virology. 1997;233:149–156. doi: 10.1006/viro.1997.8601. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann H, Eggers H J, Nelsen-Salz B. Molecular cloning and sequence determination of the complete genome of the virulent echovirus 9 strain Barty. Virus Genes. 1996;12:149–154. doi: 10.1007/BF00572953. [DOI] [PubMed] [Google Scholar]