Abstract
Simple Summary
Wool is a natural fibre with unique biological, chemical and physical properties, yet it struggles with issues related to having inconsistent quality. Improving the consistency of wool traits through selective breeding is hindered by our limited understanding of the mechanisms, genetic or otherwise underlying fibre variability. To improve understanding, this study focuses on characterisation of the KRTAP19-5 gene that would encode a high glycine and tyrosine containing keratin-associated protein. The study reveals variation within the gene and how that appears to influence the mean fibre curvature of the fine wool fibres from Chinese Tan sheep.
Abstract
Sheep’s wool is known to have unique biological, physical and chemical properties. The fibre primarily consists of proteins, but these have amino acid sequence variation, and at the phenotypic level wool fibre varies considerably. This can affect its utility and value. Unravelling the genetic factors that underpin the protein and phenotypic variability is crucial if we are to contemplate improving wool quality. Accordingly, this study investigates the high glycine and tyrosine content keratin-associated protein 19-5 gene (KRTAP19-5) in sheep. PCR-single strand confirmation polymorphism analysis, coupled with DNA sequencing of a region spanning whole coding sequence, revealed six sequence variants containing seven single nucleotide polymorphisms (SNPs). Five of the SNPs were located within the coding region, with four leading to amino acid changes if expressed. In 247 Chinese Tan sheep derived from 10 sire-lines, and renowned for their distinct ‘spring-like’ crimped wool at up to approximately 35 days after birth, one of the variants was found to be associated with decreased curvature of the fine wool fibres in the fleece. No associations were detected with other fibre traits or with variation in the heterotypic hair fibres of the Tan sheep. While these findings may be useful for developing gene markers to alter mean wool fibre curvature and improve sheep breeding, many other genes and environmental factors are known to contribute to variation in fibre traits.
Keywords: keratin-associated protein, KAP19-5, variation, wool traits, fine wool, heterotypic hair fibre, Chinese Tan sheep
1. Introduction
Wool is a natural fibre renowned for possessing unique properties [1], but as a natural fibre it can be is quite variable. This can restrict its use, and thus it influences its value. Wool fibre is primarily composed of two types of protein, the wool keratins and the keratin-associated proteins (KAPs) [2]. Understanding the genes regulating the production of these proteins is essential to improving our understanding of wool variability and thus the quality of wool.
The keratin-associated proteins have a diverse composition and comprise numerous individual genes and proteins [2,3]. They can be broadly categorised into three groups based on their amino acid composition: the high sulphur (HS) proteins, the ultra-high sulphur (UHS) proteins and the high glycine and tyrosine (HGT) proteins [4]. While the exact number of ovine KAP genes (designated KRTAPs) is unknown, studies in humans reveal 89 KRTAPs. These have been assigned into 12 HS-KAP families (containing 25 KRTAPs), 6 UHS-KAP families (containing 47 KRTAPs) and 7 HGT-KAP families (containing 17 KRTAPs) [5,6,7].
The quantity of HGT-KAPs present in wool fibres varies, from under 1% in Lincoln sheep wool, to 4–12% in Merino sheep wool [8]. The HGT-KAPs are found at a greater abundance in the orthocortex of the wool fibre, compared to paracortex, and a decrease in HGT-KAP content appears to be at least partially responsible for the felting lustre mutant observed in Merino sheep [9]. The identification of five additional HGT-KAP genes in sheep, which are not found in humans [10,11,12,13], suggests the ovine HGT-KAP family may be more diverse than in humans. Together, the evidence suggests that HGT-KAPs may play a role in regulating wool characteristics.
Gene members of all the known HGT-KAP families except for the KAP19 family have been identified and investigated in sheep [14]. In humans, KAP19 is acknowledged to be the largest HGT-KAP family, and it consists of seven genes [15], thus it is surprising that none of the KRTAP19 genes has been investigated in sheep. While genome constructs and databases identify KAP genes in other animals including alpaca, llama, cattle, yaks, dogs and goats, humans [15] are the only species where the KAP19 family has been characterised.
The Tan sheep, a breed indigenous to China, is renowned for producing wool with a distinctive ‘spring-like’ crimp, especially up to approximately 35 days after birth, in what is traditionally referred to as ‘Er-mao’ [16]. In this setting, this study sought to identify ovine KRTAP19-5, ascertain whether the gene was variable, and investigate whether variation if detected, is associated with wool traits in Chinese Tan sheep.
2. Materials and Methods
2.1. Sheep Investigated and Wool Trait Measurement
Two separate groups of sheep were investigated in this study. The first group compromised 68 sheep selected from various farms. They were chosen so as to represent unrelated individuals of differing breed, which have been selected historically for meat, wool and dual-purpose production system, and so as to create a diverse base to ascertain the likely extent of DNA sequence variation in KRTAP19-5. This group was solely used for screening for variation in ovine KRTAP19-5, and was not subjected to association analyses, given that wool samples had not been collected, and neither had wool trait information.
The second group comprised 247 Chinese Tan lambs derived from ten sire-lines, and these were chosen to enable the relationship between variation in KRTAP19-5 variation and variation in selected fibre traits to be tested. Most of the lambs were born as singles, but six of them (three pairs) were born as twins. To prevent potential confounding effects, the twins were excluded from the analyses, leaving 241 single lambs for the association study.
Wool samples were collected from the mid-side region of the Chinese Tan lambs at Er-mao (day 35 post-partum). For each sample, fine wool fibres and heterotypic hair fibres were manually separated from each other based on the noticeable difference in fibre diameter and length. This separation was achieved using a flannel board that the wool samples could be spread out on. Using a rigid card to press the base of all the fibres against the board, the typically longer and higher fibre diameter heterotypic hair fibres could be pulled out of the wool sample. This process was repeated to ensure all the heterotypic hair fibres were isolated from the fine wool fibres.
The fine and heterotypic fibres from each sample were then measured for mean fibre diameter (MFD), fibre diameter standard deviation (FDSD), coefficient of variation of fibre diameter (CVFD) and mean fibre curvature (MFC). The measurements on the heterotypic fibres were undertaken by the New Zealand Wool Testing Authority, Napier, New Zealand using International Wool Textile Organisation endorsed tests, while the measurement of the fine fibres was undertaken by Pastoral Measurements Limited (Timaru, New Zealand).
Blood samples from each sheep were collected onto TFN paper (Munktell Filter AB, Sweden) and the cards dried for storage. For analysis of KRTAP19-5, punches of 1.2 mm in diameter were taken from the blood spot on the TFN paper, and DNA for PCR amplification (which has attached to the TFN paper), was prepared using a two-step washing process [17]. This method involved incubating the punches in 20 mM NaOH solution for 30 min at room temperature, removal of the NaOH and a subsequent single wash with 1× TE−1 buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.0).
2.2. PCR Amplification and Single Strand Conformation Analysis
A pair of PCR primers were designed to amplify ovine KRTAP19-5 based on the KRTAP19-5 sequence ENSOARG00020032146 (Ensembl, KRTAP19-5 sequence derived from chromosome 1 of the ovine genome assembly ARS-UI_Ramb_v2.0:CM028704.1; 3 February 2021). The sequences of these primers were 5′-TCTGACCAGCAGCAGCAGC-3′ (forward primer) and 5′-ATCTTGGCCTTAATCTTAGAC-3′ (reverse primer), and they were synthesised by Integrated DNA Technologies (Coralville, IA, USA). The PCR amplifications were conducted in 15-μL reactions. These contained the cleansed genomic DNA on a single punch of the TFN paper, 150 μM of each deoxynucleotide triphosphate (dNTP; Bioline, London, UK), 0.25 μM of each primer, 2.5 mM Mg2+, 0.5 U of Taq DNA polymerase (Qiagen, Hilden, Germany), and 1× the reaction buffer supplied with the enzyme. The thermal profile for amplification included an initial denaturation step for 2 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 58 °C and 30 s at 72 °C, and with a final extension step for 5 min at 72 °C. The thermal cycling was carried out in S1000 thermal cyclers (Bio-Rad, Hercules, CA, USA). A positive control (sheep genomic DNA) and negative control (no DNA) were also run with the amplification reactions.
The PCR amplicons produced were analysed using a single strand conformation polymorphism (SSCP) approach. For this, a 0.7-μL aliquot of each amplicon was mixed with 7 μL of gel loading dye (0.025% bromophenol blue, 0.025% xylene-cyanol, 98% formamide, and 10 mM EDTA). After denaturation of the double-stranded DNA at 95 °C for 5 min, the samples were cooled on wet ice and loaded on 16 cm × 18 cm, 10% acrylamide: bisacrylamide (37.5:1) (Bio-Rad) gels that contain 5% glycerol. Electrophoresis of the samples was conducted at 390 volts and 7 °C in Protean II xi cells (Bio-Rad), for 19 h using 0.5× TBE run buffer. Upon completion of the electrophoresis, the SSCP gels were fixed and stained in a solution containing 10% ethanol, 0.5% acetic acid, and 0.2% silver nitrate for 10 min. The gels were rinsed once with distilled water and then developed with a solution of 3% NaOH and 0.1% HCOH until dark staining bands appeared on the yellow background. At that point, development was stopped by removing the developing solution and addition of a solution containing 10% ethanol and 0.5% acetic acid. With the PCR-SSCP approach used, different variant sequences of KRTAP19-5 were expected to produce different banding patterns on the gels, with each DNA sequence producing two bands that correspond to the two strands of the DAN for any given variant. Homozygous sheep are therefore expected to produce two bands and heterozygous sheep four bands.
2.3. DNA Sequencing and Sequence Analysis
PCR amplicons displaying different SSCP banding patterns (or sequence variants) from sheep seemingly homozygous for the gene region amplified, were directly sequenced in both directions at the Lincoln University DNA sequencing facility (Lincoln University, Lincoln, New Zealand). Typically three sheep per homozygous variant, if available, were chosen for sequencing, and with sequencing being undertaken in both directions on an Applied Biosystems Model 3500XL Genetic Analyzer (Waltham, MA, USA) using POP7 polymer and a 24 capillary array. The raw sequence reads were aligned using DNAMAN XL (version 10, Lynnon BioSoft, Vaudreuil, QC, Canada). Individual nucleotide calls are expected to match across all six raw reads using this approach. Variants that were only detected in heterozygous sheep underwent sequencing using a gel separation-based sequencing approach [18]. In this approach, a gel slice corresponding to a SSCP band of the variant was excised form the polyacrylamide gel, macerated, and then used as a template for re-amplification with the original primers. This resulting second amplicon, was checked to insure it produced the expected ‘homozygous’ pattern using the PCR-SSCP gel system, then sequenced directly as above for the homozygous sheep. Once again, this approach was used with three separate sheep and each variant isolated using this approach was sequenced in triplicate in both directions. Once final sequences had been obtained, the open reading frame was determined and translation undertaken using DNAMAN XL. The finalized sequences were then subjected to a phylogenetic analysis, using the same software.
2.4. Statistical Analyses
Statistical analyses were conducted using Minitab version 16 (Minitab Inc., State College, PA, USA). General linear models (GLMs) were used to assess the impact of the presence or absence of the ovine KRTAP19-5 variants on the various wool traits that were measured. To determine whether sire or gender would be included in the models, a univariate Pearson Chi-square test was performed to explore the association between these variables and the measure wool traits. Sire was identified to have an influence on all the wool traits measured and hence was included as an explanatory factor in all the models, while gender was identified as a factor affecting some of the wool traits. The linear model was:
| Yjkl = µ + Vj + Gk + Sl + ejkl |
where Yjkl is the phenotypic value for the jklth sheep, µ is the group raw mean for the trait, Vj is the effect of the jth variant (presence and absence), Gk is the effect of gender, Sl is the effect of the lth sire, and ejkl is the random residual effect. Significance was accepted at p < 0.05.
3. Results
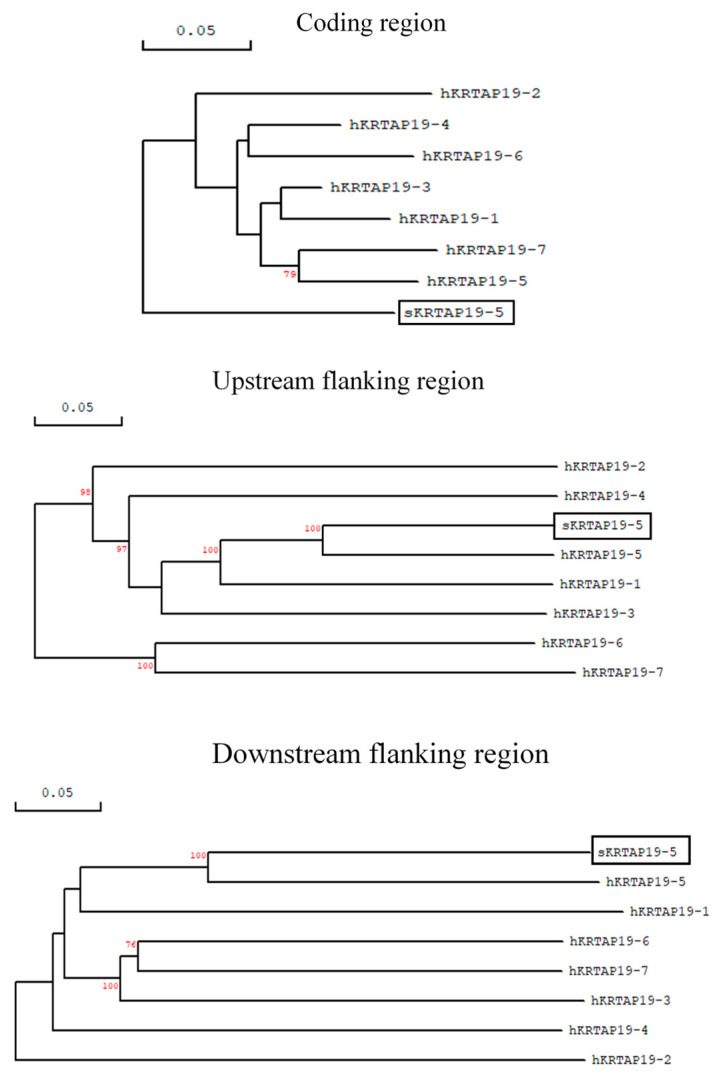
The ovine KRTAP19-5 sequence derived from the ovine genome assembly ARS-UI_Ramb_v2.0:CM028704.1, and as presented in Ensembl with the identification number ENSOARG00020032146, is an intronless gene with a coding sequence that spans 222 base pairs. Despite sharing 72.3–80.8% sequence homology with human KRTAP19-n sequences, this coding sequence does not exhibit a close relationship with any specific human KRTAP19 genes. However, the upstream and downstream flanking sequences of the coding sequence have close identity to human KRTAP19-5 (Figure 1). This suggests that the Ensembl-identified ovine KRTAP19-5 sequence represents the ortholog of human KRTAP19-5.
Figure 1.
Phylogenetic analyses of sheep KRTAP19-5 with known gene members of the human KAP19 family. The sheep KRTAP19-5 sequence is labelled with the prefix “s” and highlighted in a box, while human genes are denoted by prefix “h”. Analyses are conducted for three different regions: the coding region, as well as the 1-kb upstream and downstream flaking regions. Bootstrap confidence values are indicated at the forks, with only values over 70% shown. Scale bars represent a rate of 0.05 nucleotide substitution per site.
The ovine KRTAP19-5 sequence ENSOARG00020032146 also exhibits similarities to expressed sequence tags obtained from sheep wool follicles or skin (Supplementary Table S1), which suggests that the gene is expressed.
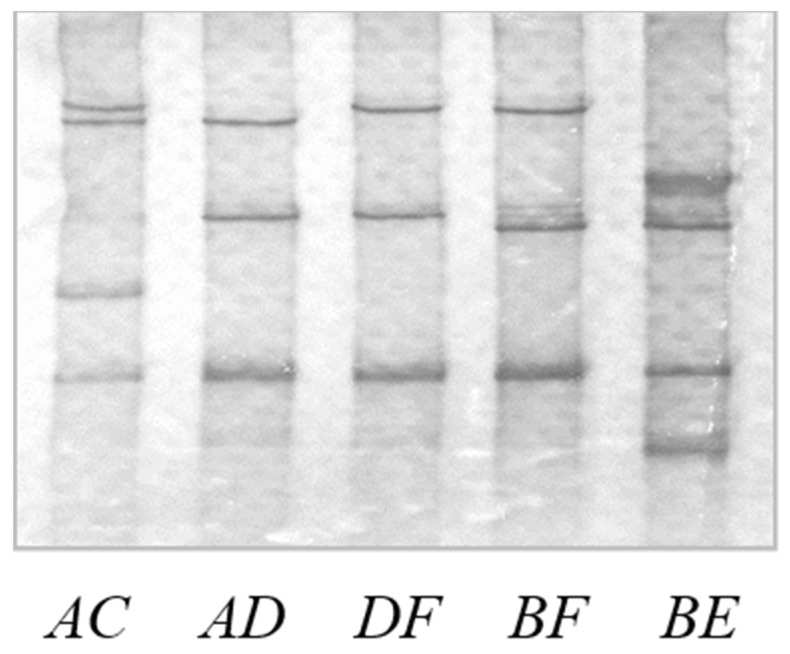
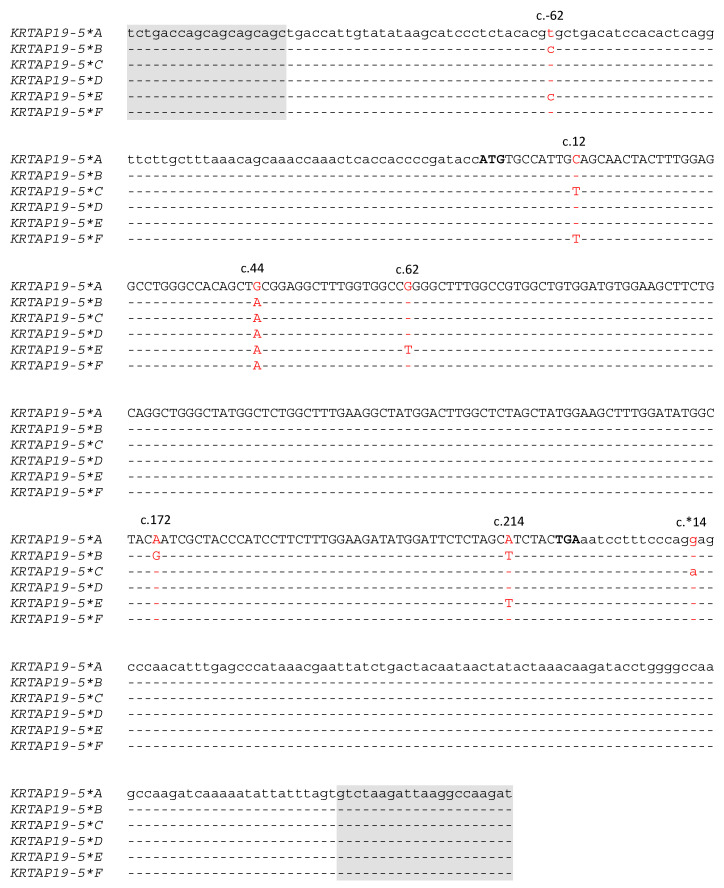
The PCR-SSCP analysis of ovine KRTAP19-5, including 112 bp upstream and 132 bp downstream of the intronless coding sequence, revealed six distinct banding patterns (Figure 2), which upon corresponded to six unique, but closely-related sequence variants or haplotypes (Figure 3). A total of seven single nucleotide polymorphisms (SNPs) were identified, with five located in the coding region. Among the SNPs in the exons, four were non-synonymous (c.44G/A, c.62G/T, c.172A/G and c.214A/T) and would result in the amino acid substitutions p.Tyr15Cys, p.Arg21Leu, p.Asn58Asp and p.Ile72Phe respectively. The DNA sequence of variant F was identical to ENSOARG00020032146.
Figure 2.
PCR-SSCP patterns of ovine KRTAP19-5. Six different banding patterns (A to F), corresponding to six different DNA haplotypes are observed in heterozygous forms. Different variant sequences of KRTAP19-5 are expected to produce different banding patterns on the gels, with each DNA sequence producing two bands that correspond to the two strands of the DAN for any given variant.
Figure 3.
Alignment of the six ovine KRTAP19-5 variant sequences using DNAMAN XL. The positions of seven SNPs identified among the six variant sequences (A to F) are indicated, with nucleotides shown in red. Nucleotides within the coding region are shown in upper case, while those outside the coding region are in lower case. The start codon and stop codon are shown in bold. Dashes represent nucleotide sequences identical to the top sequence. The primer binding regions are shaded.
In the 68 sheep used for variation screening, the variant frequencies observed were 19.4%, 41.9%, 10.5%, 19.4%, 5.6%, and 3.2% for variants A to F, respectively.
In the 241 Chinese Tan sheep used for the association study, all the variants (A to F) were detected, with frequencies of 34.0%, 18.5%, 27.2%, 8.7%, 3.3% and 8.3% respectively. Variant E was detected at a frequency below 5% and was therefore of insufficient sample size to subsequently include in the association analyses, because the sheep carrying that variant could potentially bias the outcome of the models.
The variation detected in KRTAP19-5 was associated with variation in MFC for the fine wool fibres of the Tan sheep (Table 1), with the presence of variant B being associated with decreased MFC (absent 65.3 ± 1.19°/mm vs. present 62.3 ± 1.45°/mm, p = 0.039). However, no associations were observed for other fibre traits, or with the heterotypic hair fibres.
Table 1.
Association of KRTAP19-5 variants with wool traits in fine wool fibres of Chinese Tan sheep.
| Trait 1 | Variant 2 | Mean ± SE 3 | p Value | |
|---|---|---|---|---|
| Absent | Present | |||
| MFD (µm) | A | 16.7 ± 0.22 | 16.7 ± 0.20 | 0.707 |
| B | 16.7 ± 0.19 | 16.7 ± 0.23 | 0.968 | |
| C | 16.6 ± 0.21 | 16.7 ± 0.20 | 0.729 | |
| D | 16.7 ± 0.18 | 16.6 ± 0.29 | 0.521 | |
| F | 16.7 ± 0.18 | 17.0 ± 0.30 | 0.250 | |
| FDSD (µm) | A | 4.2 ± 0.16 | 4.2 ± 0.14 | 0.711 |
| B | 4.2 ± 0.14 | 4.2 ± 0.17 | 0.823 | |
| C | 4.1 ± 0.15 | 4.2 ± 0.14 | 0.481 | |
| D | 4.2 ± 1.13 | 4.1 ± 0.21 | 0.421 | |
| F | 4.2 ± 0.13 | 4.3 ± 0.21 | 0.394 | |
| CVFD (%) | A | 25.0 ± 0.73 | 24.8 ± 0.66 | 0.754 |
| B | 24.9 ± 0.63 | 24.9 ± 0.78 | 0.999 | |
| C | 24.6 ± 0.70 | 25.1 ± 0.66 | 0.486 | |
| D | 25.0 ± 0.59 | 24.3 ± 0.96 | 0.421 | |
| F | 24.8 ± 0.59 | 25.3 ± 0.99 | 0.606 | |
| MFC (°/mm) | A | 63.3 ± 1.37 | 65.1 ± 1.24 | 0.218 |
| B | 65.3 ± 1.19 | 62.3 ± 1.45 | 0.039 | |
| C | 64.6 ± 1.33 | 64.1 ± 1.24 | 0.689 | |
| D | 64.2 ± 1.12 | 63.6 ± 1.83 | 0.643 | |
| F | 64.2 ± 1.11 | 65.1 ± 1.87 | 0.585 | |
1 MFD—mean fibre diameter; FDSD—fibre diameter standard deviation; CVFD—coefficient of variation of fibre diameter; MFC—mean fibre curvature. 2 Among the 225 sheep (excluding those carrying the rare variant E), variant A was absent in 90 and present in 135 animals; variant B was absent in 147 and present in 78 animals; variant C was absent in 113 and present in 112 animals; variant D was absent in 185 and present in 40 animals; and variant F was absent in 187 and present in 38 animals. 3 Predicted means and standard errors derived from GLMs, with p < 0.05 being presented in bold.
4. Discussion
This study describes the identification of a member of the ovine KAP19 family. While resolving its identity solely based on its coding sequence is difficult, the high similarity of the upstream and downstream sequences flanking that coding sequence to the human genome, suggests it is likely an orthologue to human KRTAP19-5. This also suggests that unexplained evolutionary processes may have occurred in producing the human and sheep loci. In this respect, differences in evolutionary patterns between the coding region and flanking regions has been previously observed in the KRTAP1 gene family [19], likely driven by distinct evolutionary forces. The evolution of the upstream and downstream regions of genes ensures beneficial regulation and expression of a gene, whereas the coding region evolves to maintain the preferred structure and function of the expressed protein.
Bioinformatics analyses of KRTAP coding sequences across 22 mammalian species suggests that HS-, UHS- and HGT-KRTAPs may have followed distinct evolutionary paths [20]. For example, the HS- and UHS-KRTAPs exhibit high rates of concerted evolution, likely driven by gene conversion and recombination events [20], with the KRTAP1 family serving as a well-studied example [19,21]. In contrast, the HGT-KRTAPs display a more dynamic evolutionary pattern, characterised by signatures of positive selection and less evidence of gene conversion or recombination [20]. Given that KRTAP19 belongs to the HGT group, future characterisation of additional KRTAP19 family members in sheep and sequence analyses of these family members may provide further information into the evolution of the KRTAP19 gene family and the HGT-KRTAPs.
A distinction also seems to arise between the sheep and human orthologs regarding the location of expression of KRTAP19-5. In sheep, KRTAP19-5 appears to be expressed in wool follicles, as evidenced by the isolation of ESTs bearing similarities to the KRTAP19-5 sequence from wool follicles or skin. In contrast, the expression of KRTAP19-5 was not detected in human hair follicles by either cDNA library screening or in-situ hybridization studies [15].
The identification of six sequence variants in the region amplified of ovine KRTAP19-5 suggests a high level of sequence diversity. The detection of seven SNP results in a calculated SNP density of 17.2 SNPs/kb in the region amplified, excluding the primer binding sequences. This SNP density is comparable to the densities found in KRTAP1-2 [22], KRTAP8-1 [23], KRTAP28-1 [24] and KRTAP36-2 [13], but less than the densities found in KRTAP1-3 [25], KRTAP1-4 [26], KRTAP2-1 [27] and KRTAP20-1 [28]. For context, the average SNP density in the sheep genome has been cited to be approximately 4.9 SNPs/kb [29], but our findings suggest that ovine KRTAP19-5 has nucleotide variation at close to four times that rate. The non-synonymous SNPs identified in ovine KRTAP19-5 may induce structural alterations in the protein, whereas synonymous SNPs and those outside the coding region may affect mRNA stability, with this potentially influencing gene expression.
The factors controlling wool fibre curvature remains elusive, with various factors suggested, such as the relative abundance and distribution of orthocortical and paracortical cells [30], the relative length of these cells [31], or the structural arrangement of keratin intermediate filaments (KIFs) [32]. Given that HGT-KRTAPs are preferentially expressed in orthocortical cells [33], then variation in KRTAP19-5 expression may affect the abundance and proportion of orthocortical and paracortical cells. Structural alterations in the KAP19-5 protein could also affect interactions with the KIFs and cross-linking processes in the fibre matrix, potentially altering the structural arrangement of KIFs and thus fibre properties like curvature.
While the mechanism(s) by which an individual KAP can affect fibre curvature remains uncertain, research has revealed connections between fibre curvature and the abundance of HGT-KAP proteins. For example, in Merino wool, a decrease in HGT-KAPs has been linked to the felting lustre mutation [34], and differences in the abundance of HGT-KAP proteins in wool fibres have been observed between wild-type and crimp mutant-type twins in both the Merino and Romney breeds [35]. These findings suggest that the levels of HGT-KAPs may indeed play a role in influencing fibre curvature. It has been suggested that there is a relationship between fibre curvature and fibre diameter, and that as fibre diameter increases staple crimp (reflecting changes in curvature) tends to decrease. Sumner [36] suggests that there are breed and selection lines that defy this generalization, but that the trait seems to be ‘due to the effect of a small number of genes’.
Plowman et al. [37] reported that the Merino wool fibres (fine wool) had a higher content of certain HGT-KAP proteins compared to the wool from Bordaleiro (medium wool) and Churro (coarse wool) sheep, and suggesting that the content of HGT-KAPs may decrease with larger fibres. If this holds true for KAP19-5, the content of KAP19-5 would likely be lower in the heterotypic hair fibres, with this possibly explaining why the effect on curvature was only observed in the fine wool fibres from the Chinese Tan sheep. Further research is certainly warranted to validate this observation.
The variant associated with decreased MFC (variant B), exhibited different frequencies in the two groups of sheep investigated in this study. Among the sheep of differing breed, variant B occurred at a frequency of 41.9%, but in the Chinese Tan sheep, this variant was detected at a much low frequency of 18.5%. This lower frequency observed in the Tan sheep may align with the high degree of ‘crimpiness’ that is characteristic of Tan sheep wool [16]. The identification of this variant may also present an opportunity to optimize wool curvature through selective breeding, with the removal of B from the population potentially increasing crimp.
The identification of seven KRTAP19 genes within the human KAP19 family [15] leads us to hypothesize that there are of multiple KRTAP19 genes in sheep as well. However, as other KRTAP19 genes have not yet been identified in sheep, and need to be, their impact on wool traits remains uncertain. This makes it challenging to ascertain whether the association observed for KRTAP19-5 is because of this specific gene, or is the result of linkage with hypothetically nearby, yet un-characterised KRTAP19 genes. Further identification and investigation of these genes would provide valuable information in this respect. The KRTAP19-n genes are likely flanked by KRTAP36-2 [13] and KRTAP15-1 [37]. However, variation in KRTAP36-2 was found to affect wool yield [13], while variation in KRTAP15-1 was associated with wool yield and FDSD [38]. These findings suggest that the association detected for KRTAP19-5 is less likely due to the influence of closely positioned genes from other KAP families.
5. Conclusions
This study identified a previously unknown KAP gene in sheep and reports variation in this gene. These findings add to our knowledge of wool protein genetics. The observed variation in KRT19-5 was associated with wool mean fibre curvature. Further research is needed to confirm and validate these findings and also to further explore the relationship between KRTAP19-5 and other wool traits, especially given the small population of sheep studied in the association part of this study, and the limited number of wool traits measured. These findings may be useful for the development of gene markers and thus enable the identification and selection of sheep for either increased or decreased fibre curvature. It should be part of an ongoing broader initiative to investigate the effect of wool protein genes on fibre traits.
Acknowledgments
The authors thank Freeman Fang and Andrea Hogan for providing technique support and assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14152155/s1, Table S1: ESTs showing high similarity to ovine KRTAP19-5 sequence ENSOARG00020032146.
Author Contributions
Conceptualization, H.Z., J.T. and J.G.H.H.; formal analysis, L.B., H.Z. and W.L.; investigation, L.B. and W.L.; methodology, L.B., H.Z. and J.T.; Supervision, H.Z., J.T. and J.G.H.H.; writing—original draft, L.B. and H.Z.; writing—review and editing, H.Z., J.T. and J.G.H.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to that the collection of sheep blood drops by the nicking of their ears was covered by Section 7.5 Ani-mal Identification, in: Code of Welfare: Sheep and Beef Cattle (2016); a code of welfare issued under the Animal Welfare Act 1999 (New Zealand Government).
Informed Consent Statement
Informed consent was obtained from sheep farm owners.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Ningxia Hui Autonomous Region Key Research and Development Program (2023BCF01007), the Qinghai Province Science and Technology International Cooperation Special Project (2023-HZ-809) and the Anhui Province Science and Technology Aid to Youth Special Project (2023j11020003).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Leeder J.D. Wool: Nature’s Wonder Fibre. Australia Textiles Publishers; Ocean Grove, Australia: 1984. [Google Scholar]
- 2.Powell B.C., Rogers G.E. The role of keratin proteins and their genes in the growth, structure and properties of hair. EXS. 1997;78:59–148. doi: 10.1007/978-3-0348-9223-0_3. [DOI] [PubMed] [Google Scholar]
- 3.Rogers M.A., Langbein L., Praetzel-Wunder S., Winter H., Schweizer J. Human hair keratin-associated proteins (KAPs) Int. Rev. Cytol. 2006;251:209–263. doi: 10.1016/S0074-7696(06)51006-X. [DOI] [PubMed] [Google Scholar]
- 4.Powell B.C., Rogers G.E. Hard keratin IF and associated proteins. In: Goldman R.D., Steinert P.M., editors. Cellular and Molecular Biology of Intermediate Filaments. Plenum Press; New York, NY, USA: 1990. pp. 267–300. [Google Scholar]
- 5.Rogers M.A., Langbein L., Praetzel Wunder S., Giehl K. Characterization and expression analysis of the hair keratin associated protein KAP26.1. Br. J. Dermatol. 2008;159:725–729. doi: 10.1111/j.1365-2133.2008.08743.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers M.A., Schweizer J. Human KAP genes, only the half of it? Extensive size polymorphisms in hair keratin-associated protein genes. J. Invest. Dermatol. 2005;124:vii–ix. doi: 10.1111/j.0022-202X.2005.23728.x. [DOI] [PubMed] [Google Scholar]
- 7.Rogers M.A., Winter H., Langbein L., Wollschläger A., Praetzel-Wunder S., Jave-Suarez L.F., Schweizer J. Characterization of human KAP24.1, a cuticular hair keratin-associated protein with unusual amino-acid composition and repeat structure. J. Invest. Dermatol. 2007;127:1197–1204. doi: 10.1038/sj.jid.5700702. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie J.M. The proteins of hair and other hard α-keratins. In: Goldman R.D.S., Peter M., editors. Cellular and Molecular Biology of Intermediate Filaments. Plenum; New York, NY, USA: 1990. pp. 95–128. [Google Scholar]
- 9.Rogers G.E. Biology of the wool follicle: An excursion into a unique tissue interaction system waiting to be re-discovered. Exp. Dermatol. 2006;15:931–949. doi: 10.1111/j.1600-0625.2006.00512.x. [DOI] [PubMed] [Google Scholar]
- 10.Gong H., Zhou H., Dyer J.M., Hickford J.G.H. The sheep KAP8-2 gene, a new KAP8 family member that is absent in humans. SpringerPlus. 2014;3:528. doi: 10.1186/2193-1801-3-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong H., Zhou H., Wang J., Li S., Luo Y., Hickford J.G.H. Characterisation of an ovine keratin associated protein (KAP) gene, which would produce a protein rich in glycine and tyrosine, but lacking in cysteine. Genes. 2019;10:848. doi: 10.3390/genes10110848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H., Gong H., Wang J., Dyer J.M., Luo Y., Hickford J.G.H. Identification of four new gene members of the KAP6 gene family in sheep. Sci. Rep. 2016;6:24074. doi: 10.1038/srep24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H., Li W., Bai L., Wang J., Luo Y., Li S., Hickford J.G.H. Ovine KRTAP36-2: A new keratin-associated protein gene related to variation in wool yield. Genes. 2023;14:2045. doi: 10.3390/genes14112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H., Gong H., Wang J., Luo Y., Li S., Tao J., Hickford J.G.H. The complexity of the ovine and caprine keratin-associated protein genes. Int. J. Mol. Sci. 2021;22:12838. doi: 10.3390/ijms222312838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers M.A., Langbein L., Winter H., Ehmann C., Praetzel S., Schweizer J. Characterization of a first domain of human high glycine-tyrosine and high sulfur keratin-associated protein (KAP) genes on chromosome 21q22.1. J. Biol. Chem. 2002;277:48993–49002. doi: 10.1074/jbc.M206422200. [DOI] [PubMed] [Google Scholar]
- 16.Tao J., Zhou H., Yang Z., Gong H., Ma Q., Ding W., Li Y., Hickford J.G.H. Variation in the KAP8-2 gene affects wool crimp and growth in Chinese Tan sheep. Small Rumin. Res. 2017;149:77–80. doi: 10.1016/j.smallrumres.2017.01.001. [DOI] [Google Scholar]
- 17.Zhou H., Hickford J.G.H., Fang Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006;354:159–161. doi: 10.1016/j.ab.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Gong H., Zhou H., Plowman J.E., Dyer J.M., Hickford J.G.H. Analysis of variation in the ovine ultra-high sulphur keratin-associated protein KAP5-4 gene using PCR-SSCP technique. Electrophoresis. 2010;31:3545–3547. doi: 10.1002/elps.201000301. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H., Visnovska T., Gong H., Schmeier S., Hickford J., Ganley A.R. Contrasting patterns of coding and flanking region evolution in mammalian keratin associated protein-1 genes. Mol. Phylogenet. Evol. 2019;133:352–361. doi: 10.1016/j.ympev.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Khan I., Maldonado E., Vasconcelos V., Stephen J., Johnson W.E., Antunes A. Mammalian keratin associated proteins (KRTAPs) subgenomes: Disentangling hair diversity and adaptation to terrestrial and aquatic environments. BMC Genom. 2014;15:779. doi: 10.1186/1471-2164-15-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers G.R., Hickford J.G.H., Bickerstaffe R. Polymorphism in two genes for B2 high sulfur proteins of wool. Anim. Genet. 1994;25:407–415. doi: 10.1111/j.1365-2052.1994.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 22.Gong H., Zhou H., Hodge S., Dyer J.M., Hickford J.G.H. Association of wool traits with variation in the ovine KAP1-2 gene in Merino cross lambs. Small Rumin. Res. 2015;124:24–29. doi: 10.1016/j.smallrumres.2015.01.009. [DOI] [Google Scholar]
- 23.Gong H., Zhou H., Plowman J.E., Dyer J.M., Hickford J.G.H. Search for variation in the ovine KAP7-1 and KAP8-1 genes using polymerase chain reaction–single-stranded conformational polymorphism screening. DNA Cell Biol. 2012;31:367–370. doi: 10.1089/dna.2011.1346. [DOI] [PubMed] [Google Scholar]
- 24.Bai L., Wang J., Zhou H., Gong H., Tao J., Hickford J.G.H. Identification of ovine KRTAP28-1 and its association with wool fibre diameter. Animals. 2019;9:142. doi: 10.3390/ani9040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itenge-Mweza T.O., Forrest R.H., McKenzie G.W., Hogan A., Abbott J., Amoafo O., Hickford J.G.H. Polymorphism of the KAP1.1, KAP1.3 and K33 genes in Merino sheep. Mol. Cell. Probes. 2007;21:338–342. doi: 10.1016/j.mcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Gong H., Zhou H., Hickford J.G.H. Polymorphism of the ovine keratin-associated protein 1-4 gene (KRTAP1-4) Mol. Biol. Rep. 2010;37:3377–3380. doi: 10.1007/s11033-009-9925-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Zhou H., Hickford J.G.H., Luo Y., Gong H., Hu J., Liu X., Li S., Song Y., Ke N. Identification of the ovine keratin-associated protein 2-1 gene and its sequence variation in four Chinese sheep breeds. Genes. 2020;11:604. doi: 10.3390/genes11060604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong H., Zhou H., Bai L., Li W., Li S., Wang J., Luo Y., Hickford J.G.H. Associations between variation in the ovine high glycine-tyrosine keratin-associated protein gene KRTAP20-1 and wool traits. J. Anim. Sci. 2019;97:587–595. doi: 10.1093/jas/sky465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kijas J.W., Townley D., Dalrymple B.P., Heaton M.P., Maddox J.F., McGrath A., Wilson P., Ingersoll R.G., McCulloch R., McWilliam S. A genome wide survey of SNP variation reveals the genetic structure of sheep breeds. PLoS ONE. 2009;4:e4668. doi: 10.1371/journal.pone.0004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro W., Carnaby G. Wool-fibre crimp part I: The effects of microfibrillar geometry. J. Text. Inst. 1999;90:123–136. doi: 10.1080/00405009908690618. [DOI] [Google Scholar]
- 31.Harland D.P., Vernon J.A., Woods J.L., Nagase S., Itou T., Koike K., Scobie D.A., Grosvenor A.J., Dyer J.M., Clerens S. Intrinsic curvature in wool fibres is determined by the relative length of orthocortical and paracortical cells. J. Exp. Biol. 2018;221:jeb172312. doi: 10.1242/jeb.172312. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell J.P., Mastronarde D.N., Woods J.L., Bryson W.G. The three-dimensional arrangement of intermediate filaments in Romney wool cortical cells. J. Struct. Biol. 2005;151:298–305. doi: 10.1016/j.jsb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Powell B.C., Nesci A., Rogers G.E. Regulation of keratin gene expression in hair follicle differentiation. Ann. N. Y. Acad. Sci. 1991;642:1–20. doi: 10.1111/j.1749-6632.1991.tb24376.x. [DOI] [PubMed] [Google Scholar]
- 34.Li S.W., Ouyang H.S., Rogers G.E., Bawden C.S. Characterization of the structural and molecular defects in fibres and follicles of the merino felting lustre mutant. Exp. Dermatol. 2009;18:134–142. doi: 10.1111/j.1600-0625.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 35.Plowman J.E., Paton L.N., Bryson W.G. The differential expression of proteins in the cortical cells of wool and hair fibres. Exp. Dermatol. 2007;16:707–714. doi: 10.1111/j.1600-0625.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 36.Sumner R.M.W. Effect of divergent selection for wool bulk on live weight and wool characteristics in Perendale sheep. Proc. N. Z. Soc. Anim. Prod. 2007;67:80–186. [Google Scholar]
- 37.Plowman J.E., Harland D.P., Campos A.M., e Silva S.R., Thomas A., Vernon J.A., van Koten C., Hefer C., Clerens S., de Almeida A.M. The wool proteome and fibre characteristics of three distinct genetic ovine breeds from Portugal. J. Proteom. 2020;225:103853. doi: 10.1016/j.jprot.2020.103853. [DOI] [PubMed] [Google Scholar]
- 38.Li W., Gong H., Zhou H., Wang J., Liu X., Li S., Luo Y., Hickford J.G.H. Variation in the ovine keratin-associated protein 15-1 gene affects wool yield. J. Agric. Sci. 2018;156:922–928. doi: 10.1017/S0021859618000953. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.