Abstract
Introduction
Yellow fever (YF) and dengue (DEN) viruses are important re-emerging mosquito-borne viruses sharing similar vectors and reservoirs. The last documented YF outbreak in Kenya occurred in 1992-95. However, YF virus is re-emerging in bordering countries including Uganda, Ethiopia and South Sudan with the potential for spread to the neighboring regions in Kenya. Dengue is endemic in Kenya with outbreaks being detected in various towns in the north and the coast. This study reports on the Aedes (Stegomyia) mosquito species occurrence, diversity, and blood feeding patterns, as means of measuring the risk of transmission of YF and DEN in Kacheliba sub-county, West Pokot County, which borders previous YF outbreak areas in eastern Uganda.
Methodology
Adult mosquitoes were collected using CO2-baited BG Sentinel traps at three time points during the rainy season. Mosquitoes were identified to the species level. Species abundance during the three sampling periods were compared, with emphasis on Aedes aegypti and other Stegomyia species, using generalized linear models that included mosquito diversity. Individually blood-fed mosquitoes were analyzed by DNA amplification of the 12S rRNA gene followed by sequencing to determine the source of blood meal.
Results
Overall, 8605 mosquitoes comprising 22 species in 5 genera were collected. Sampled Stegomyia species included Ae. aegypti (77.3%), Ae. vittatus (11.4%), Ae. metallicus (10.2%) and Ae. unilineatus (1.1%). Ae. aegypti dominated the blood-fed specimens (77%, n=68) and were found to have fed mostly on rock hyraxes (79%), followed by goats (9%), humans and cattle (each 4%), with a minor proportion on hippopotamus and rock monitor lizards (each comprising 1%).
Conclusion
Our findings reveal the presence of important Stegomyia species, which are known potential vectors of YF and DEN viruses. In addition, evidence of more host feeding on wild and domestic animals (hyrax and goat) than humans was observed. How the low feeding on humans translates to risk of transmission of these viruses, remains unclear, but calls for further research including vector competence studies of the mosquito populations for these viruses. This forms part of a comprehensive risk assessment package to guide decisions on implementation of affordable and sustainable vaccination (YF) and vector control plans in West Pokot County, Kenya.
Keywords: Yellow fever, Dengue, Stegomyia mosquito abundance and diversity, Blood feeding
Introduction
Yellow fever virus (YFV) and dengue virus (DENV) are among the most important mosquito-borne viruses of the family Flaviviridae and genus Flavivirus. Yellow fever virus was first isolated in West Africa in 1927 (Barrett and Higgs, 2007). The recent re-emergence of YFV with major outbreaks in countries bordering Kenya (Uganda, Ethiopia and South Sudan), and regionally in two countries - Angola (with cases of travelers being confirmed in Kenya) and the Democratic Republic of Congo - is of great concern to public health (Kraemer et al., 2017). There is an estimated annual YF incidence of 200,000 cases, and 30,000 deaths, mostly in West and sub-Saharan Africa in which 33 countries and over 500 million people are at risk (WHO, 2014, Garske et al., 2014, Kraemer et al., 2017). In East Africa, the frequency of YF outbreaks is increasing after the first report in Kenya in 1992-95 (Sanders et al., 1998, Reiter et al., 1998), Sudan in 2003, 2005 and 2010 (Onyango et al., 2004, WHO, 2005) and Uganda in 2011 and 2016 (WHO, 2011, WHO, 2016 and InterHealth Worldwide, 2016). The number of cases has increased over the past two decades (WHO, 2016, Kraemer et al., 2017), perhaps because of decreased population immunity to this infection, deforestation, urbanization, population migration from rural to urban areas and vice versa, and the appearance of new vectors (Neiderud, 2015). It is therefore, crucial to carry out vector ecological studies to assess the risk of disease transmission in order to facilitate prioritization of rational regional vaccination programs considering the costs and availability of vaccines.
Dengue outbreaks have also increased in frequency in the region with reports in Tanzania, Somalia, Djibouti, Eritrea, Sudan and Kenya (Sang, 2006; Konongoi et al., 2016; Vairo et al., 2016). Dengue, one of the most important re-emerging arboviruses worldwide, was first reported in Kenya in 1982 during an outbreak in the coastal region (Johnson et al., 1982). In 2011, a second dengue outbreak occurred in Mandera in North Eastern Kenya. Subsequently between 2013 and 2014, DEN outbreak occurred in Mombasa (Coastal Kenya), involving co-circulation of serotypes DEN-1, 2 and 3 (Konongoi et al., 2016). With the expanding distribution of the principal vector, Aedes aegypti, combined with rapid population growth, unplanned urbanization, and increased international travel, extensive transmission of DENV is likely (Gubler, 2004).
Both YF and DEN share a niche in the ecosystem and focusing research efforts on both viruses is scientifically and financially prudent. DENV and YFV are primarily transmitted by Stegomyia mosquitoes. Both viruses originated in sylvatic cycles, are maintained in non-human primates and forest-dwelling Aedes mosquitoes, and have a history of successful emergence and sustained transmission among humans by Ae. aegypti (Hanley et al., 2013). Emergence of urban YF could be mediated by the adaptation of sylvatic YFV to domestic vectors as has been previously suggested for dengue, which shares similar vectors and vertebrate hosts. The adaptation of sylvatic vectors to rural or urban settings could similarly lead to emergence of these viruses in rural and urban areas (Weaver and Reisen, 2010, Coffey et al., 2013). The scenario underscores the importance of understanding the types of vectors present in these ecological interfaces.
Mosquito biting frequency and how bites are distributed among humans and other vertebrate host species can influence transmission risk of a vector-borne disease (Adams and Kapan, 2009). An improved understanding of mosquito host blood feeding preferences would refine knowledge of the entomological processes supporting pathogen transmission and could reveal targets for minimizing risk and breaking pathogen transmission cycles (Harrington et al., 2014). Many mosquito species express inherent traits in host preference, presence and abundance of available hosts. In addition, wild and domestic animal grazing areas create ideal conditions that facilitate virus amplification and transmission by mosquitoes from reservoir animals native to such habitats to susceptible domestic animals and human populations (O–Brien et al., 2011). Thus, the need to determine the mosquito hosts blood feeding preferences in the area.
Surveillance for YF and dengue vectors is a critical component of assessing risk of transmission and outbreak occurrence. This requires knowledge on the distribution of potential vectors involved, including the critical aspects of their ecology such as abundance and their competence in transmitting and sustaining these viruses. Knowledge about their host blood feeding preferences can reveal information about potential reservoirs of disease and risk of transmission to susceptible populations. With more outbreaks being reported regionally, there is need to identify and evaluate vectors in locations in Kenya that border areas with reports of recent outbreaks, which may be at increased risk of epidemic transmissions. This study reports findings on the vector species occurrence, composition and blood feeding patterns of the Stegomyia species, potential vectors, to assess the risk of transmission of YF and DEN in West Pokot County which borders recent YF outbreak areas in Uganda.
Methods
Ethical Considerations
Approval for this study was provided by Kenya Medical Research Institute (KEMRI) Centre Scientific Committee (CSC), the KEMRI Scientific and Ethics Review Unit (SERU), (under protocol number KEMRI-SERU 2787) and University of Pretoria.
Study site
This study was conducted in West Pokot County, Kacheliba sub-county, in Kenya. The county borders Uganda (Figure 1), which experienced the most recent outbreaks of YF in 2011 and 2016 in areas bordering the county. The area is characterized by hot and dry weather most of the year with an annual mean temperature of 10 – 30 °C. Rainfall is erratic and unpredictable with the annual mean ranging from about 300-400 mm; falling to less than 150 mm in the arid central parts. The driest months are January through to March, while the wettest are April through June, with the other months receiving little or no rainfall. Bush density also decreases with decrease in rainfall (Climate –data.org, 2018). The mean daily rainfall during the sampling period was 3.94mm, 1.3mm and 5.55mm in the months of May 2015, December 2015 and May 2016, respectively.
Figure 1:
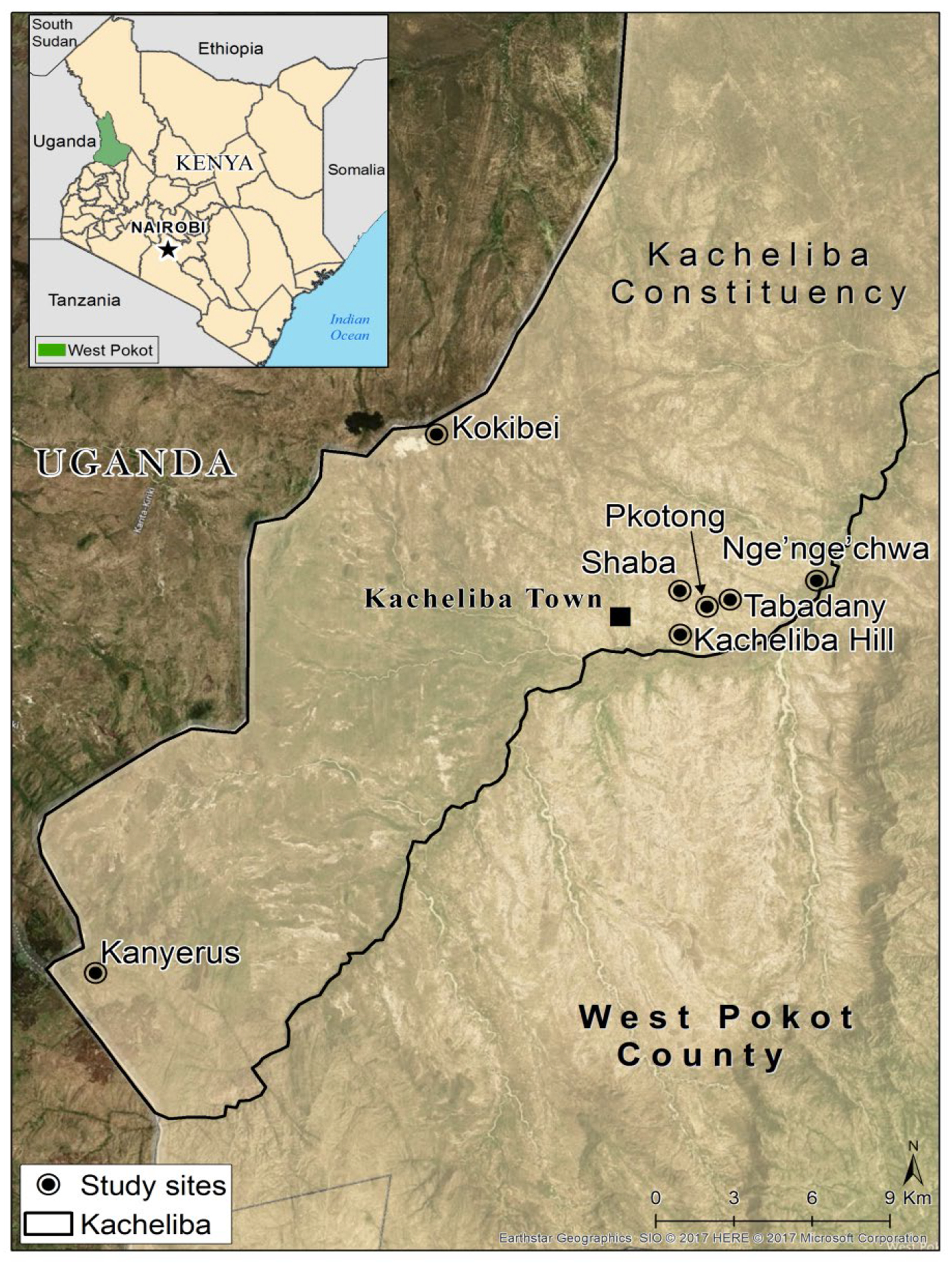
Map showing the sites sampled within Kacheliba sub-County during the May 2015, December 2015 and May 2016 sampling periods.
The human activity in West Pokot, whose population is approximately 512,690 (KNBS, 2009), is mainly nomadic pastoralism. The herders usually move between Kacheliba and the neighboring Uganda in search of water, food and pasture. This practice usually puts them at risk of exposure to YF and other exotic viruses, and has potential to lead to cross boarder exchange of diseases between the two countries. Currently, however, there is increasing tendency for the people to migrate to urban centers, where they adopt sedentary lifestyles creating a suitable environment for breeding of mosquitoes. There is also risk of viremic persons returning, to initiate local transmission in Kenya with potential for more widespread activity.
Mosquitoes were collected from peridomestic areas in Kacheliba sub-county. These included Pkotong, Shaba, Kacheliba and Kokibei hills that comprise of caves which form good habitats for rock dwelling animals such as rock hyraxes. In addition, there are several rock pools that collect water creating ideal conditions for mosquito breeding long after the rains. Pkotong, Shaba and Kacheliba hills are between 1 and 2 km apart and all neighbour the Kacheliba town. Kokibei is situated about 15 kms northwest of Kacheliba town and is situated right on the Kenya-Uganda border. Sampling was also conducted in Tabadany, Nge’nge’chwa and Kanyerus all characterized by flat terrain, open grasslands with presence of small bushes, shrubs and acacia trees. While Tabadany and Nge’nge’chwa are proximal to Kacheliba town, Kanyerus is situated about 30 kms southwest of Kacheliba town, and like Kokibei, lies close to the Kenya-Uganda border.
Mosquito sampling, processing and identification
Mosquito sampling was conducted for 10 days during each of two long rainy seasons (May 3-12, 2015 and May 14-23, 2016) and the short rainy season of 2015 from December 10-19. Adult collection was done using BG Sentinel traps (ten traps per site) baited with CO2 supplied in the form of dry ice and dispensed in Thermos flasks (~2L capacity). The ten traps were set at 0600 hours and retrieved at 1800 hours each day during the sampling period. The collected mosquitoes were sorted into vials by collection site, date, and stored in liquid nitrogen awaiting transportation to the icipe Emerging Infectious Diseases (EID) laboratory. The collected mosquitoes were identified by morphology using taxonomic keys (Edwards, 1941; Jupp, 1986; Huang, 1981; Huang, 1979; Huang, 2001). Blood fed mosquitoes were stored singly for mosquito host blood meal analysis.
Host blood feeding preference
Individual blood fed mosquito abdomens were separated from the rest of the body using a scapel which was sterilized before every successive decapitation. Each separated abdomen was transferred into a sterile 1.5 ml microcentrifuge tube and triturated in 500 μl of phosphate buffered saline (PBS). The DNA was then extracted using the DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer’s instructions. The DNA obtained was used as the template in a standard polymerase chain reaction (PCR) to amplify the 12S mitochondrial rRNA gene using published primer sets 12S3F [5′-GGGATTAGATACCCCACTATGC-3′] and 12S5R [5′-TGCTTACCATGTTACGACTT-3′] (Roca et al., 2004). All amplification products (~ 500 bp) were resolved in 1% agarose gels stained with ethidium bromide. All amplicons were purified using a PCR purification kit (Promega) following the manufacturer’s instructions. Bidirectional Sanger sequencing was done by Inqaba (Pretoria, South Africa). The sequences were cleaned and analysed using Molecular Evolutionary Genetics Analysis version 6.0 (MEGA). Sequences were assigned to particular species by Blastn analysis of the GenBank DNA sequence database (National Center for Biotechnology Information (NCBI), 2008) and the Barcode of Life Data (BOLD) Systems database (http://www.boldsystems.org/views/login.php). Positive identification and host species assignment were based on exact or near exact matches (>98%).
Statistical analysis
Mosquitoes were pooled for each trapping period: May 2015, December 2015 and May 2016. We determined mosquito diversity for each sampling period, by estimating the Shannon diversity index (‘diversity’) using the vegan package (Oksanen et al., 2015) in R version 3.3.1 (R development Core Team). We compared the abundance patterns (response variable) for all mosquito species and for individual species (Ae. aegypti, Ae. metallicus and Ae. vittatus) across the sampling periods (predictor variable) using generalized linear models (GLMs) with negative binomial error structure. We focused on Ae. aegypti, Ae. metallicus and Ae. vittatus as they were fairly represented across the sampling periods. The diversity trends across the sampling periods were compared after log-transformation. This was ascertained using normal GLMs with diversity specified as main factor. The short rain season (December 2015) was taken as a reference sampling period. Data normality for diversity data was confirmed by performing Shapiro–Wilk tests on model residuals. All data were analyzed at α=0.05 level of significance.
Results
Presence and abundance of Aedes stegomyia species
A total of 8605 mosquitoes, comprising 22 species in 5 genera, were collected during the different sampling periods (Table 1). Aedes aegypti was most predominant and was represented in all sampling periods. There were significantly more Stegomyia mosquito captures during the May 2016 sampling period; 12-fold higher compared to May 2015 and 3-fold higher compared to December 2015 (Table 1). Overall, the region was dominated by five genera Aedes, Anopheles, Culex, Mansonia and Coquillettidia. The Stegomyia genus was most represented in the mosquito captures (11 species), followed by Anopheles genus (5 species), and then Culex (4 species). Mansonia and Coquillettidia were each represented by 1 species (Table 1).
Table 1:
Summary of mosquito species in Kacheliba sub-county sampled during the May 2015, December 2015 and May 2016 sampling periods
| Sampling Periods | |||
|---|---|---|---|
| Long rain season | Short rain season | Long rain season | |
| Species | May 2015 | December 2015 | May 2016 |
| Aedes aegypti | 385 | 1551 | 4726 |
| Aedes vittatus | 4 | 115 | 323 |
| Aedes metallicus | 3 | 6 | 295 |
| Aedes hirsutus | 1 | 0 | 13 |
| Aedes mcintoshi | 1 | 0 | 3 |
| Aedes sudanensis | 0 | 0 | 7 |
| Aedes tarsalis | 0 | 0 | 23 |
| Aedes tricholabis | 0 | 0 | 2 |
| Aedes unilineatus | 0 | 0 | 21 |
| Aedes chaussieri | 0 | 1 | 0 |
| Aedes species | 0 | 0 | 2 |
| Anopheles coustani | 0 | 0 | 1 |
| Anopheles funestus s.l. | 0 | 7 | 369 |
| Anopheles gambiae s.l. | 1 | 2 | 16 |
| Anopheles nili | 0 | 18 | 0 |
| Anopheles rufipes | 0 | 0 | 5 |
| Coquillettidia aurites | 0 | 0 | 1 |
| Culex pipiens | 14 | 14 | 375 |
| Culex tigripes | 0 | 0 | 1 |
| Culex univittatus | 4 | 1 | 26 |
| Culex zombaensis | 0 | 0 | 263 |
| Mansonia uniformis | 0 | 0 | 5 |
| Total | 413 | 1715 | 6477 |
Data for Pkotong, Shaba and Kacheliba hill, and that of Tabadany and Nge’nge’chwa sites, were merged because of their proximity to each other, the sites were presented as K1 and K2, respectively. A higher number of potential YFV and DENV vectors were collected in K1 (Pkotong, Shaba and Kacheliba hill sites), compared to K2 (Tabadany and Nge’nge’chwa) and K3 (Kokibei) (Table 2).
Table 2:
Distribution of potential DENV and YFV vectors collected from sampling sites during different sampling periods
| Sites | Season | Ae. chaussieri | Ae. aegypti | Ae. metallicus | Ae. unilineatus | Ae. vittatus |
|---|---|---|---|---|---|---|
| K1 (Pkotong, Shaba and Kacheliba hill) | Long rain, May 2015 | 0 | 384 | 3 | 0 | 4 |
| Short rain, Dec 2015 | 1 | 1551 | 6 | 0 | 115 | |
| Long rain, May 2016 | 0 | 3521 | 218 | 20 | 284 | |
| Total | 1 | 5456 | 227 | 20 | 403 | |
| K2 (Tabadany and Nge’nge’chwa) | Long rain, May 2015 | 0 | 1 | 0 | 0 | 0 |
| Short rain, Dec 2015 | 0 | 0 | 0 | 0 | 0 | |
| Long rain, May 2016 | 0 | 1186 | 69 | 0 | 39 | |
| Total | 0 | 1187 | 69 | 0 | 39 | |
| K3 (Kokibei) | Long rain, May 2015 | 0 | 0 | 0 | 0 | 0 |
| Short rain, Dec 2015 | ns | ns | ns | ns | ns | |
| Long rain, May 2016 | 0 | 19 | 8 | 0 | 0 | |
| Total | 0 | 19 | 8 | 0 | 0 | |
ns- not sampled
Mosquito diversity based on Shannon diversity index showed significant differences across the sampling periods (p< 0.0001). Mean diversity ranged from 0.38 to 1.12, with the highest being 3-fold higher compared to the lowest. The highest species diversity was recorded during the May 2016 sampling period (Figure 2).
Figure 2:
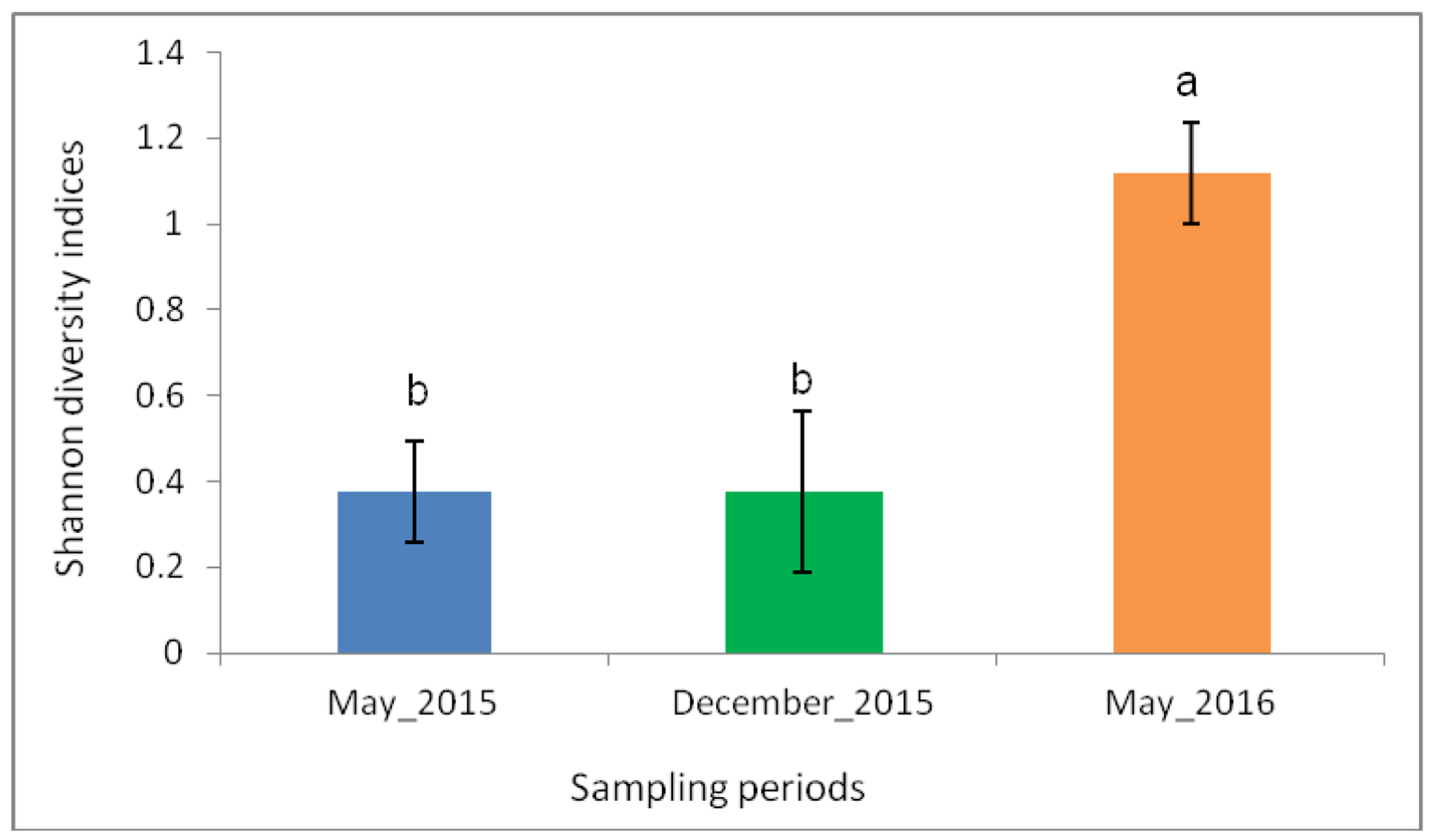
Shannon diversity indices across the three sampling periods. There was significantly high species diversity during the May 2016 sampling period. Error bars denote the standard error. Bars followed by different letters denote significant difference.
Total mosquito abundance and the abundance of potential vectors of DEN and YF, showed significant variation across the sampling periods (Table 3).
Table 3:
Negative binomial results comparing the abundance of the mosquitoes across the sampling periods
| Total Abundance | Aedes aegypti | Aedes metallicus | Aedes vittatus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| estimate ± se | Z value | p-value | estimate ± se | Z value | p-value | estimate ± se | Z value | p-value | estimate±se | Z value | p-value | |
| December 2015- short rain | 1 | 1 | 1 | 1 | ||||||||
| May 2015- Long rain | −1.760 ± 0.584 | −3.016 | 0.003 ** | −1.730±0.708 | −2.442 | 0.015 * | −1.030±0.793 | −1.299 | 0.194 | −3.695±1.275 | −2.899 | 0.004 ** |
| May 2016- Long rain | 0.741 ± 0.554 | 1.337 | 0.181 | 0.526 ± 0.673 | 0.782 | 0.4343 | 3.307± 0.536 | 6.176 | <0.001*** | 0.445±1.119 | 0.398 | 0.691 |
se - Standard error
*- Significant codes;
p<0.05;
p<0.01;
p<0.001
Highest abundance and diversity were recorded during the May 2016 collection, while the lowest abundance was recorded in May 2015 (Figure 3). The lowest diversity was recorded in the short rain season sampling, December 2015.
Figure 3:
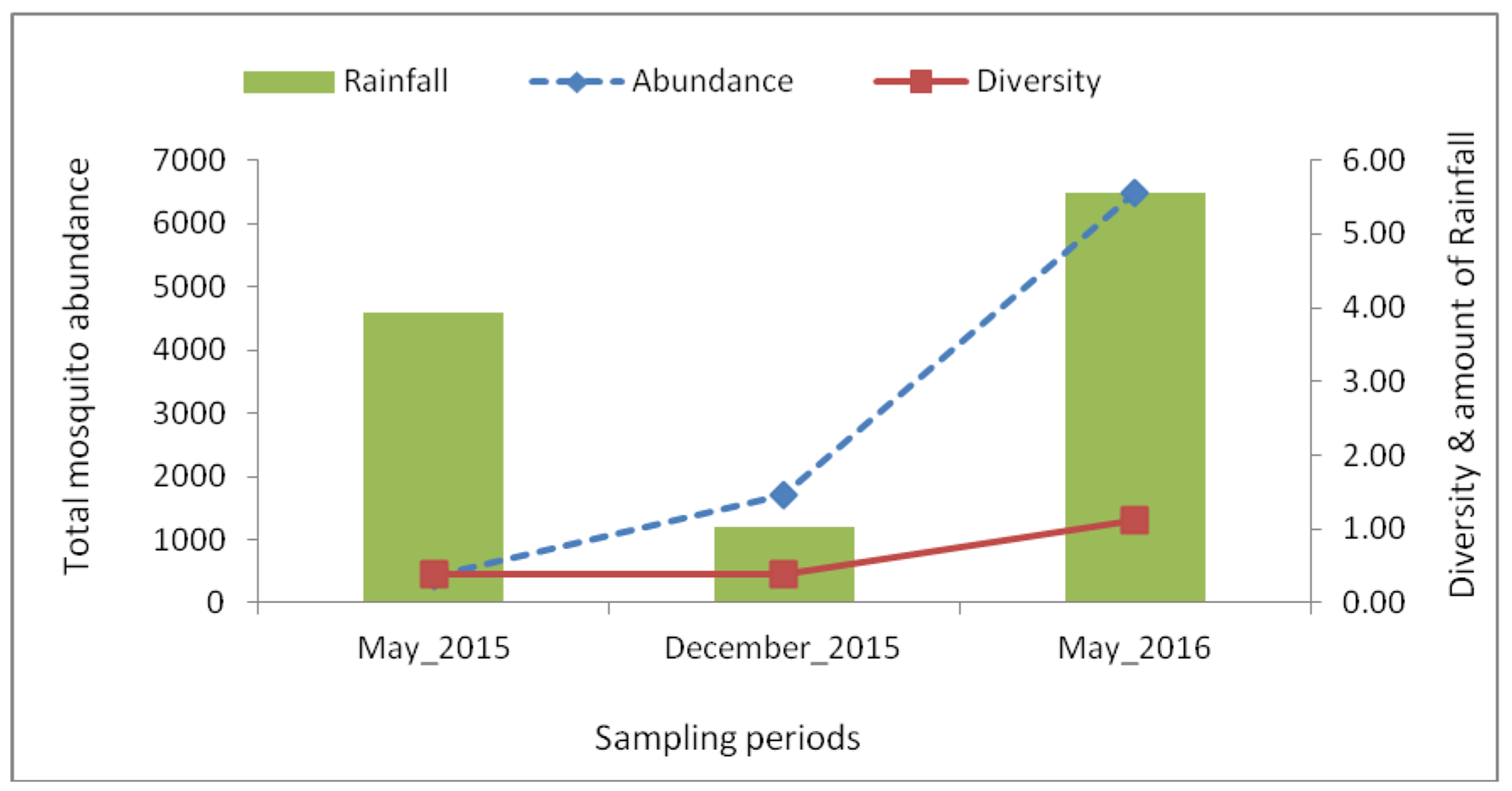
The total abundance and mean species diversity of mosquitoes collected, and the amount of rainfall (mm) during the sampling periods. The abundance and diversity increased with increased amount of rainfall.
Among host-seeking females of potential DENV and YFV vectors, Ae. aegypti was predominant (n=6662, 77.42%), followed by Ae. vittatus (n=442, 5.14%) and Ae. metallicus (n=304, 3.53%) (Figure 4). Aedes unilineatus had the least number sampled only during the long rain season in May 2016.
Figure 4:
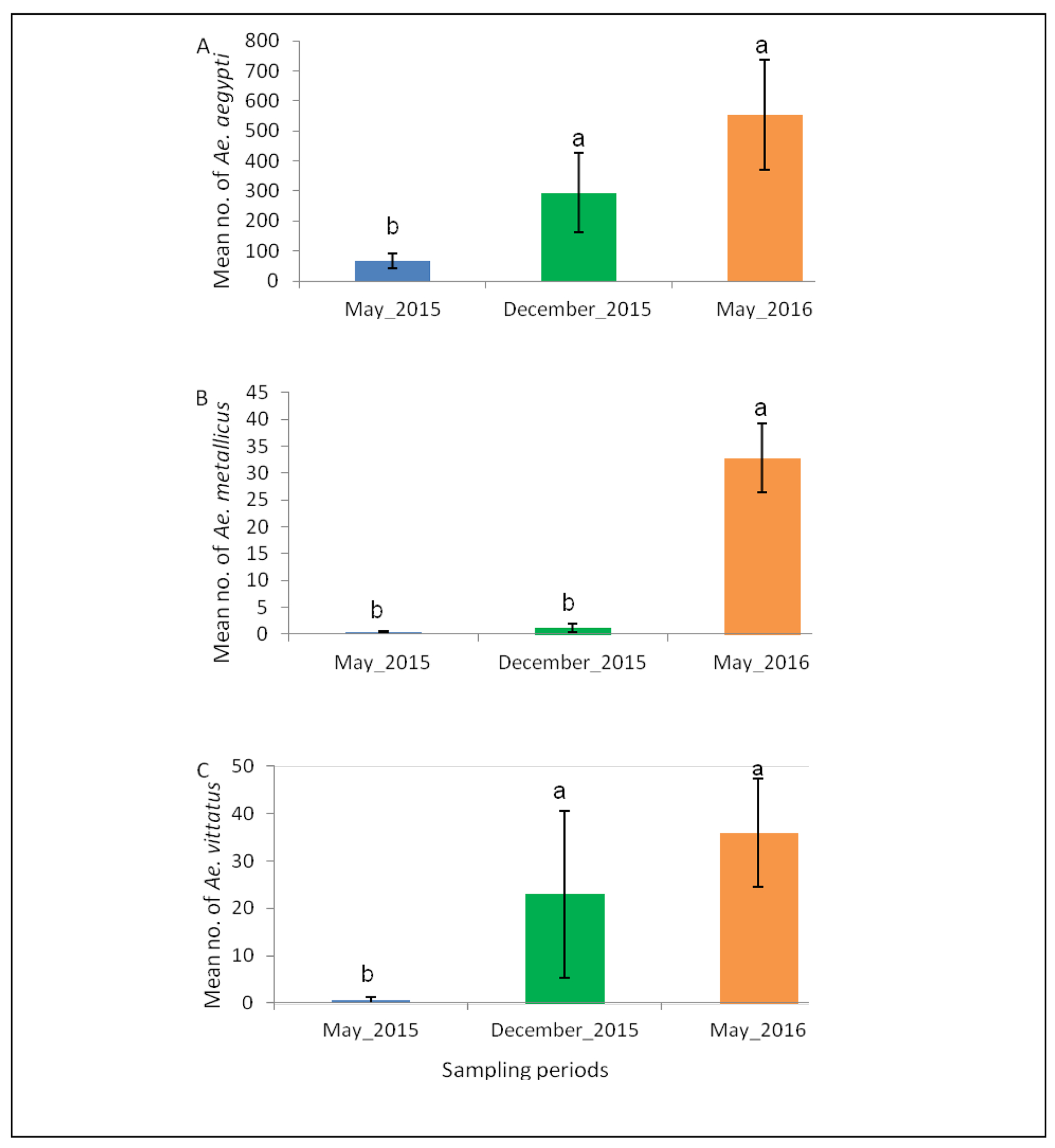
The abundance of (A) Ae. aegypti, (B) Ae. metallicus and (C) Ae. vittatus, potential vectors of DENV and YFV across different sampling periods in West Pokot, Kenya. Error bars denote the standard error. Bars followed by different letters denote significant difference.
Host blood feeding preference
Eighty eight (88) blood-fed mosquitoes, potential vectors of DENV and YFV, were found: Ae. aegypti: 68, Ae. metallicus: 9, Ae. unilineatus: 1 and Ae. vittatus: 10 (Figure 5). The majority of Ae. aegypti had fed on rock hyrax (n=54, 79%), followed by goats (n=6, 9%), humans and cattle (n=3, 4% each), and two had fed on hippopotamus and rock monitor lizard (each n=1, 1%). Most Ae. vittatus collected had fed on goats (n=4, 40%), followed by cattle (n=3, 30%), human (n=2, 20%) and sheep (n=1, 10%). As for Ae. metallicus, a similar number had fed on goat and sheep (n=3, 33.3%), while 22.2% (n=2) had fed on rock hyrax and 11.1% (n=1) on human. The single blood-fed Ae. unilineatus had fed on rock hyrax.
Figure 5:
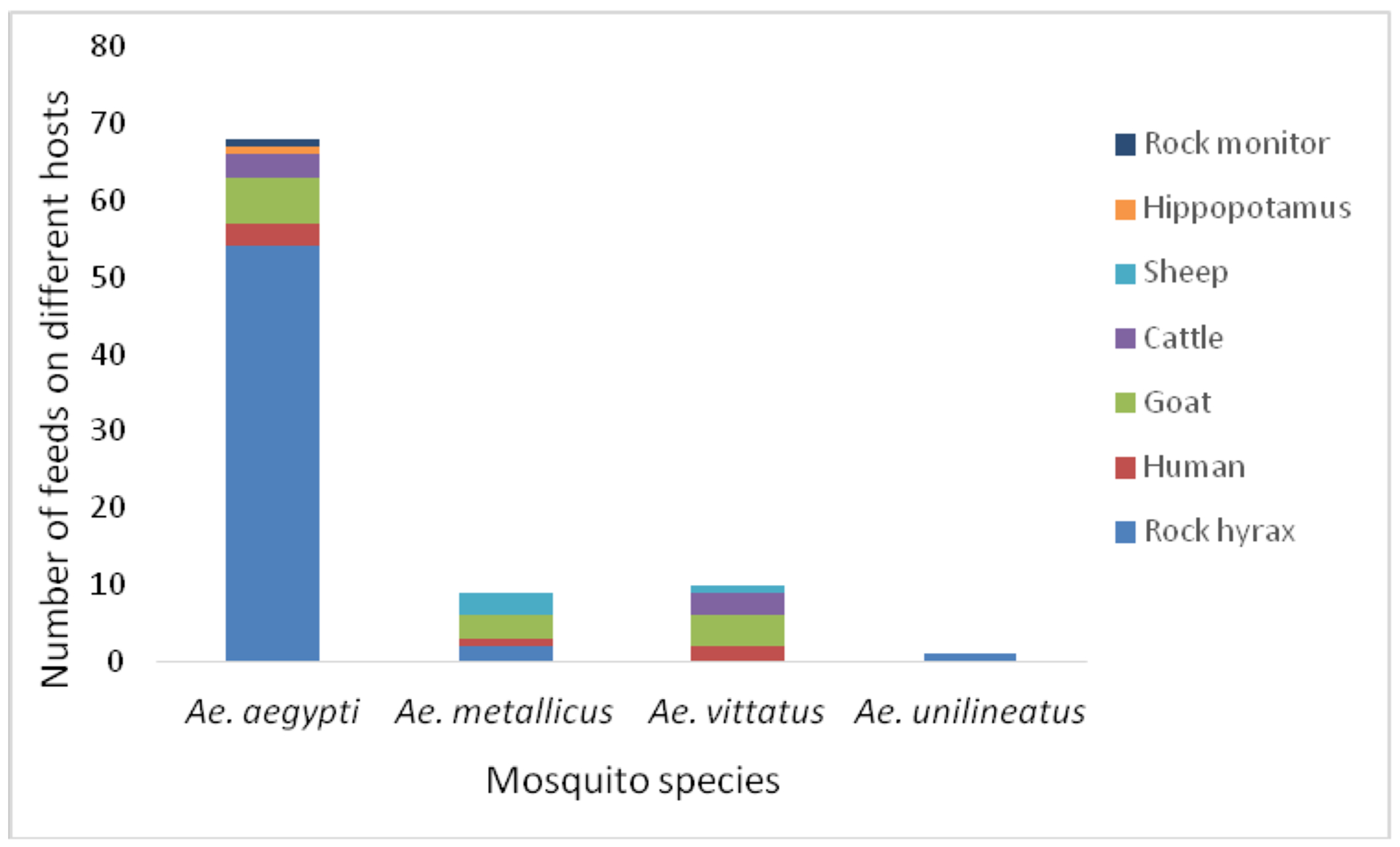
Host blood feeding patterns for the potential vectors of DENV and YFV in West Pokot County, Kenya. Total number analyzed, n=88.
Discussion
The surveillance of vectors (vector presence and their associated bionomic parameters, including abundance and blood feeding preferences) is essential for assessing the risk of pathogen transmission and occurrence of outbreaks. Our findings show that the mosquito fauna in the study area in West Pokot County, Kenya, bordering Uganda, is dominated by Stegomyia species, notably Ae. aegypti, Ae. vittatus and Ae. metallicus. These species are of medical significance having been implicated in the transmission of YFV/DENV in different settings (Cordellier, 1991; Barrett and Higgs, 2007; Ngoagouni et al., 2012; Hanley et al., 2013). Therefore, their presence in the study area would signify potential risk of transmission of YF and DEN viruses.
Total mosquito abundance correlated with a period of highest rainfall (May 2016). The effect of rainfall on mosquito abundance is well recognized (Arum et al., 2015; Agha et al., 2017a; Agha et al., 2017b). Rainfall increases the availability of natural and artificial sites for mosquito breeding and development. Risk of virus transmission and potential for outbreaks has been found to be high during such periods of abundant rainfall (Aitken et al., 1968; de Kruijf, 1972). An analogous pattern was observed for individual species including Ae. aegypti, Ae. metallicus and Ae. vittatus. Coincidentally, mosquito diversity and species richness showed a significant variation across the sampling periods and correlated with the period of highest rainfall.
The mosquito fauna of the study area seems to be less diverse compared to other sites in Kenya (Arum et al., 2015; Agha et al., 2017a; Agha et al., 2017b). Diversity estimates may however be affected by the type of sampling tool deployed. BG Sentinel traps were used in the current study, which are known to have bias for Stegomyia species (Barrera et al., 2013). To overcome this limitation, traps were baited with CO2, which is a universal attractant for most hematophagous insects, including mosquitoes (Dekker et al., 2005). Human landing collection may be the most effective method for sampling sylvatic Aedes (Diallo et al., 2012), but it was not possible to obtain ethical clearance for this collection method for the current study. Although our approach was aimed at collecting Aedes species of potential importance to DENV/YFV epidemiology, a combination of other tools including larval sampling may be more appropriate to determine species diversity. Overall, our study provides a useful baseline and first report on the mosquito fauna inhabiting the ecology of our study area in Kenya.
Host feeding pattern is an important component of vectorial capacity. This can reveal information about vector-host contact rates and potential reservoirs important in the amplification of a virus, and disease epidemiology. Based on our findings, the mosquitoes displayed a more zoophagic tendency, with most Stegomyia feeding on rock hyraxes, goats, cattle and sheep, and few feeding on humans. This may be due to the availability of particular hosts, such as rock hyraxes, which are abundant compared to the other hosts. However, other possible adaptive preferences cannot be discounted. We showed that Ae. aegypti, Ae. metallicus and Ae. vittatus feed on multiple hosts, including humans, indicating a potential to transmit viruses to humans. This assertion will require data on their ability to transmit the viruses, and vector competence studies that are ongoing. Additional sampling and testing of adult samples for viruses using a combination of methods including culture and sequencing would be valuable.
Aedes aegypti is the main DEN/YF virus vector in most urban settings (Barrett and Higgs, 2007; Ngoagouni et al., 2012; Hanley et al., 2013; Agha et al., 2017a). The zoophilic tendency observed for this species in this study, although striking, mirrors previous findings reported in West Africa (Diallo et al., 2012) associated with the sylvatic form, Ae. aegypti subspecies formosus. The likelihood that this form dominates the sylvatic setting characteristic of our study sites remains high, although further studies are recommended to ascertain this. The low feeds on humans may suggest low risk of transmission of these viruses especially YFV by this species. In the Kenyan YF outbreak of 1992–1993, Ae. aegypti was not the incriminated vector; rather, Ae. africanus, Ae. bromeliae and Ae. keniensis were identified as vectors through virus isolation during the outbreak (Reiter et al., 1998). Furthermore, in the sylvatic setting in West Africa, this species is considered not to play any role in the transmission of important viruses such as Chikungunya virus (Diallo et al., 2012). This form is thought to feed mainly on wild animals, especially non-human primates (Diallo et al., 2012), important in the natural transmission cycle of YFV and DENV. Surprisingly, we did not find any blood feeds on non-human primates in any of the mosquito species examined, although they were observed to be present in sampling area. Seasonal migratory patterns among non-human primates in search of food are well recognized (Alberts and Altmann, 2001). The lack of blood feeding from this host could be attributed to mosquito trapping coinciding with periods when the non-human primates had migrated to other localities within the vast West Pokot County or across the border to Uganda, probably in search of food.
Conclusion
The presence of important Stegomyia species, which are known vectors of YFV and DENV, in Kacheliba, West Pokot County, Kenya, shows the risk for transmission of these viruses and possible outbreaks of YF and DEN in this area. However, the Stegomyia species displayed a strong zoophagic preference, mainly feeding on wild and domestic animals (hyrax and goat), suggesting a reduced potential for disease transmission to human. The findings from our study confirm the need for further bionomic and surveillance studies on mosquitoes in this area. Further research, including vector competence of the Stegomyia species, is required to guide implementation of affordable and sustainable vector control and vaccination programs.
Acknowledgments
We acknowledge the contribution of Caroline Tigoi (icipe, Nairobi) for project management. We recognize the technical support of John Gachoya (KEMRI, Nairobi) and James Wauna (icipe), in mosquito collection and identification. We are grateful to Jackson Kimani, GIS support unit, icipe for producing the map of the study area. We are also grateful for the support from the local chief as well as community members of Kacheliba, West Pokot County. We thank Dr. Robert Skilton, Capacity Building, icipe for the review of the manuscript.
Funding Statement
EC was supported by a German Academic Exchange Service (DAAD) In-Region Postgraduate Scholarship and a L’Oreal-UNESCO for Women in Science Scholarship. We gratefully acknowledge the financial support for this research by the following organizations and agencies: National Institutes of Health (NIH), Grant No. 1R01AI099736-01A1 to RS; UK Aid from the UK Government; Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); and the Kenyan Government. The views expressed herein do not necessarily reflect the official opinion of the donors.
Footnotes
Competing Interests
The authors have no competing interests
References
- Barrett AD, Higgs S. Yellow fever: A disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–29. [DOI] [PubMed] [Google Scholar]
- Kraemer MU, Faria NR, Reiner RC Jr, Golding N, Nikolay B, Stasse S, et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015-16: a modelling study. Lancet Infect Dis. 2017;17(3):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [Internet] Yellow fever facts sheet no 100. http://www.who.int/mediacentre/factsheets/fs100/en/.2014 [cited 2014 March].
- Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, et al. Yellow fever in africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014;11(5):e1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EJ, Marfin AA, Tukei PM, Kuria G, Ademba G, Agata NN, et al. First recorded outbreak of yellow fever in kenya, 1992-1993. I. Epidemiologic investigations. Am. J. Trop. Med. Hyg 1998;59(4):644–9. [DOI] [PubMed] [Google Scholar]
- Reiter P, Cordellier R, Ouma JO, Cropp CB, Savage HM, Sanders EJ, et al. First recorded outbreak of yellow fever in kenya, 1992-1993. Ii. Entomologic investigations. Am. J. Trop. Med. Hyg 1998;59(4):650–6. [DOI] [PubMed] [Google Scholar]
- Onyango CO, Ofula VO, Sang RC, Konongoi SL, Sow A, De Cock KM, et al. Yellow fever outbreak, imatong, southern sudan. Emerg Infect Dis. 2004;10(6):1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Yellow fever outbreak in south kordofan, sudan. Weekly Epidemiological Record; 2005. [Google Scholar]
- World Health Organization. Yellow fever, uganda. Weekly epidemiological record; 2011. p. 37–44. [Google Scholar]
- World Health Organisation (2016). Yellow fever-Uganda. http://www.who.int/csr/don/02-may-2016-yellow-fever-uganda/en/ [Google Scholar]
- InterHealth Worldwide (2016). Yellow fever outbreak in Uganda. https://www.interhealthworldwide.org/home/health-resources/health-alerts/2016/april/26/yellow-fever-outbreak-in-uganda/
- Neiderud CJ. How urbanization affects the epidemiology of emerging infectious diseases. Infection Ecology & Epidemiology, 2015; 5, 10.3402/iee.v5.27060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang RC Dengue in africa. (a review). Report on dengue. Scientific working group. Geneva Switzerland. Special Programme for Research & Training in Tropical Diseases (TDR) World Health Organization. 2006. [Google Scholar]
- Konongoi L, Ofula V, Nyunja A, Owaka S, Koka H, Makio A, et al. Detection of dengue virus serotypes 1, 2 and 3 in selected regions of Kenya: 2011–2014. Virology Journal. 2016;13:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairo F, Mboera L, De Nardo P, Oriyo NM, Meschi S, Rumisha SF et al. Clinical, Virologic, and Epidemiologic Characteristics of Dengue Outbreak, Dar es Salaam, Tanzania, 2014. Emerging Infectious Diseases, 2016; 22(5), 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Ocheng D, Gichogo A, Okiro M, Libondo D, Kinyanjui P, et al. Epidemic dengue fever caused by dengue type 2 virus in kenya: Preliminary results of human virological and serological studies. East Afr Med J. 1982;59(12):781–4. [PubMed] [Google Scholar]
- Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: Full circle? Comp Immunol Microbiol Infect Dis. 2004;27(5):319–30. [DOI] [PubMed] [Google Scholar]
- Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, and Reisen WK. Present and Future Arboviral Threats. Antiviral Research, 2010;85(2), 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey LL, Forrester N, Tsetsarkin K, Vasilakis N, & Weaver SC. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiology, 2013;8(2), 155–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B, Kapan DD. Man Bites Mosquito: Understanding the Contribution of Human Movement to Vector-Borne Disease Dynamics. PLoS ONE 2009; 4(8): e6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Fleisher A, Ruiz-Moreno D, Vermeylen F, Wa CV, Poulson RL, et al. Heterogeneous feeding patterns of the dengue vector, Aedes aegypti, on individual human hosts in rural thailand. PLoS Negl Trop Dis. 2014;8(8):e3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien VA, Moore AT, Young GR, Komar N, Reisen WK, Brown CR. An enzootic vector-borne virus is amplified at epizootic levels by an invasive avian host. Proceedings of the Royal Society of London B: Biological Sciences. 2011;278(1703):239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climate-data.org, (Internet, cited on 2018 April). https://en.climate-data.org/region/1718/
- Kenya National Bureau of Statistics. Census report, 2009. [Google Scholar]
- Edwards FW. Mosquitoes of the Ethiopian region. Iii.-culicine adults and pupae. Mosquitoes of the Ethiopian Region. III.-Culicine Adults and Pupae. 1941. [Google Scholar]
- Jupp PG. Mosquitoes of Southern Africa. Ecogilde, South Africa 1986:156. [Google Scholar]
- Huang Y-M, Ward RA. A pictorial key for the identification of the mosquitoes associated with yellow fever in africa. DTIC Document, 1981. [Google Scholar]
- Huang Y-M. Aedes (stegomyia) simpsoni complex in the ethiopian region with lectotype designation for simpsoni (theobald)(diptera: Culicidae). DTIC Document, 1979. [Google Scholar]
- Huang Y-M. A pictorial key for the identification of the subfamilies of culicidae, genera of culicinae, and subgenera of Aedes mosquitoes of the afrotropical region (diptera: Culicidae): Walter Reed Biosystematics Unit; 2001. [Google Scholar]
- Roca AL, Kahila Bar-Gal G, Eizirik E, Helgen KM, Maria R, Springer MS, et al. Mesozoic origin for West Indian insectivores.Nature 2004;429: 649–651. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara R, et al. Package ‘vegan’, Community ecology package, version 2.2–1. 2015. [Google Scholar]
- Cordellier R,. L’épidémiologie de la fièvre jaune en Afrique de l’Ouest. Bulletin of the World Health Organization, 1991;69(1), p.73. [PMC free article] [PubMed] [Google Scholar]
- Ngoagouni C, Kamgang B, Manirakiza A, Nangouma A, Paupy C, Nakoune E. et al. Entomological profile of yellow fever epidemics in the Central African Republic, 2006–2010. Parasites & vectors, 2012;5(1), p.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken THG, Worth CB, and Tikasingh ES. Arbovirus studies in Bush Bush forest, Trinidad, W. I. September 1959-December 1964. III. Entomologic studies. Am. J. Trop. Med. Hyg 1968;17:253–268. [DOI] [PubMed] [Google Scholar]
- de Kruijf HAM.. Aspects of the ecology of mosquitoes in Surinam. III. Seasonal distribution and abundance of some populations. Stud. Fauna Surinam Other Guyanas XIII 1972;51:30–48. [Google Scholar]
- Arum SO, Weldon CW, Orindi B, Landmann T, Tchouassi DP, Affognon HD, & Sang R. Distribution and diversity of the vectors of Rift Valley fever along the livestock movement routes in the northeastern and coastal regions of Kenya. Parasites & Vectors, 2015; 8, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha SB, Tchouassi DP, Bastos ADS, Sang R. Assessment of risk of dengue and yellow fever virus transmission in three major Kenyan cities based on Stegomyia indices. PLOS Neglected Tropical Diseases 2017a; 11(8): e0005858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha SB, Tchouassi DP, Bastos ADS, Sang R. Dengue and yellow fever virus vectors: Seasonal abundance, diversity and resting preferences in three kenyan cities. Parasites & Vectors. 2017b;10(1):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Mackay AJ, & Amador M. An Improved Trap To Capture Adult Container-Inhabiting Mosquitoes. Journal of the American Mosquito Control Association, 2013; 29(4), 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker T, Geier M, Cardé RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. Journal of Experimental Biology. 2005;208(15):2963. [DOI] [PubMed] [Google Scholar]
- Diallo D, Sall AA, Buenemann M, Chen R, Faye O, et al. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl Trop Dis 2012;6: e1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts SC and Altmann J. Immigration and hybridization patterns of yellow and anubis baboons in and around Amboseli, Kenya. American Journal of Primatology, 2001: 53, 139–154. [DOI] [PubMed] [Google Scholar]


