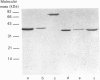
Abstract
The annexins are a family of structurally similar, Ca2(+)-dependent, phospholipid-binding proteins. We compared six members of this family (calpactin I heavy chain, lipocortins I and III, endonexin II, p68 and protein II) to determine their phospholipid-binding specificities, as well as their ability to promote aggregation and fusion of phospholipid vesicles. The Ca2+ requirement for all of the proteins was lowest for binding to vesicles composed of phosphatidic acid, followed by phosphatidylserine and then phosphatidylinositol. Only protein II, p68, lipocortin III and endonexin II bound to vesicles composed of phosphatidylethanolamine, and none bound to phosphatidylcholine. Both calpactin I heavy chain and lipocortin I promoted aggregation of phosphatidylserine- or phosphatidylinositol-containing vesicles in the presence of less than 10 microM-Ca2+. Lipocortin I promoted fusion of liposome membranes by lowering threshold Ca2+ concentrations. Although calpactin I heavy chain did not affect threshold Ca2+ concentrations, it did increase the rate and extent of spontaneous fusion. In contrast, p68 inhibited fusion at threshold Ca2+ concentrations. Whereas previous reports have emphasized properties that the annexins have in common, these findings reveal quantitative and qualitative differences among the annexins which may relate to distinct intracellular functions.
Full text
PDF

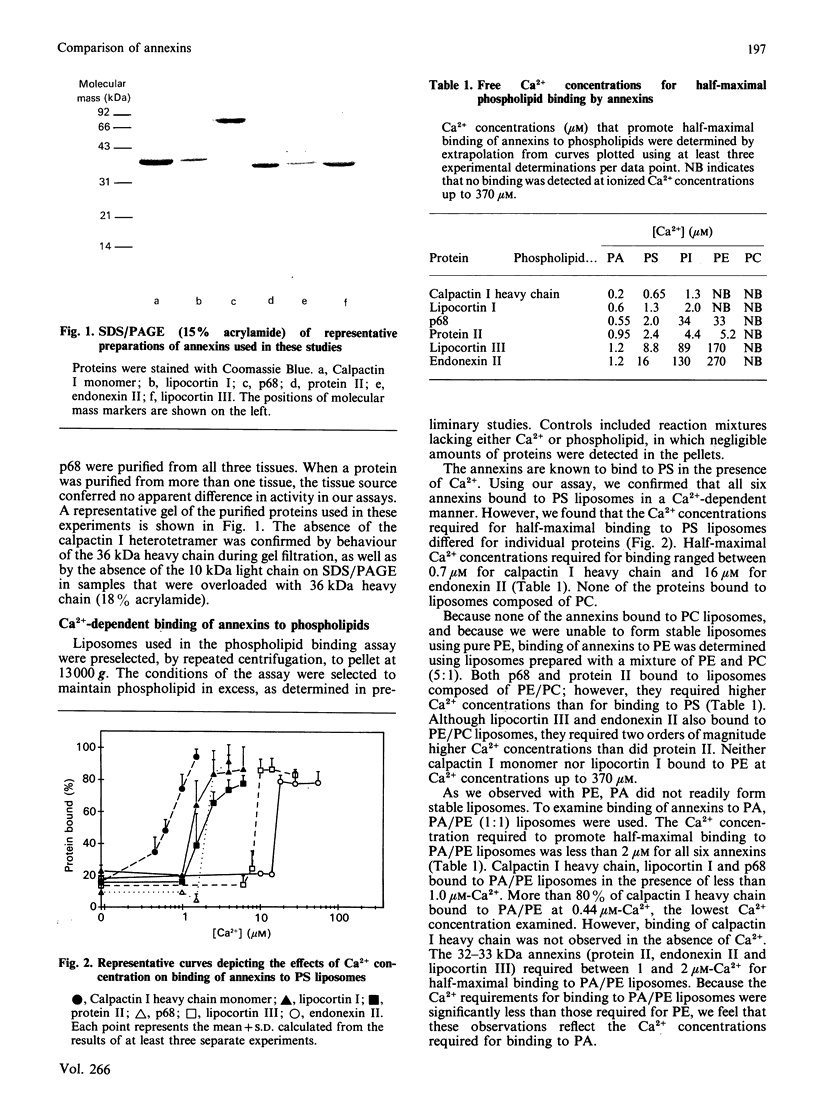
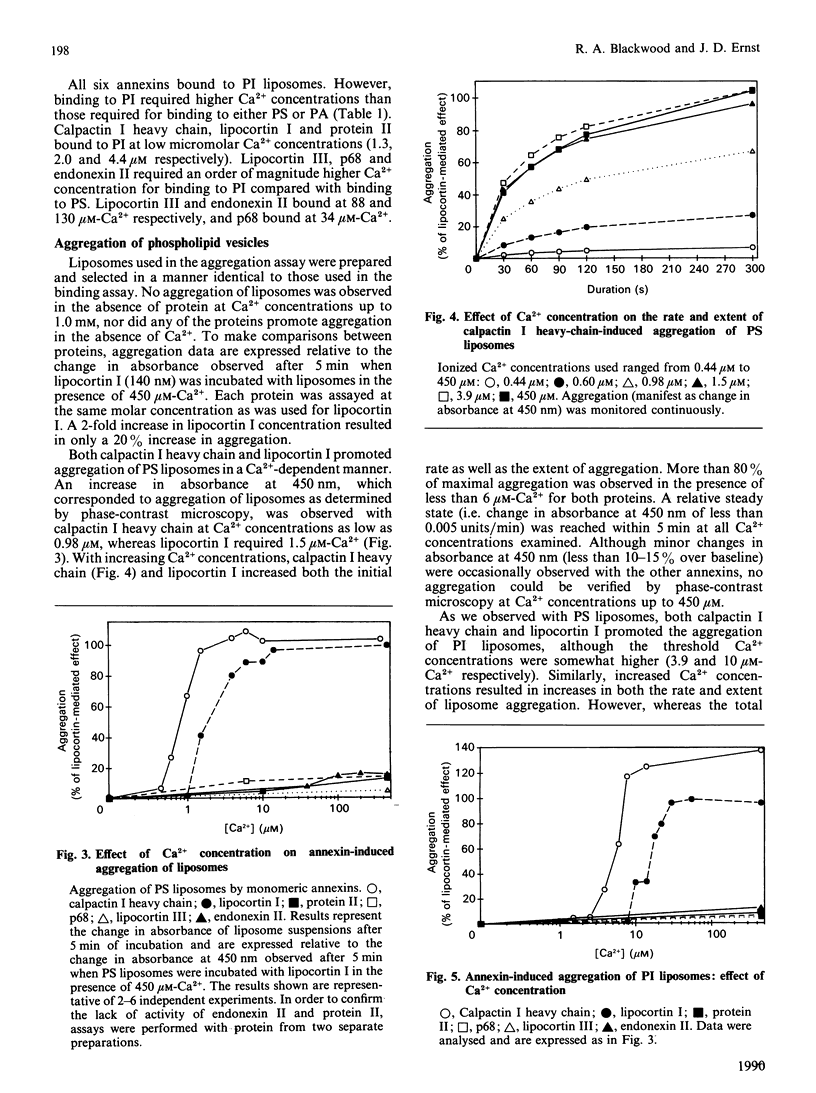
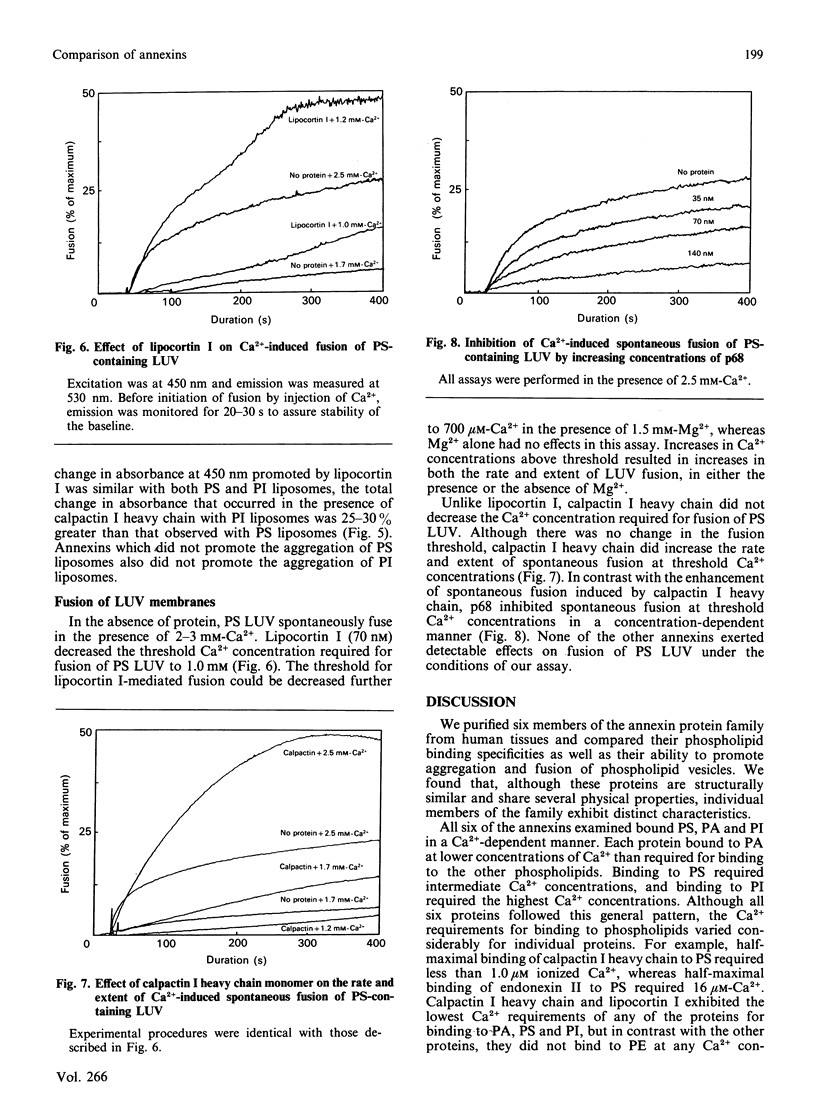

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Brugge J. S. The p35/p36 substrates of protein-tyrosine kinases as inhibitors of phospholipase A2. Cell. 1986 Jul 18;46(2):149–150. doi: 10.1016/0092-8674(86)90729-4. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Calpactin in exocytosis. Nature. 1988 Jan 7;331(6151):20–20. doi: 10.1038/331020a0. [DOI] [PubMed] [Google Scholar]
- Burns A. L., Magendzo K., Shirvan A., Srivastava M., Rojas E., Alijani M. R., Pollard H. B. Calcium channel activity of purified human synexin and structure of the human synexin gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3798–3802. doi: 10.1073/pnas.86.10.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Self-association of synexin in the presence of calcium. Correlation with synexin-induced membrane fusion and examination of the structure of synexin aggregates. J Biol Chem. 1979 Jan 25;254(2):553–558. [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Crompton M. R., Owens R. J., Totty N. F., Moss S. E., Waterfield M. D., Crumpton M. J. Primary structure of the human, membrane-associated Ca2+-binding protein p68 a novel member of a protein family. EMBO J. 1988 Jan;7(1):21–27. doi: 10.1002/j.1460-2075.1988.tb02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Allen T. M., Fedor J., Papahadjopoulos D. Lipid mixing during membrane aggregation and fusion: why fusion assays disagree. Biochemistry. 1987 Dec 15;26(25):8435–8442. doi: 10.1021/bi00399a061. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Hong K., Fraley R., Perry C., Friend D. S., James T. L., Papahadjopoulos D. Physicochemical characterization of large unilamellar phospholipid vesicles prepared by reverse-phase evaporation. Biochim Biophys Acta. 1983 Jul 13;732(1):289–299. doi: 10.1016/0005-2736(83)90214-6. [DOI] [PubMed] [Google Scholar]
- Ernst J. D., Hoye E., Blackwood R. A. Use of a novel strategy for the preparation and characterization of an antipeptide antibody capable of recognizing members of the annexin family. Biochem Biophys Res Commun. 1989 Jun 30;161(3):959–964. doi: 10.1016/0006-291x(89)91336-3. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Walker J. H., Boustead C., Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987 Apr;7(4):289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- Glenney J. Two related but distinct forms of the Mr 36,000 tyrosine kinase substrate (calpactin) that interact with phospholipid and actin in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4258–4262. doi: 10.1073/pnas.83.12.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Fitch J. M., Jones J. M., Schlaepfer D. D. Two lipocortin-like proteins, endonexin II and anchorin CII, may be alternate splices of the same gene. Trends Biochem Sci. 1989 Feb;14(2):48–50. doi: 10.1016/0968-0004(89)90041-8. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Ekerdt R., Papahadjopoulos D. Synexin facilitates fusion of specific phospholipid membranes at divalent cation concentrations found intracellularly. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4642–4644. doi: 10.1073/pnas.79.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Meers P., Ernst J. D., Düzgünes N., Hong K. L., Fedor J., Goldstein I. M., Papahadjopoulos D. Synexin-like proteins from human polymorphonuclear leukocytes. Identification and characterization of granule-aggregating and membrane-fusing activities. J Biol Chem. 1987 Jun 5;262(16):7850–7858. [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Slaughter C. A., Leznicki I., Barjon P., Reynolds G. A. Human 67-kDa calelectrin contains a duplication of four repeats found in 35-kDa lipocortins. Proc Natl Acad Sci U S A. 1988 Feb;85(3):664–668. doi: 10.1073/pnas.85.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Johnsson N., Plessmann U., Van P. N., Söling H. D., Ampe C., Vandekerckhove J. The amino acid sequence of protein II and its phosphorylation site for protein kinase C; the domain structure Ca2+-modulated lipid binding proteins. EMBO J. 1987 Jun;6(6):1599–1604. doi: 10.1002/j.1460-2075.1987.tb02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]