Abstract
Background
Correctly diagnosing dizziness in children is essential for appropriate management; nevertheless, healthcare professionals face challenges due to children’s limited ability to describe their symptoms and their cooperation during physical examination. The objective of this study is to describe the first 100 patients seen at a newly established pediatric vertigo center.
Methods
This is a retrospective review of a consecutive series of 100 patients seen at our pediatric vertigo clinic in a tertiary referral center from August 2019 until June 2022. Comprehensive clinical data were collected. The diagnoses were established by 2 pediatric otolaryngologists based on validated diagnostic criteria. Trends in diagnosis, investigation, and treatment of these patients were analyzed.
Results
A total of 100 children were included in the study. Vestibular migraine was the most common diagnosis (20%) followed by benign paroxysmal vertigo of childhood (14%). Eleven patients had combined pathologies. Fifteen out of 70 children (21%) had abnormal audiograms, 30 out of 48 children (62.5%) had abnormal vestibular testing, and 6 out of 31 (19%) patients had abnormal imaging. Fifty-one children received medical treatment, 23 received vestibular physiotherapy, and 9 patients had particle repositioning maneuvers; moreover, 17 of these patients received multimodal treatment.
Conclusions
Our analysis suggests that imaging and audiology testing have relatively low yield in the assessment of pediatric vertigo. On the other hand, vestibular testing detected a high proportion of abnormalities, such as saccadic pursuit, vertical nystagmus, central positional nystagmus, and abnormal directional preponderance, particularly associated with vestibular migraine. Given the complexity of diagnosing vertigo in children, it is critical to establish multidisciplinary specialized centers capable of providing accurate diagnosis and treatment for these children.
Keywords: vertigo, dizziness, children, migraine, vestibular testing, audiological testing, radiological testing, diagnosis
Graphical Abstract.
Background
It is well-established that children and adolescents experience vertigo and dizziness, with up to a 15% prevalence reported during the school-age stage of development (6-12 years). 1 Although the incidence and presentation of vertigo differs between children and adults, the range of diagnoses is similar. 2 Migraine is considered to be one of the most common causes of pediatric vertigo with a prevalence ranging between 25% and 30%. 3 Vertigo in childhood may result in delayed postural control, gross motor development, and abnormal coordination, which can affect quality of life as well as school and daytime performance. 4 Therefore, it is critical to accurately diagnose the cause of vertigo in children for optimal and timely management. Although a proper history and physical examination is often sufficient to make an accurate diagnosis of pediatric vertigo, healthcare providers are still presented with a number of challenges. The first challenge is obtaining a reliable history because children are often unable to accurately describe their symptoms, and the second challenge is obtaining cooperation of the child during the physical examination.
There are minimal high-quality data on pediatric vertigo in the literature, with only a few large series published. The objective of this study is to determine the prevalence of the causes of pediatric vertigo and describe their presentation, diagnostic assessment, and management.
Methods
The study received approval from the McGill University Health Center Research Ethics Boards (MUHC-REB).
Study Design and Population
We conducted a retrospective case series of the first 100 pediatric patients (<18 years) who presented to a newly established vertigo center at the Montreal Children’s Hospital with the main complaint of vertigo/dizziness, starting from August 2019 until June 2022. The following data were collected: age, sex, history of present illness, physical and vestibular examination findings, vestibular testing results, audiological data, imaging results, and treatment offered.
Vertigo Clinic Assessment
Our team included pediatric otolaryngologists, audiologists, and vestibular lab technician. As part of our multidisciplinary approach, we had a close collaboration with the neurology department and the concussion clinic, both receiving referrals from them and making referrals from our end to manage patients with migraine and central causes.
The assessment began with a thorough medical history, tailored to the patient’s age and linguistic skills. From our observations, children older than 4 years typically articulate their symptoms, although this varies among individuals. The history will focus on vertigo description, duration, frequency, associated symptoms, and risk factors similar to taking history in adults. On the other hand, children younger than 4 years, much of the history is obtained from parents or caregivers. While similar questions are asked, younger children find it challenging to describe vertigo; instead, the focus shifts to aspects like balance, gait, physical activity, clumsiness, frequent falls, and difficulty navigating dark environments. It is crucial for this age group to assess motor milestones such as head and neck control, unsupported sitting, crawling, and walking, especially for infants.
All patients received general otolaryngology examination including an otomicroscopy examination, orthostatic vital signs, motor milestones, and cranial nerves. The vestibular examination was adapted to the patient’s age and consisted of general cranial nerves examination, nystagmus assessment, pursuit, saccade, convergence, skew, head impulse test (HIT), head shake test, dynamic visual acuity, cerebellar exam, Romberg’s, Tandem gate, unipodal standing with eyes open and closed, and Fukuda testing. Further maneuvers were performed with infrared goggles which included head shake test, Dix-Hallpike, supine roll, midline head-hang, and fistula testing.
Audiological Assessment
The assessment consisted of an age-appropriate hearing test and impedance testing. These tests were performed by 2 pediatric audiologists. Children as young as 6 months old received otoacoustic emission, auditory brainstem response, or behavioral observation audiometry; children 6 months to 3 years received visual response audiometry in addition to the previous tests; children 3 to 5 years received condition play audiometry, and children 6 years and older received standard audiometry.
Imaging
Computed tomography (CT) and/or magnetic resonance imaging (MRI) of the brain and temporal bone were performed for vertigo with central features, sudden sensorineural hearing loss (SNHL), asymmetrical hearing loss, atypical presentation, neurological deficit, headache with red flag features, or post-head trauma if suspected inner ear fistula.
Vestibular Battery Assessment
While not all tests are necessary or suitable for every child, our approach to vestibular testing varies based on the patient’s age and level of cooperation. The vestibular battery assessment consisted of videonystagmography (VNG), video head impulse test (vHIT), and cervical vestibular evoked myogenic potential (cVEMP) testing. Detailed description of the vestibular testing and their interpretations can be found in Supplemental Appendix 1.
VNG/caloric testing is often performed for children aged 6 years and above. It can be performed using either water or air. However, using air is preferred as we can quickly stop the stimulus and avoid wetting the patient. cVEMP can be performed regardless of the child’s age as supported by the literature.5,6 Our approach is to offer cVEMP testing as soon as the child can hold their head up. vHIT testing depends heavily on the child’s cooperation and is typically offered to those older than 4 years. However, some studies performed it for children as young as 3 months using remote video method or 3 years using the traditional goggles.7,8
Vertigo Diagnostic Criteria
Two experienced pediatric otolaryngologists (M.K.A. and J.G.) reviewed the charts independently to confirm the vertigo diagnoses based on validated diagnostic criteria and, in the case of disagreement, they collectively discussed until they reached a complete agreement. The diagnostic criteria were adopted from the International Classification of Headache Disorder, American Academy of Otolaryngology-Head and Neck Surgery Equilibrium Committee, International Classification of Vestibular Disorders, American Autonomic Society, and American Academy of Neurology. A detailed summary of the criteria for each diagnosis can be found in Supplemental Appendix 2.
Statistical Analysis
Descriptive statistics were performed using Microsoft Excel to estimate the prevalence of the various causes of vertigo, the findings of the investigations, and the treatment.
Results
The retrospective review included 100 children (60 female and 40 male) with mean age of 11.13 ± 4.4 years (range: 1-17 years).
Vertigo Diagnosis
The diagnoses of pediatric vertigo in our series are summarized in Figure 1. Out of 100 total patients, the most common diagnosis was vestibular migraine with a total of 20 children. Nine children were found to have vestibular hypofunction on clinical and objective vestibular testing which did not meet any of the aforementioned criteria or diagnoses. Two patients were diagnosed with autoimmune inner ear disease (AIED). Two children had otitis media associated dizziness. One patient had congenital nystagmus confirmed on vestibular testing. One patient had dizziness associated with a newly diagnosed hypothyroidism; symptoms resolved after the initiation of thyroid hormone replacement therapy. Three patients had no clear diagnoses based on the selected diagnostic criteria adopted in this study. Eleven children had combined pathologies (See details in Figure 1).
Figure 1.
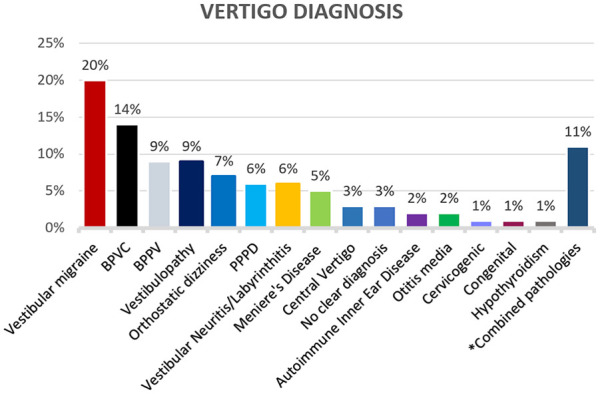
Vertigo diagnosis. BPVC, benign paroxysmal vertigo of childhood; BPPV, benign paroxysmal positional vertigo; PPPD, persistent postural perceptual dizziness.
Combined pathologies: 4 patients had vestibular migraine + orthostatic dizziness, 2 patients had vestibular migraine + BPPV, PPPD + BPPV, vestibulopathy + PPPD, vestibular migraine + PPPD, labyrinthitis + PPPD, vestibular migraine + PPPD + orthostatic dizziness.
Audiological Assessment
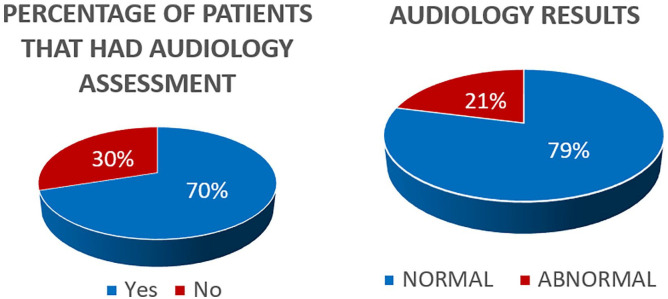
Audiological assessment was performed on 70 children (Figure 2). Among them, 55 had a normal hearing assessment (79%). The abnormal findings in the other 15 patients can be seen in Table 1.
Figure 2.
Audiological assessment results.
Table 1.
Audiological Findings.
| Abnormal audiological findings | Diagnosis |
|---|---|
| Mild—severe SNHL | 6 children with vestibular hypofunction confirmed on vestibular testing |
| Fluctuating SNHL | 2 children with Meniere’s 2 children with AIED |
| Sudden onset SNHL | 3 children with labyrinthitis |
| Profound SNHL (>90 dB) | Child with enlarged vestibular aqueduct and absent cochlear nerve |
Abbreviations: AIED, autoimmune inner ear disease; SNHL, sensorineural hearing loss.
Imaging Assessment
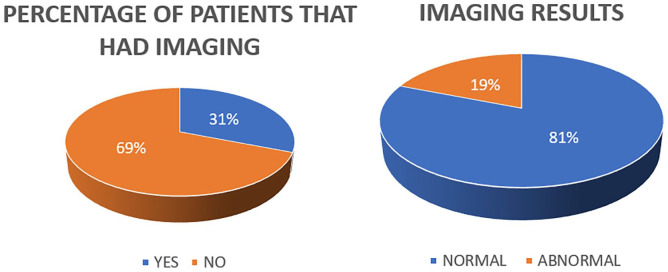
Thirty-one children underwent CT and/or MRI scans (Figure 3). Among these, 25 had normal imaging (81%), while 6 patients had abnormal findings (19%), including conditions such as absent cochlear nerve, vestibule dysplasia, bilateral enlarged vestibular aqueduct, cochleovestibular hypoplasia, otic sparing temporal bone fracture, and Chiari malformation.
Figure 3.
Imaging results.
Vestibular Assessment
Vestibular assessment, which involved conducting VNG, vHIT, and cVEMP tests, was conducted on 48 children. Among these, 13 had low thresholds on cVEMP (27%). However, no patient had third window symptoms or signs. Abnormal VNG and vHIT findings are summarized in Table 2.
Table 2.
Summary of Abnormal VNG and vHIT Results.
| Diagnosis | Number of patients with abnormal results | VNG results | vHIT results |
|---|---|---|---|
| Vestibular migraine | 8 | 3 cases had pure vertical nystagmus. 1 case had abnormal saccadic pursuit. 1 case had reduced calorics + pure vertical nystagmus. 1 case had abnormal saccadic pursuit + pure vertical nystagmus. 1 case had abnormal DP. 1 case had abnormal DP + positioning nystagmus. |
1 case exhibited reduced gains. |
| BPPV | 5 | All cases had posterior canal BPPV with geotropic torsional upbeating nystagmus. | Normal |
| Vestibular neuritis/Labyrinthitis | 3 | 1 case exhibited reduced calorics + pure vertical nystagmus. | 1 case had reduced left posterior semicircular canal gain with overt saccades. 1 case had reduced LARP gain with overt saccades. |
| Central vertigo | 3 | 1 case had abnormal saccadic pursuit + pure vertical nystagmus. 1 case had abnormal DP (normal LP) + pure vertical nystagmus. 1 case had pure vertical nystagmus. |
Normal |
| Vestibular hypofunction not meeting specific diagnostic criteria | 4 | 1 case had bilateral reduced calorics (<12°/s) + saccadic pursuit. 3 cases had reduced calorics. |
1 case had reduced gains in all semicircular canals. |
| PPPD | 3 | 1 case had positional nystagmus. 1 case had vertical nystagmus in all positions + posterior BPPV. |
1 case had left lateral semicircular canal reduced gains with overt saccades. |
| AIED | 2 | 1 case had reduced calorics + positional nystagmus. 1 case had reduced calorics. |
1 case had reduced gains in right lateral/posterior/superior semicircular canals. 1 case had covert saccade with normal gains. |
| Meniere’s | 1 | 1 case had reduced calorics on the right side. | Normal |
| Congenital | 1 | 1 case had sinusoidal nystagmus. | Normal |
Abbreviations: AIED, autoimmune inner ear disease; BPPV, benign paroxysmal positional vertigo; DP, directional preponderance; LP, labyrinthine preponderance; PPPD, persistent postural-perceptual dizziness; vHIT, video head impulse test; VNG, videonystagmography.
Treatment
A total of 9 patients received repositioning maneuvers for benign paroxysmal positional vertigo (BPPV; Figure 4). Fifty-one patients received medications along with lifestyle modification (Table 3). Twenty-three patients, most of whom had vestibular hypofunction and persistent postural perceptual dizziness (PPPD), underwent vestibular physiotherapy. Seventeen patients had multimodal therapy (Figure 4).
Figure 4.
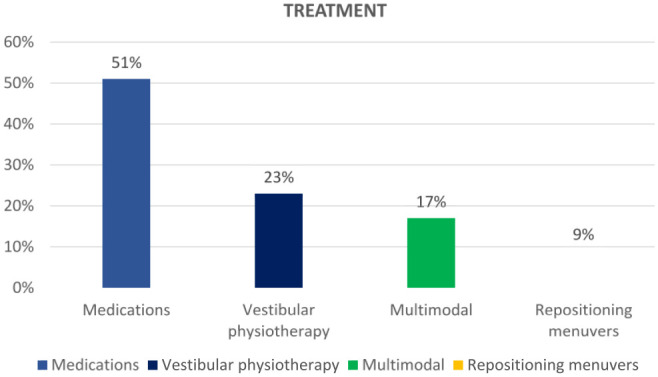
Treatment.
Table 3.
Class of Medical Treatment Offered Based on the Diagnosis.
| Diagnosis | Medical treatment |
|---|---|
| Vestibular migraine | Vitamin B2 Magnesium Tricyclic antidepressant Propranolol Cyproheptadine |
| BPVC | Cyproheptadine |
| Vestibular neuritis/labyrinthitis | Systemic steroids ± antibiotics |
| Meniere’s | Betahistine |
| AIED | Systemic steroids 1 patient had methotrexate 1 patient had mycophenolate |
| PPPD | SSRIs |
| Hypothyroidism | Synthroid |
Abbreviations: AIED, autoimmune inner ear disease; BPVC, benign paroxysmal vertigo of childhood; PPPD, persistent postural-perceptual dizziness; SSRI, selective serotonin reuptake inhibitor.
Discussion
Vertigo in children has a wide differential diagnosis that includes a variety of diseases that differ from those seen in adults. Common conditions such as BPPV and Meniere’s disease (MD) in adults are less common in children. 9 The prevalence of the different causes of vertigo in our study correlated with what has been published in the literature thus far.2,10,11
Vestibular Migraine and Benign Paroxysmal Vertigo of Childhood
The most common diagnoses in our series are vestibular migraine/migraine-related and benign paroxysmal vertigo of childhood (BPVC; 20% and 14%, respectively). BPVC is more common in children younger than 6 years and vestibular migraine is more common in children older than 10 years.12,13 Vestibular migraine was associated with high degree of central features on vestibular testing such as vertical nystagmus, central positional nystagmus, abnormal smooth pursuit, and/or directional preponderance (DP). Our treatment regimen for vestibular migraine/migraine-related included diet and lifestyle modifications and the use of vitamin B2 as a first-line treatment. Magnesium was then prescribed if the first line of treatment failed. For unresponsive or severe cases, tricyclic antidepressants, propranolol, or cyproheptadine were initiated (Table 3). BPVC was primarily treated with observation and cyproheptadine when pharmacotherapy was indicated.
Benign Paroxysmal Positional Vertigo
Although BPPV is less common in children, it was the third most common cause in our series (9%), compared to an estimated 5% in the literature. 11 All our cases except for one had posterior canal BPPV, and they received Epley’s reposition maneuver with excellent recovery. One patient had initially ageotropic variant of posterior BPPV who received quick liberatory maneuver converted to geotropic nystagmus and received Epley’s maneuver. Subsequent examination follow-up showed a transition to horizontal canal BPPV and received barbecue roll repositioning maneuver.
Persistent Postural Perceptual Dizziness
PPPD is a recently defined diagnosis characterized by chronic dizziness, unsteadiness, and/or non-spinning vertigo. 14 Dizziness in PPPD is exacerbated by upright posture, active or passive motion, or exposure to moving visual stimuli or complex visual patterns. 14 In our series, children with PPPD often had fear of falling and felt uncomfortable in open and crowded spaces. PPPD is considered one of the most common causes of chronic dizziness in adults but reports in children are few. 15 Wang et al 16 found that 7.3% of their pediatric series met the diagnostic criteria for PPPD. Similar results were found in our study with 11 children experiencing PPPD. PPPD is often triggered by conditions that cause vertigo, unsteadiness, or dizziness. In our study, 5 children presented with concurrent BPPV or labyrinthitis (Figure 1). The management of PPPD in our cohort included addressing precipitating disorders; for example, those with BPPV underwent particle repositioning maneuvers. PPPD was primarily treated with vestibular physiotherapy, while patients experiencing significant distress received selective serotonin reuptake inhibitors as needed.
Orthostatic Dizziness
It is important to check the orthostatic vitals in vertigo clinics as it is often skipped or missed. It has been reported about 5% of patients seen in specialized vertigo clinic have orthostatic dizziness. 17 Our series had similar results with a prevalence of 7%. It is important for these patients to have cardiology assessment and workup if any associated chest pain or true syncope to rule out any serious cardiac conditions.
Vestibular Neuritis/Labyrinthitis
Six patients in our series were diagnosed with vestibular neuritis/labyrinthitis based on history, physical examination, and objective vestibular battery assessment, which is slightly lower than previous reports in the literature (16%). 13 All patients were given high doses of oral prednisone, and those who had labyrinthitis were given additional antibiotics (Table 3). All patients received vestibular physiotherapy to help with compensation and rehabilitation.
Meniere’s Disease
MD is uncommon in children, diagnosed in about 1% to 4% of all cases. 18 In our series, 5 patients were diagnosed with MD (2 definite MD; 3 probable MD) and all were teenagers, with the youngest being 13 years old. The treatment regimen included salt restriction and betahistine. Betahistine doses ranging from 8 mg to 24 mg yielded positive responses in 2 patients. One patient did not tolerate the betahistine because of nausea and gastric upset, another received betahistine but was lost to follow-up, and another did not require medical therapy because the vertigo attacks were brief and sporadic. In a meta-analysis of MD adult patients, betahistine was found to reduce vertigo by 56% when compared to placebo. 19 The included studies in the analysis, however, showed statistical and clinical heterogeneity. The BEMED trial, a phase 3 multicenter, double blind, randomized, placebo controlled, betahistine dose defining superiority trial, compared the effects of betahistine at low and high doses (48 mg/day, 144 mg/day, respectively) versus placebo. Authors reported a significant reduction in vertigo attacks across all groups over the 9 month treatment period. Their study found that using low- or high-dose betahistine for 9 months did not change the mean number of vertigo attacks related to MD when compared to placebo. 20 Most studies in the literature are on adults and optimal treatment modality for pediatric MD remains to be determined. In our study, a lower dose of betahistine reduced vertigo attacks in 2 out of 4 symptomatic Meniere’s patients. However, the data are insufficient to draw any conclusions.
Central Vertigo
Three patients had central vertigo confirmed on vestibular battery testing. The most common vestibular findings were pure vertical nystagmus and central positional nystagmus. Only one patient had brainstem and cerebellar hypoplasia on radiological investigation and the remaining 2 had normal imaging, and their presentation did not match any specific diagnostic criteria. They are currently undergoing a neurological assessment.
Otitis Media
Two patients presented with imbalance and disequilibrium associated with otitis media with effusion (OME) on physical examination. The symptoms in one of them resolved after myringotomy and pressure equalized tube insertion. Literature has shown evidence of vertigo in both OME and acute or chronic otitis media. Grace and Pfleiderer reported that 22% of children with OME had vestibular symptoms, and 85% of them had complete resolution after pressure-equalizing tube insertion. 21
Autoimmune Inner Ear Disease
Two patients had AIED, which is a disorder characterized by progressive and fluctuating SNHL caused by abnormal immune response.22,23 It is commonly seen in women in their third and sixth decades of life.19,23 Both of our patients were 14- and 15-year-old females. One of them had systemic lupus erythematosus (SLE). The diagnosis was confirmed by progressive SNHL responding to a high-dose steroid challenge. They had vertigo with clinical evidence of vestibular hypofunction and confirmed on VNG and vHIT. Both patients’ symptoms improved after receiving tapered doses of oral prednisone followed by administering mycophenolate mofetil for the patient with SLE and methotrexate for the other patient.
Cervicogenic Vertigo
One patient was diagnosed with cervicogenic vertigo, presenting with vertigo associated with neck flexion and had normal radiological and vestibular investigations. There is controversy regarding whether cervicogenic vertigo is an independent entity. The Barany Society and a study by Yacovino and Hain 24 described the possibility of association between vertigo and neck pain with migraine. 25
Hypothyroidism
One patient was found to have vertigo after a recent diagnosis of hypothyroidism. Vertigo resolved after the initiation of thyroid hormone replacement therapy. Hsu et al reported an association of hypothyroidism with tinnitus and vertigo in the adult population. 26
Vestibulopathy
Nine children in our cohort had evidence of vestibular hypofunction on clinical and/or objective vestibular testing, but they did not meet any specific diagnostic criteria. We labeled those patients with a diagnosis of vestibulopathy. The pediatric vertigo literature lacks a clear definition for this term and it is often used interchangeably with other known entities such as vestibular neuritis. 27 Based on our data, we used vestibulopathy to denote the following; patients presenting with vertigo and fulfilling A or B, and C:
(A) Abnormal objective vestibular battery assessment such as reduced labyrinthine preponderance (LP) on VNG calorics, reduced gains with saccades on vHIT, and/or abnormal rotary chair testing.
(B) SNHL or asymmetrical SNHL that does not meet MD’s criteria or AIED.
(C) Condition should not meet any known diagnostic criteria such as migraine, vestibular migraine, BPVC, BPPV, PPPD, vestibular neuritis/labyrinthitis, MD, AIED, central causes and with no evidence of retrocochlear/brainstem pathologies on radiological investigations.
Post-Concussion
Mucha et al 28 listed the different causes and mechanisms of vestibular dysfunction following concussion. It can be caused by BPPV, semicircular canal pathology, otolith organ dysfunction, perilymphatic fistula, posttraumatic endolymphatic hydrops, central vestibular dysfunction, posttraumatic migraine, or labyrinthine concussion causing vestibular loss. 28 In our study, 8 patients experienced vertigo after concussion. Two of them developed BPPV, 2 developed PPPD, 2 developed vestibulopathy, 1 developed vestibular migraine, and 1 developed vestibulopathy and PPPD.
Audiological Assessment and Imaging Yield
Seventy-eight percent of our cohort had a normal hearing assessment. It is worth noting that hearing tests were helpful in diagnosing labyrinthitis, MD, and AIED. Among the 9 patients diagnosed with vestibulopathy according to our study’s criteria, 6 patients had SNHL on their audiograms (67%). However, our study revealed an overall low yield of hearing assessment in children with vertigo, proving beneficial primarily when hearing loss was evident or when suspicions of MD, AIED, or labyrinthitis arose. Peterson and Brodsky 29 reported that audiometry is important in children because of the association with inner ear anomalies and SNHL, but it has a much lower yield in adolescents, especially those with no ear concerns.
Neuroimaging was conducted for 31% of our patients, with 81% of these yielding normal results. Our study highlighted a limited effectiveness of neuroimaging in cases with vertigo. The indications for imaging, based on our study, included atypical headache, symptoms/signs, or objective vestibular testing suggestive of a central cause, neurological deficit, sudden SNHL, and asymmetrical SNHL.
Vestibular Testing Yield
Vestibular assessment in children requires a detailed history and physical examination. Directing attention to the physical examination, research indicates that specific assessments such as single leg balance testing, the Romberg test, the HIT, and diagnostic positional maneuvers, have a high sensitivity and specificity in detecting vestibular abnormalities in children.30-32 Our center employs objective vestibular batteries including VNG, vHIT, and cVEMP, which have demonstrated their diagnostic value in evaluating vertigo in children.33,34 We observed that VNG and vHIT helped diagnose vestibular hypofunction. VNG also aided in the diagnosis of BPPV and other central causes of vertigo. In cVEMP testing, 31% of patients had unilateral low threshold. However, it’s worth noting that at our center, cVEMP had a high false positive rate; indicating abnormally low thresholds without clinical or imaging evidence of third window lesions. As a result, it was not particularly useful in establishing a diagnosis.
In our study, 30% of patients with vestibular migraine had abnormal central-type features on VNG. These included pure vertical nystagmus and central positional nystagmus on positioning testing, saccadic pursuit, and abnormal DP with normal LP. In a case-control study of vestibular migraine in adults, Vivek et al 35 reported a statistically significant difference between abnormal vertical smooth pursuit and abnormal eye movement on right Dix-Hallpike positional testing on VNG. Fu et al 36 reported that 73% of adult vestibular migraine patients had abnormal vestibular function testing, including abnormal cVEMP, ocular VEMP, vHIT, and caloric test that were found in 20%, 44%, 32%, and 56% of patients, respectively. They also reported abnormal findings in oculomotor function testing including spontaneous nystagmus, positional nystagmus, gaze-evoked nystagmus, and abnormal smooth pursuit. 36 Other studies reported similar findings in vestibular migraine (8%-60%).36-38 Our study is among the few reports that have described comparable results in children with vestibular migraine, with VNG findings similar to those reported in adult literature.35-38 In addition, it is worth noting that one patient exhibited borderline reduced gains on vHIT and reduced thresholds on cVEMP.
Limitations
The study’s limitations include its retrospective nature, the lack of objective assessment of vertigo such as the Dizziness Handicap Inventory, and performing the vestibular testing at an adult vestibular laboratory. The current study, on the other hand, offers advantages such as the large number of children, the use of validated diagnostic criteria for the different causes of vertigo, reporting the findings of vestibular battery testing in children, being one of the first to describe PPPD in children, and proposing a definition for vestibulopathy in children.
Conclusions
Vestibular migraine and BPVC were the most common causes of vertigo in our series. We found an association between vestibular migraine and central positional nystagmus, which has been previously described in adults, but not yet in children. Our findings also indicate that imaging and auditory testing may be less useful in the diagnosis of pediatric vertigo than previously thought. Larger prospective studies are needed to evaluate the diagnostic yield of imaging and auditory assessment in dizzy children. Finally, the need for a universally accepted diagnostic classification system for pediatric vertigo to ensure more consistent and effective communication about these children.
Supplemental Material
Supplemental material, sj-docx-1-ohn-10.1177_19160216241265685 for The First 100 Children Treated in a Newly Established Pediatric Vertigo Center by Mohammed K. Alnoury, Samer Salameh, Aleksandra Ostrovska and Joshua Gurberg in Journal of Otolaryngology - Head & Neck Surgery
Supplemental material, sj-docx-2-ohn-10.1177_19160216241265685 for The First 100 Children Treated in a Newly Established Pediatric Vertigo Center by Mohammed K. Alnoury, Samer Salameh, Aleksandra Ostrovska and Joshua Gurberg in Journal of Otolaryngology - Head & Neck Surgery
Acknowledgments
Not applicable.
Footnotes
Author Contributions: M.K.A.: Designed the study, conducted data collection, participated in data analysis, and manuscript writing. S.S.: Conducted data collection and participated in manuscript writing. A.O.: Interpreted the vestibular testing and participated in manuscript writing. J.G.: Designed the study, interpreted results, critically revised the manuscript, and approved the final and submitted version. All authors read and approved the final manuscript.
Availability of Data and Materials: The datasets used in the current study are available; however, it has to follow McGill University Health Center Research Ethics Boards regulations and requirements.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent for Publication: Not applicable.
Ethics Approval and Consent to Participate: The study received approval from the McGill University Health Center Research Ethics Boards (MUHC-REB).
ORCID iDs: Mohammed K. Alnoury  https://orcid.org/0000-0001-9222-8501
https://orcid.org/0000-0001-9222-8501
Joshua Gurberg  https://orcid.org/0000-0002-4511-8884
https://orcid.org/0000-0002-4511-8884
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Russell G, Abu-Arafeh I. Paroxysmal vertigo in children an epidemiological study. Int J Pediatr Otorhinolaryngol. 1999;49(suppl 1):S105-S107. doi: 10.1016/s0165-5876(99)00143-3 [DOI] [PubMed] [Google Scholar]
- 2. Jahn K. Vertigo and balance in children—diagnostic approach and insights from imaging. Eur J Paediatr Neurol. 2011;15:289-294. doi: 10.1016/j.ejpn.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 3. Wiener-Vacher SR. Vestibular disorders in children. Int J Audiol. 2008;47:578-583. doi: 10.1080/14992020802334358 [DOI] [PubMed] [Google Scholar]
- 4. Inoue A, Iwasaki S, Ushio M, et al. Effect of vestibular dysfunction on the development of gross motor function in children with profound hearing loss. Audiol Neurootol. 2013;18:143-151. doi: 10.1159/000346344 [DOI] [PubMed] [Google Scholar]
- 5. Sheykholeslami K, Megerian CA, Arnold JE, Kaga K. Vestibular-evoked myogenic potentials in infancy and early childhood. Laryngoscope. 2005;115(8):1440-1444. [DOI] [PubMed] [Google Scholar]
- 6. Kelsch TA, Schaefer LA, Esquivel CR. Vestibular evoked myogenic potentials in young children: test parameters and normative data. Laryngoscope. 2006;116(06):895-900. [DOI] [PubMed] [Google Scholar]
- 7. Hamilton SS, Zhou G, Brodsky JR. Video head impulse testing (VHIT) in the pediatric population. Int J Pediatr Otorhinolaryngol. 2015;79(08):1283-1287. [DOI] [PubMed] [Google Scholar]
- 8. Wiener-Vacher SR, Wiener SI. Video head impulse tests with a remote camera system: normative values of semicircular canal vestibulo-ocular reflex gain in infants and children. Front Neurol. 2017;8:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nedzelski JM, Barber HO, McIlmoyl L. Diagnoses in a dizziness unit. J Otolaryngol. 1986;15:101-104. [PubMed] [Google Scholar]
- 10. Devaraja K. Vertigo in children; a narrative review of the various causes and their management. Int J Pediatr Otorhinolaryngol. 2018;111:32-38. doi: 10.1016/j.ijporl.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 11. Jahn K, Langhagen T, Heinen F. Vertigo and dizziness in children. Curr Opin Neurol. 2015;28:78-82. doi: 10.1097/WCO.0000000000000157 [DOI] [PubMed] [Google Scholar]
- 12. Lee JD, Kim CH, Hong SM, et al. Prevalence vestibular and balance disorders in children and adolescents according to age: a multi-center study. Int J Pediatr Otorhinolaryngol. 2017;94:36-39. doi: 10.1016/j.ijporl.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 13. Casani AP, Dallan I, Navari E, Franceschini SS, Cerchiai N. Vertigo in childhood: proposal for a diagnostic algorithm based upon clinical experience. Acta Otorhinolaryngol Ital. 2015;35:180-185. [PMC free article] [PubMed] [Google Scholar]
- 14. Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Barany Society. J Vestib Res. 2017;27:191-208. doi: 10.3233/VES-170622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HJ, Lee JO, Choi JY, Kim JS. Etiologic distribution of dizziness and vertigo in a referral-based dizziness clinic in South Korea. J Neurol. 2020;267:2252-2259. doi: 10.1007/s00415-020-09831-2 [DOI] [PubMed] [Google Scholar]
- 16. Wang A, Fleischman KM, Kawai K, Corcoran M, Brodsky JR. Persistent postural-perceptual dizziness in children and adolescents. Otol Neurotol. 2021;42:e1093-e1100. doi: 10.1097/MAO.0000000000003212 [DOI] [PubMed] [Google Scholar]
- 17. Jahn K. Vertigo and dizziness in children. Handb Clin Neurol. 2016;137:353-363. doi: 10.1016/B978-0-444-63437-5.00025-X [DOI] [PubMed] [Google Scholar]
- 18. Gioacchini FM, Alicandri-Ciufelli M, Kaleci S, Magliulo G, Re M. Prevalence and diagnosis of vestibular disorders in children: a review. Int J Pediatr Otorhinolaryngol. 2014;78:718-724. doi: 10.1016/j.ijporl.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 19. Murdin L, Hussain K, Schilder AGM. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;2016(6):CD010696. doi: 10.1002/14651858.CD010696.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adrion C, Fischer CS, Wagner J, Gürkov R, Mansmann U, Strupp M; BEMED Study Group. Efficacy and safety of betahistine treatment in patients with Meniere’s disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. 2016;352:h6816. doi: 10.1136/bmj.h6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grace AR, Pfleiderer AG. Dysequilibrium and otitis media with effusion: what is the association? J Laryngol Otol. 1990;104:682-684. doi: 10.1017/s0022215100113611 [DOI] [PubMed] [Google Scholar]
- 22. Mccabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2004;113:526-520. doi: 10.1177/000348940411300703. [DOI] [PubMed] [Google Scholar]
- 23. Vambutas A, Pathak S. AAO: autoimmune and autoinflammatory (disease) in otology: what is new in immune-mediated hearing loss. Laryngoscope Investig Otolaryngol. 2016;1:110-115. doi: 10.1002/lio2.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yacovino DA, Hain TC. Clinical characteristics of cervicogenic-related dizziness and vertigo. Semin Neurol. 2013;33:244-255. doi: 10.1055/s-0033-1354592 [DOI] [PubMed] [Google Scholar]
- 25. Seemungal BM, Agrawal Y, Bisdorff A, et al. The Bárány Society position on ‘Cervical Dizziness’. J Vestib Res. 2022;32:487-499. doi: 10.3233/VES-220202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu A, Tsou YA, Wang TC, Chang WD, Lin CL, Tyler RS. Hypothyroidism and related comorbidities on the risks of developing tinnitus. Sci Rep. 2022;12:3401. doi: 10.1038/s41598-022-07457-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strupp M, Bisdroff A, Furman J, et al. Acute unilateral vestibulopathy/vestibular neuritis: diagnostic criteria. J Vestib Res. 2022;32:389-406. doi: 10.3233/VES-220201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mucha A, Fedor S, DeMarco D. Vestibular dysfunction and concussion. Handb Clin Neurol. 2018;158:135-144. doi: 10.1016/B978-0-444-63954-7.00014-8 [DOI] [PubMed] [Google Scholar]
- 29. Peterson JD, Brodsky JR. Evaluation and management of paediatric vertigo. Curr Opin Otolaryngol Head Neck Surg. 2022;30:431-437. doi: 10.1097/MOO.0000000000000849 [DOI] [PubMed] [Google Scholar]
- 30. Cushing SL, Papsin BC. Taking the history and performing the physical examination in a child with hearing loss. Otolaryngol Clin North Am. 2015;48:903-912. doi: 10.1016/j.otc.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 31. Halmágyi GM, Curthoys IS. Vestibular contributions to the Romberg test: testing semicircular canal and otolith function. Eur J Neurol. 2021;28:3211-3219. doi: 10.1111/ene.14942 [DOI] [PubMed] [Google Scholar]
- 32. Brodsky JR, Lipson S, Wilber J, Zhou G. Benign paroxysmal positional vertigo (BPPV) in children and adolescents: clinical features and response to therapy in 110 pediatric patients. Otol Neurotol. 2018;39:344-350. doi: 10.1097/MAO.0000000000001673 [DOI] [PubMed] [Google Scholar]
- 33. Gedik-Soyuyuce O, Gence-Gumus Z, Ozdilek A, et al. Vestibular disorders in children: a retrospective analysis of vestibular function test findings. Int J Pediatr Otorhinolaryngol. 2021;146:110751. doi: 10.1016/j.ijporl.2021.110751 [DOI] [PubMed] [Google Scholar]
- 34. Ciolek PJ, Kang E, Honaker JA, et al. Pediatric vestibular testing: tolerability of test components in children. Int J Pediatr Otorhinolaryngol. 2018;113:29-33. doi: 10.1016/j.ijporl.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 35. Vivek S, Prem G, Dorasala S, Faizal B, Joy M, Nair AS. Videonystagmography (VNG) findings in patients with vestibular migraine: a hospital-based study. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 3):4290-4297. doi: 10.1007/s12070-021-02953-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fu W, Wang Y, He F, Wei D, Bai Y, Han J, Wang X. Vestibular and oculomotor function in patients with vestibular migraine. Am J Otolaryngol. 2021;42:103152. doi: 10.1016/j.amjoto.2021.103152 [DOI] [PubMed] [Google Scholar]
- 37. Power L, Shute W, McOwan B, Murray K, Szmulewicz D. Clinical characteristics and treatment choice in vestibular migraine. J Clin Neurosci. 2018;52:50-53. doi: 10.1016/j.jocn.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 38. Teggi R, Colombo B, Bernasconi L, Bellini C, Comi G, Bussi M. Migrainous vertigo: results of caloric testing and stabilometric findings. Headache. 2009;49:435-444. doi: 10.1111/j.1526-4610.2009.01338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ohn-10.1177_19160216241265685 for The First 100 Children Treated in a Newly Established Pediatric Vertigo Center by Mohammed K. Alnoury, Samer Salameh, Aleksandra Ostrovska and Joshua Gurberg in Journal of Otolaryngology - Head & Neck Surgery
Supplemental material, sj-docx-2-ohn-10.1177_19160216241265685 for The First 100 Children Treated in a Newly Established Pediatric Vertigo Center by Mohammed K. Alnoury, Samer Salameh, Aleksandra Ostrovska and Joshua Gurberg in Journal of Otolaryngology - Head & Neck Surgery