Abstract
As a result of the ongoing opioid epidemic, physicians have encountered increasing rates of drug-use-related native tricuspid valve infective endocarditis (DU-TVIE), a complex multi-faceted disease that is best managed by interdisciplinary teams. Despite the large number of patients with DU-TVIE, there is little data to support the optimal treatment strategy with respect to medical and surgical therapy. The recent introduction of percutaneous mechanical aspiration of tricuspid valve vegetations has added another treatment modality that is also of uncertain benefit. Here we review the literature on the management of DU-TVIE and highlight the multi-step treatment approach developed by the multidisciplinary endocarditis team at the University of Kentucky.
Keywords: endocarditis, multidisciplinary teams, substance use disorder, tricuspid valve disease
Introduction
Isolated native tricuspid valve infective endocarditis (TVIE) accounts for ~5%–10% of all cases of infective endocarditis (IE) and is associated with lower in-hospital mortality compared to left-sided IE. 1 As many as 90% of patients with TVIE may have a history of injection drug use.2,3 With the ongoing opioid epidemic, there has been a corresponding increase in rates of drug-use-associated IE, which can vary geographically.3,4 In certain cases, TVIE may significantly damage the integrity of the native tricuspid valve, resulting in severe regurgitation, and potentially right-sided heart failure, leading providers to pursue surgical tricuspid valve replacement (TVR). Historically, tricuspid valve surgery for severe regurgitation has been associated with improved outcomes compared to medical therapy; however, the data were acquired from older patient cohorts with chronic heart failure. 5 By contrast, patients who inject drugs that develop TVIE are often under 40 years of age with fewer medical comorbidities, which may account for the lower mortality with TVIE than left-sided IE.1,4 In addition, the limited literature comparing medical and surgical therapy for TVIE has not demonstrated superior mortality outcomes with valve operations. 6 Longitudinal data demonstrate that 5-year survival for persons who inject drugs (PWIDs) with severe tricuspid regurgitation (TR) secondary to endocarditis can be as high as 94%. 7
A particular concern in this population is the potential for re-infection of a newly placed prosthetic valve which can be challenging to treat effectively. Despite this, many patients with isolated TVIE are still referred for valve surgery, in part because current American Heart Association (AHA) Guidelines provide a class IIa recommendation for valve surgery in patients with right-sided IE and the following complications: right heart failure secondary to severe TR with poor response to medical therapy, sustained infection caused by difficult-to-treat organisms or lack of response to appropriate antimicrobial therapy, and tricuspid valve vegetations that are ⩾2.0 cm in diameter and recurrent pulmonary emboli despite antimicrobial therapy.8,9 What remains clear is that the existing literature has not identified the ideal treatment pathway for patients with acute isolated TVIE.
The scope of the problem
Multiple national population studies in the United States have demonstrated a rise in drug-use-related TVIE (DU-TVIE) since the late 2000s. 4 Certain areas of the country have seen a more dramatic rise in cases, particularly Kentucky, Tennessee, and West Virginia. 10 At the University of Kentucky, the number of patients with DU-TVIE increased 11-fold between 2009–2010 and 2017–2018. 3 Providers, particularly in these high-volume areas, are now confronted with managing an abundance of patients with this complex, multi-faceted disease. Although replacing the tricuspid valve may appear to be a straightforward solution, complex factors limit the success of this approach. Many social barriers exist that limit postoperative care and follow-up, including but not limited to: lack of housing or reliable transportation, geographic isolation, and potential ongoing exposure to substance use. While the vast majority of younger patients who develop severe TR will ultimately require valve replacement, this population may need multiple valve replacements given their age, rates of prosthetic valve failure, and re-infection. These patients may also tolerate severe TR for several years before surgical intervention is necessary. Even with the advent of multidisciplinary endocarditis teams (MDETs), which have been shown to decrease in-hospital and short-term mortality for patients with IE, and the recently published AHA position statement on the management of IE in people who use drugs (PWUDs), the optimal longitudinal support and ideal timing of TVR in this population has not been established.9,11–13
Development of the MDET
In response to the massive increase in patients with IE, the University of Kentucky Healthcare created an MDET and cardiovascular infectious diseases (CVID) consult service in September 2021. The MDET is comprised of providers from infectious diseases, cardiac surgery, cardiology, addiction medicine, neurosurgery, neurology, physical medicine and rehabilitation, palliative care, and ethics. The group formally meets weekly to discuss all inpatients with IE and document its recommendations in the electronic medical record. Cases for the conference are identified primarily by the infectious diseases consult service in collaboration with the cardiac surgery service. Frequent communication between MDET providers and clinical primary teams also occurs outside of scheduled meeting times. Decisions regarding percutaneous mechanical aspiration (PMA) of the tricuspid valve and valve replacement are made at the weekly conference. The CVID consult service is an interdisciplinary team housed in the division of infectious diseases and comprised of an attending physician, advanced practice provider, nurse navigator, pharmacist, and social worker. The CVID service consults on all inpatients with IE and follows them throughout their hospitalization and transition to outpatient. The CVID team coordinates the weekly MDET meetings and works with other specialties to schedule follow-up and outpatient testing.
The University of Kentucky MDET’s approach was developed in response to the sudden, substantial increase in the number of patients presenting with DU-TVIE. Initially, all patients with indications were offered surgical interventions. However, it soon became very difficult to intervene on all patients with indications due to patient volume, staffing issues, and operating room availability. In addition, our experience suggested that the majority of medically managed patients survived to discharge and patients who underwent valve replacement acutely had high rates of relapsed substance use and prosthetic valve endocarditis. Moreover, we witnessed several adverse outcomes in patients undergoing PMA. Consequently, physicians in cardiac surgery, cardiology, infectious diseases, and addiction medicine adopted an approach that emphasized initial medical therapy with treatment of patients’ underlying substance use disorder (SUD) and close outpatient follow-up to schedule elective valve surgery if deemed necessary (Figure 1).
Figure 1.
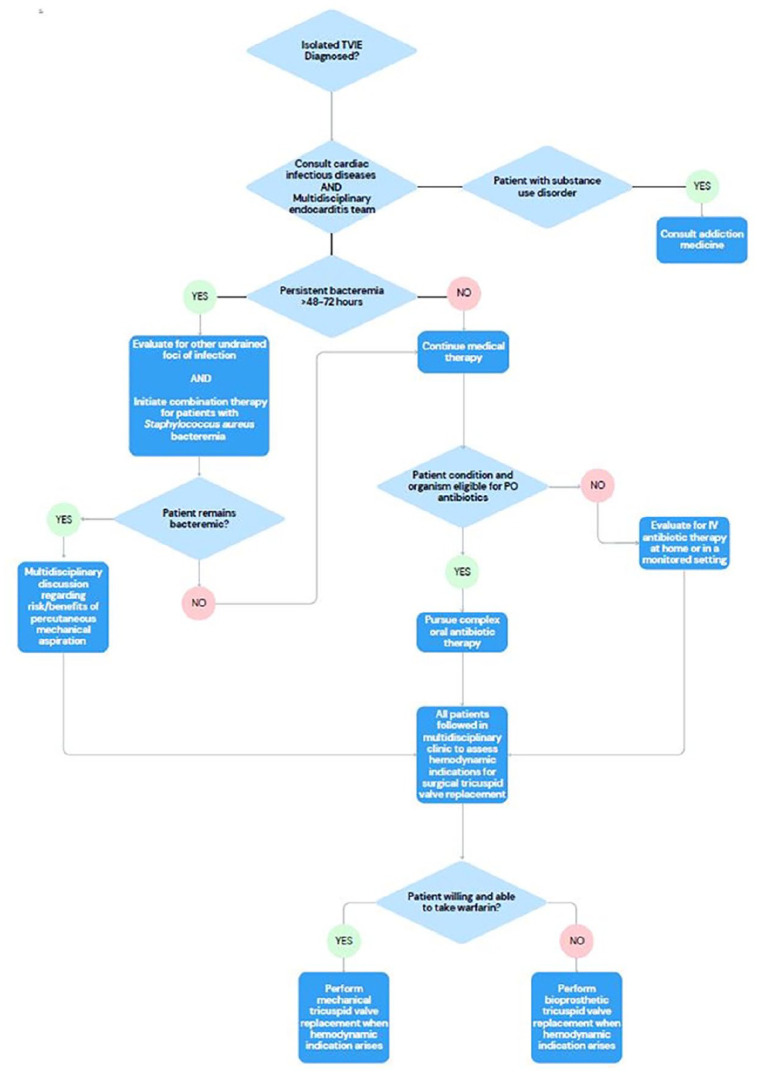
Treatment pathway for patients with isolated native tricuspid valve endocarditis.
Initial management
The diagnosis of TVIE is made utilizing the modified Duke Criteria as well as the recently published 2023 Duke-ISCVD criteria.14,15 Once the diagnosis is confirmed, patients are ideally managed by an MDET. If a diagnosing hospital does not have an MDET, consultation with a regional center with either an MDET or that cares for a high volume of patients with TVIE to discuss transfer is advised. 13 Previous literature has demonstrated that even patients with IE managed medically have reduced in-hospital mortality if their care involves an MDET. 11 This is particularly important in cases of DU-TVIE when medication for opioid use disorder (MOUD) is not available at the referring facility. 12 Patients who receive addiction medicine consultation during their hospitalization have been shown to have lower rates of mortality post-discharge and it is crucial to treat the underlying cause of the IE to improve long-term outcomes. 16 Early involvement with addiction medicine is important as many patients with opioid use disorder experience significant opioid withdrawal symptoms upon admission to the hospital that may lead to patient-directed discharge if not adequately addressed. 17
The multidisciplinary team can discuss the patient’s case and determine whether further testing is required to evaluate for additional factors complicating the TVIE such as undrained foci of infection, left-sided vegetations, or a patent foramen ovale (PFO), and to ensure optimal antimicrobial therapy. High-volume centers may consider employing an infectious diseases provider with additional expertise in cardiovascular infections. 18 For patients with persistent Staphylococcus aureus bacteremia lasting >48 to 72 h, combination antimicrobial therapy can be initiated to eradicate the bloodstream infection. Previous data have suggested that clinical outcomes, including attributable mortality, are worse in patients with persistent S. aureus bacteremia. 19 Early initiation of combination therapy with daptomycin and ceftaroline for methicillin-resistant S. aureus and cefazolin or oxacillin with ertapenem for methicillin-susceptible S. aureus (MSSA) may decrease time to blood culture clearance and mortality.20–23
If, despite addressing all other possible sources, patients continue to experience ongoing signs of active infection defined by persistent bacteremia, fevers, or other signs of sepsis, it would be reasonable to consider PMA of the tricuspid valve. However, given the limited data comparing this approach to medical or open surgical management, as well as the lack of clear indications for the procedure, the authors’ opinion is that PMA should be reserved as a last-resort treatment option. 24 Decisions regarding PMA are best made by an MDET. Our practice is to consider PMA only in patients who have persistent bacteremia for ⩾7 days on maximal antibiotic therapy provided that other foci of infection are addressed. Patients with vegetations > 2 cm who are clinically stable with clear blood cultures are not referred for PMA. In our experience, some patients undergoing PMA develop progression of their sepsis and refractory vasoplegia after the procedure. Our institutional review board-approved dataset of 215 patients demonstrated no difference in 1-year mortality outcomes for individuals receiving medical therapy, PMA, or valve surgery. 25 While surgical valve replacement has been associated with decreased in-hospital mortality in some published series, it has also been correlated with higher rates of major adverse cardiovascular events. 26 In addition, long-term data have demonstrated that IE in PWUDs is associated with poor long-term survival and high rates of recurrent IE, particularly in patients who undergo surgery. 27 For these reasons as well as the challenges with treating recurrent prosthetic valve endocarditis, the evidence demonstrating the long-term tolerability of severe TR in younger PWUDs, and in the absence of complicating factors such as PFO or left-sided endocarditis with surgical indications, we advocate that TVR should not be performed during the index hospitalization.
Discharge planning
Once a patient’s bacteremia has resolved and they are clinically stable, a critical piece of their care is discharge planning and coordination of outpatient follow-up. Treatment of PWUDs with intravenous antibiotics has been a long-standing concern in the medical field and often leads to many patients remaining hospitalized to complete prolonged 4–6 weeks courses of therapy. However, randomized controlled trial data have demonstrated comparable outcomes for patients with IE secondary to MSSA, coagulase-negative staphylococci, streptococci, and Enterococcus faecalis transitioned to oral antibiotics after at least 10 days of intravenous treatment when compared to full courses of IV antibiotics. 28 Patients who do not fall into this category should still be evaluated for completion of outpatient parenteral antibiotic therapy (OPAT) as no randomized controlled trial data have demonstrated inferior outcomes in PWUDs eligible for OPAT compared to persons who do not inject drugs. In addition to helping facilitate earlier discharge, this approach may allow PWUDs to enter SUD treatment programs sooner.
Appropriate treatment of the underlying SUD is crucial to preventing future episodes of endocarditis as well as decreasing overall mortality.16,29 If a patient has elected to initiate MOUD, they should be connected to an outpatient provider or program that can continue this therapy. The addiction medicine team can also collaborate with local SUD treatment facilities to help patients discharge directly to a rehabilitation program if desired. Ideally, all required outpatient follow-up appointments are made prior to discharge and provided to the patient, along with contact information for the respective clinics. Individual MDETs may consider employing a nurse navigator who can provide patients with these appointments as well as follow-up phone calls and appointment reminders. 30
Post-discharge follow-up
Patients, particularly those receiving OPAT or oral antibiotics, may benefit from follow-up with an infectious diseases provider within 2 weeks of discharge, as a means of decreasing readmissions. 31 This individual would ideally treat other co-occurring infections such as HIV, hepatitis B, and/or C which can contribute to morbidity and mortality in PWUDs. An infectious diseases provider who also provides MOUD can be a valuable asset as this treatment can be integrated into their overall care and limit the number of appointments patients must attend. The creation of multi-specialty clinics involving infectious diseases physicians with additional training in addiction medicine and cardiac surgeons can allow the multidisciplinary model to extend to the outpatient setting, and again, decrease the number of appointments for patients. 32
Timing of surgery
Rather than performing surgery acutely during the index hospitalization, we advocate for outpatient re-evaluation to determine the best timing for surgical TVR. We recommend that patients undergo serial assessments, including echocardiographic evaluations of their tricuspid valve, degree of regurgitation, right ventricular size, and systolic function, as well as a thorough appraisal of their physical symptoms. In addition, the status of patients’ SUD is re-evaluated, in particular, whether they are injecting substances. Rather than requiring complete abstinence from substance use (particularly if that is not the patient’s goal) prior to surgical intervention, we favor a harm reduction approach and offer surgery to patients who have active use provided they are no longer injecting substances, as this is the primary risk factor for subsequent episodes of endocarditis.25,33 Patients may be followed longitudinally at intervals ranging from 1 to 6 months. Only once they develop symptoms of right-sided heart failure or worsening right ventricular dilation on echocardiogram do we advocate scheduling surgery. These recurring appointments may improve provider–patient rapport, particularly if they are conducted in a non-judgmental manner, and provide opportunities for additional patient education about their valve disease and harm-reduction methods. The visits also serve as an opportunity to re-address socioeconomic barriers to care or gauge a patient’s levels of commitment to proceeding with a major surgical intervention. Multiple missed appointments may be related to housing or transportation issues or may be a sign that the patient is not yet ready to undergo a valve replacement, particularly with a mechanical valve.
Choosing the valve – Preoperative management
To reduce the amount of implanted prosthetic material, we favor pursuing tricuspid valve repair if feasible. However, in many cases of TVIE, there is significant destruction of the valve leaflets that precludes valve repair. Existing literature has not demonstrated a significant difference in short- or long-term outcomes for patients receiving mechanical or bioprosthetic TVRs. 34 However, there are currently no surgically implanted prosthetic valves designed exclusively to be placed in the tricuspid position, which may contribute to high rates of premature valve failure post-replacement. There is little data on the optimal choice of valve for younger patients, particularly those with SUD. In patients aged <60 years without contraindications for warfarin anticoagulation, our preference is to offer a mechanical valve given the associated longer durability. 35 However, in older individuals and those who are unable or unwilling to take warfarin or where compliance is a concern, we favor the placement of a bioprosthetic valve. For all women of reproductive age, preoperative contraceptive counseling is provided as well as referrals to obstetrics and gynecology (OB/GYN) for treatment if needed. This is particularly crucial given the teratogenicity of warfarin and the high rates of morbidity and mortality for pregnant individuals with mechanical valves.36,37
Patients are counseled about the possibility of requiring a pacemaker postoperatively given the risk of high-grade atrioventricular (AV) block associated with TVR. 38 Prior to surgery, we advise consultation with an electrophysiologist (who is ideally a part of the MDET) to discuss potential postoperative pacing strategies. Placement of transvenous pacemaker leads across a bioprosthetic tricuspid valve is associated with higher rates of valve failure. 38 Alternative treatment options include placing a transvenous lead in the coronary sinus instead of the right ventricle, placement of permanent epicardial leads at the time of the valve surgery, or insertion of a leadless pacemaker postoperatively. In PWUDs receiving bioprosthetic valves requiring postoperative pacing, particularly those who continue to inject substances, a leadless pacemaker may be preferable given their very low reported rates of infection. 39 The patient can then be upgraded to a permanent transvenous system in the future if needed. This approach allows the patient more time to effectively treat their SUD before placing a significant amount of prosthetic material that may make them even more prone to cardiovascular infection. Leadless pacemakers are generally not placed in patients with mechanical valves due to the risk of damaging the valve leaflets during implantation. However, successful placement of leadless pacemakers across bioprosthetic and mechanical valves by experienced electrophysiologists has been described.40,41
Pain control after median sternotomy can be particularly challenging in PWUDs, who may have a high tolerance of opioid medications. Prior to surgery, multidisciplinary discussions with the patient’s addiction medicine provider and anesthesiologist may be beneficial. Patients receiving buprenorphine or methadone as MOUD should be continued on these medications prior to and after surgery. 42 Preoperative or postoperative nerve blocks can be considered to alleviate the severity of postoperative pain and reduce the amount of opioid pain medication prescribed. 43
Once the decision is made to proceed with valve surgery and a date is set, we suggest that the outpatient providers and inpatient clinicians communicate to ensure a warm handoff and minimize interruptions in care.
Intra-operatively
As previously mentioned, when feasible, tricuspid valve repair is preferred to replacement. We recommend against valvectomy due to the higher rates of unplanned readmissions with this procedure, the need for subsequent valve replacement, and the risk of loss to follow-up. 44 If valve replacement is unavoidable, special considerations can be taken intra-operatively to avoid damage to the cardiac conduction system. Close attention to the anatomy of the AV node is important during the procedure. When operating in proximity to the AV node, our group advocates for the use of native valve leaflets rather than annular sutures. If the valve leaflets have been completely destroyed, we recommend superficial placement of the annular sutures to avoid damaging the AV node. All patients have temporary epicardial pacing leads placed at the time of surgery as transient complete heart block after removing the aortic cross clamp is common. 45 In certain cases, often in the setting of removing an infected transvenous pacemaker at the time of valve surgery, permanent epicardial leads may be placed intra-operatively. This decision is ideally made preoperatively in conjunction with an electrophysiologist as part of an MDET.
Postoperatively
For patients with SUD, we recommend that they are again seen by addiction medicine during their surgical admission as well as the acute pain service (if available) to manage postoperative pain. Some patients may experience anxiety regarding the re-introduction of opioid pain medication. 46 The addiction medicine team can help provide education about the receipt of opioid pain medications while receiving MOUD and help set expectations for the amount of medication that will be prescribed on discharge. OB/GYN referral can also be completed inpatient for individuals who would like to start oral contraceptives or receive a subdermal implant. If pacing requirements persist beyond 5–7 days, placement of a permanent pacing device is prudent as discussed previously.
Postoperative complications
After discharge, patients are again followed longitudinally in a multidisciplinary manner to manage and potentially prevent postoperative complications, including re-infection. Patients are educated about their risk of future episodes of endocarditis, even independent of further injection substance use. We have designed pocket cards that we provide to patients at their postoperative appointments that include signs and symptoms of endocarditis, recommendations to ask their providers to obtain blood cultures if they present with fever, when and what to take for antibiotic prophylaxis as well as the infectious diseases clinic contact information (Figures 2 and 3). 47 One of the primary areas of concern is premature bioprosthetic tricuspid valve failure, which is seen more commonly in younger patients. 48 Patients with mechanical valves are followed closely by an anticoagulation clinic with routine monitoring of their international normalized ratio. The 2021 joint European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines for the management of valvular heart disease also provide a class IIa recommendation for anticoagulation with warfarin for 3 months after bioprosthetic TVR. 49 Home finger-stick testing kits may be covered by patients’ insurance and allow for convenient monitoring at home without venipuncture, which may be difficult in PWUDs with damage to their peripheral veins. Meta-analysis has also demonstrated lower rates of stroke, thromboembolism, major bleeding, and emergency department visits with patient self-testing when compared to office or laboratory-based monitoring. 50
Figure 2.

Front side of the University of Kentucky patient endocarditis prevention pocket card which outlines signs and symptoms of infective endocarditis and encourages patients to ask their medical provider to collect blood cultures before starting antibiotics for the listed symptoms.
Figure 3.

Reverse side of the University of Kentucky patient endocarditis prevention pocket card which outlines indications and selection choice of antibiotic for prophylaxis as well as contact information for the infectious diseases clinic.
Special considerations – PFO
Patients with TVIE and PFO pose a unique challenge to clinicians as paradoxical embolization across a PFO in the setting of TVIE has been well described.51,52 In patients presenting with only tricuspid valve involvement on transthoracic echocardiography and evidence of systemic emboli, we have a high suspicion for potentially undetected left-sided vegetations or PFO. We advocate that these patients undergo transesophageal echocardiography (TEE) with agitated saline bubble study to further evaluate for such complications. If a PFO is identified and concomitant left-sided endocarditis has been excluded by TEE, the optimal management of patients with TVIE and systemic emboli remains unclear. The goal of procedural intervention is primarily to prevent future, potentially devastating, embolic complications. However, the risk of recurrent emboli has been demonstrated to decrease substantially after approximately 14 days of appropriate antimicrobial therapy. 53 Tricuspid valve surgery with PFO closure can be considered, particularly in patients who have hypoxia from significant right-to-left shunting, which is often exacerbated by severe TR. We have previously reported on two successful cases of TVIE with PFO and systemic emboli that were initially treated with transcatheter placement of a PFO occlusion device followed by delayed valve surgery after clinical stabilization.54,55 In both instances, the devices were placed after blood culture clearance was achieved and there was no intra-operative evidence of infection of the occlusion material at the time of valve surgery. While further studies are required to evaluate the efficacy of this approach, providers could consider the placement of an occlusion device as a salvage intervention to prevent future systemic emboli in patients with TVIE and PFO who are unable to undergo valve surgery.
The University of Kentucky’s approach
The University of Kentucky Treatment pathway, implemented in September 2021, advocates almost exclusively for initial medical therapy provided in the framework of a multidisciplinary team, for all patients with isolated native DU-TVIE. In an institutional review board-approved companion study of the first 2.5 years utilizing this treatment algorithm, we report on 72 patients with isolated native TVIE with only one reported in-hospital death (1.4%) and two deaths at 90 days (2.8%), a mortality rate comparable to or lower than rates seen in many studies that include patients receiving surgical valve replacement. 56 Only one patient underwent surgery during the index hospitalization and five underwent PMA. A further 10 patients underwent delayed TVR after outpatient follow-up.
Discussion
Despite the abundance of data supporting the use of MDETs, there has been a very slow adoption of this practice in North America. Much of this may be due to the fee-for-service model of healthcare in the United States. Currently, these inpatient multidisciplinary team meetings are not reimbursable. While there is a CPT code (CPT 99367, Under Medical Team Conference, Without Direct (Face-to-Face) Contact With Patient and/or Family) that can be entered for documentation related to these discussions, the meetings are considered as ‘Status Indicator B’ by the Center for Medicare and Medicaid Services and payment for covered services are bundled into payment for other services. 57 Consequently, busy departments may be reluctant to commit their providers’ time to an endeavor that, although improving care, is not directly revenue-generating. Endocarditis mortality outcomes are also not currently a quality metric that is measured by the Center for Medicare and Medicaid Services. Tying these outcomes to a quality measure that impacts reimbursement may cause more hospitals to allocate resources toward creating MDETs. This could be done in tandem with adjustments to the Society of Thoracic Surgeons star rating system. To achieve a 3-star status for their cardiac surgery program, hospitals could be required to demonstrate that they possess an MDET that meets regularly. 58 Without linking endocarditis outcomes or MDET implementation to reimbursement or quality metrics in a meaningful way, the slow adoption of endocarditis teams will likely continue for the foreseeable future.
Conclusion
DU-TVIE remains a challenging disease that is complicated both by the patient’s medical condition and social determinants of health. Depending on a provider’s geographic location, they may encounter large numbers of patients with DU-TVIE. While there has been new guidance in recent years on how to approach this patient population, the position statement from the AHA on the treatment of IE in PWIDs still highlights the role of valve surgery. 9 Given the existing data and the challenges in effectively caring for this patient population, we suggest that hospitals and providers consider adopting a multidisciplinary approach to the treatment of DU-TVIE that emphasizes maximum medical therapy and defers surgical intervention until other impediments to effective treatment are addressed.
Acknowledgments
None.
Footnotes
ORCID iD: Sami El-Dalati  https://orcid.org/0000-0003-2603-2951
https://orcid.org/0000-0003-2603-2951
Contributor Information
Sami El-Dalati, Division of Infectious Diseases, Department of Internal Medicine, University of Kentucky Medical Center, 3101 Beaumont Centre Circle, Lexington, KY 40513, USA.
Talal Alnabelsi, Gill Heart and Vascular Institute, University of Kentucky Medical Center, Lexington, KY, USA.
John Gurley, Gill Heart and Vascular Institute, University of Kentucky Medical Center, Lexington, KY, USA.
Kelli Cremeans, Division of Infectious Diseases, Department of Internal Medicine, University of Kentucky Medical Center, Lexington, KY, USA.
Hassan Reda, Division of Cardiovascular and Thoracic Surgery, University of Kentucky Medical Center, Lexington, KY, USA.
Tessa London-Bounds, Division of Cardiovascular and Thoracic Surgery, University of Kentucky Medical Center, Lexington, KY, USA.
Erinn Ogburn, Division of Cardiovascular and Thoracic Surgery, University of Kentucky Medical Center, Lexington, KY, USA.
Michael Sekela, Division of Cardiovascular and Thoracic Surgery, University of Kentucky Medical Center, Lexington, KY, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Sami El-Dalati: Conceptualization; Visualization; Writing – original draft; Writing – review & editing.
Talal Alnabelsi: Conceptualization; Writing – review & editing.
John Gurley: Writing – review & editing.
Kelli Cremeans: Writing – review & editing.
Hassan Reda: Writing – review & editing.
Tessa London-Bounds: Writing – review & editing.
Erinn Ogburn: Writing – review & editing.
Michael Sekela: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Clarelin A, Rasmussen M, Olaison L, et al. Comparing right- and left sided injection-drug related infective endocarditis. Sci Rep 2021; 11(1): 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreillon P, Que YA. Infective endocarditis. Lancet 2004; 363(9403): 139–149. [DOI] [PubMed] [Google Scholar]
- 3. Alnabelsi TS, Sinner G, Al-Abdouh A, et al. The evolving trends in infective endocarditis and determinants of mortality: a 10-year experience from a Tertiary Care Epicenter. Curr Probl Cardiol 2023; 48(6): 101673. [DOI] [PubMed] [Google Scholar]
- 4. Kadri AN, Wilner B, Hernandez AV, et al. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States from 2002 to 2016. J Am Heart Assoc 2019; 8(19): e012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kadri AN, Menon V, Sammour YM, et al. Outcomes of patients with severe tricuspid regurgitation and congestive heart failure. Heart 2019; 105(23): 1813–1817. [DOI] [PubMed] [Google Scholar]
- 6. Hussain ST, Witten J, Shrestha NK, et al. Tricuspid valve endocarditis. Ann Cardiothorac Surg 2017; 6(3): 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chong CZ, Cherian R, Ng P, et al. Clinical outcomes of severe TVIE related to intravenous drug abuse – a case series. Acta Cardiol 2021; 14: 1–6. [DOI] [PubMed] [Google Scholar]
- 8. Baddour L, Wilson W, Bayer A, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1–53. [DOI] [PubMed] [Google Scholar]
- 9. Baddour LM, Weimer MB, Wurcel AG, et al. Management of infective endocarditis in people who inject drugs: a scientific statement from the American Heart Association. Circulation 2022; 146(14): e187–e201. [DOI] [PubMed] [Google Scholar]
- 10. Chobufo MD, Atti V, Vasudevan A, et al. Trends in infective endocarditis mortality in the United States: 1999 to 2020: a cause for alarm. J Am Heart Assoc 2023; 12(24): e031589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El-Dalati S, Cronin D, Riddell JT, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg 2022; 113: 118–124. [DOI] [PubMed] [Google Scholar]
- 12. Roy AS, Hagh-Doust H, Azim AA, et al. Multidisciplinary teams for the management of infective endocarditis: a systematic review and meta-analysis. Open Forum Infect Dis 2023; 10(9): ofad444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delgado V, Marsan NA, de Waha S, et al. Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2023; 36: 3075–3128. [DOI] [PubMed] [Google Scholar]
- 14. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4): 633–638. [DOI] [PubMed] [Google Scholar]
- 15. Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for infective endocarditis: updating the Modified Duke Criteria. Clin Infect Dis 2023; 77(4): 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson JD, Altieri Dunn SC, Roy P, et al. Inpatient addiction medicine consultation service impact on post-discharge patient mortality: a propensity-matched analysis. J Gen Intern Med 2022; 37(10): 2521–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus 2020; 41(4): 519–525. [DOI] [PubMed] [Google Scholar]
- 18. El-Dalati S, Thornton A, Reda H, et al. Beyond a team: the comprehensive interdisciplinary endocarditis program in the United States. Int J Cardiol 2024; 397: 131638. [DOI] [PubMed] [Google Scholar]
- 19. van der Vaart TW, Prins JM, Soetekouw R, et al. All-cause and infection-related mortality in Staphylococcus aureus bacteremia, a multicenter prospective cohort study. Open Forum Infect Dis 2022; 9(12): ofac653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geriak M, Haddad F, Rizvi K, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2019; 63(5): e02483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCreary EK, Kullar R, Geriak M, et al. Multicenter cohort of patients with methicillin-resistant Staphylococcus aureus bacteremia receiving daptomycin plus ceftaroline compared with other MRSA treatments. Open Forum Infect Dis 2019; 7(1): ofz538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ulloa ER, Singh KV, Geriak M, et al. Cefazolin and ertapenem salvage therapy rapidly clears persistent methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2020; 71(6): 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El-Dalati S, Sridaran S, Uricchio M, et al. Oxacillin plus ertapenem combination therapy leads to rapid blood culture clearance and positive outcomes among patients with persistent MSSA bacteraemia: a case series. JAC Antimicrob Resist 2021; 3(3): dlab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang RS, Alam U, Maqsood MH, et al. Outcomes with percutaneous debulking of tricuspid valve endocarditis. Circ Cardiovasc Interv 2023; 16(7): e012991. [DOI] [PubMed] [Google Scholar]
- 25. El-Dalati S, Sinner G, Leung S, et al. Comparison of medical therapy, valve surgery, and percutaneous mechanical aspiration for tricuspid valve infective endocarditis. Am J Med. Epub ahead of print 2024. DOI: 10.1016/j.amjmed.2024.04.031 [DOI] [PubMed] [Google Scholar]
- 26. Siddiqui E, Alviar CL, Ramachandran A, et al. Outcomes after tricuspid valve operations in patients with drug-use infective endocarditis. Am J Cardiol 2022; 185: 80–86. [DOI] [PubMed] [Google Scholar]
- 27. Straw S, Baig MW, Gillott R, et al. Long-term outcomes are poor in intravenous drug users following infective endocarditis, even after surgery. Clin Infect Dis 2020; 71(3): 564–571. [DOI] [PubMed] [Google Scholar]
- 28. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380(5): 415–424. [DOI] [PubMed] [Google Scholar]
- 29. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open 2020; 3(2): e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vernon D, Brown JE, Griffiths E, et al. Reducing readmission rates through a discharge follow-up service. Future Healthc J 2019; 6(2): 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saini E, Ali M, Du P, et al. Early infectious disease outpatient follow-up of outpatient parenteral antimicrobial therapy patients reduces 30-day readmission. Clin Infect Dis 2019; 69(5): 865–868. [DOI] [PubMed] [Google Scholar]
- 32. El-Dalati S, Paras ML, Strnad L, et al. In plain sight: the need for a dedicated cardiovascular infectious disease subspecialty. JACC Adv 2024; 3(1): 100748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thakarar K, Nenninger K, Agmas W. Harm reduction services to prevent and treat infectious diseases in people who use drugs. Infect Dis Clin North Am 2020; 34(3): 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Negm S, Arafat AA, Elatafy EE, et al. Mechanical versus bioprosthetic valve replacement in the tricuspid valve position: a systematic review and meta-analysis. Heart Lung Circ 2021; 30(3): 362–371. [DOI] [PubMed] [Google Scholar]
- 35. Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence 2011; 5: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Hagen IM, Roos-Hesselink JW, Ruys TP, et al. Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation 2015; 132(2): 132–142. [DOI] [PubMed] [Google Scholar]
- 37. Jeejeebhoy FM. Prosthetic heart valves and management during pregnancy. Can Fam Physician 2009; 55(2): 155–157. [PMC free article] [PubMed] [Google Scholar]
- 38. Bazire B, Algalarrondo V, Dreyfus J. Permanent pacemaker implantation after tricuspid valve surgery. JACC Case Rep 2023; 13: 101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. El-Chami MF, Bonner M, Holbrook R, et al. Leadless pacemakers reduce risk of device-related infection: Review of the potential mechanisms. Heart Rhythm 2020; 17(8): 1393–1397. [DOI] [PubMed] [Google Scholar]
- 40. Hwang J, Han S, Park HS, et al. Implantation of a leadless pacemaker in a patient with mechanical tricuspid valve. Heart Rhythm Case Rep 2022; 8(4): 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morani G, Bolzan B, Pepe A, et al. Leadless pacemaker through tricuspid bioprosthetic valve: early experience. J Arrhythm 2021; 37(2): 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Acampora GA, Nisavic M, Zhang Y. Perioperative buprenorphine continuous maintenance and administration simultaneous with full opioid agonist: patient priority at the interface between medical disciplines. J Clin Psychiatry 2020; 81(1): 19com12810. [DOI] [PubMed] [Google Scholar]
- 43. Kar P, Ramachandran G. Pain relief following sternotomy in conventional cardiac surgery: a review of non neuraxial regional nerve blocks. Ann Card Anaesth 2020; 23(2): 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Protos AN, Trivedi JR, Whited WM, et al. Valvectomy versus replacement for the surgical treatment of tricuspid endocarditis. Ann Thorac Surg 2018; 106(3): 664–669. [DOI] [PubMed] [Google Scholar]
- 45. Baraka AS, Taha SK, Yazbeck, et al. Transient atrioventricular block after release of aortic cross-clamp. Anesth Analg 1995; 80(1): 54–57. [DOI] [PubMed] [Google Scholar]
- 46. St Marie B, Broglio K. Managing pain in the setting of opioid use disorder. Pain Manag Nurs 2020; 21(1): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15): 1736–1754. [DOI] [PubMed] [Google Scholar]
- 48. Burri M, Vogt MO, Hörer J, et al. Durability of bioprostheses for the tricuspid valve in patients with congenital heart disease. Eur J Cardiothorac Surg 2016; 50(5): 988–993. [DOI] [PubMed] [Google Scholar]
- 49. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021; 60(4): 727–800. [DOI] [PubMed] [Google Scholar]
- 50. Van Beek A, Moeyaert M, Ragheb B, et al. Outcomes of Warfarin Home INR monitoring vs office-based monitoring: a retrospective claims-based analysis. J Gen Intern Med 2024; 39(7): 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nii T, Yoshikawa H, Okabe T, et al. Septic pulmonary and systemic embolism in tricuspid endocarditis. BMJ Case Rep 2020; 13(2): e233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farrant O, Scozzi G, Hughes R. Systemic septic emboli in tricuspid endocarditis due to an atrial communication with a right-to-left shunt. BMJ Case Rep 2020; 13(2): e233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hubert S, Thuny F, Resseguier N, et al. Prediction of symptomatic embolism in infective endocarditis: construction and validation of a risk calculator in a multicenter cohort. J Am Coll Cardiol 2013; 62(15): 1384–1392. [DOI] [PubMed] [Google Scholar]
- 54. Shimfessel TT, El-Dalati SA, Sekela M, et al. Paradoxical embolisation in right-sided infective endocarditis and patent foramen ovale. BMJ Case Rep 2022; 15(5): e250272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tackett MS, Ahmed T, El-Dalati SA. Paradoxical embolisation to the brain in right-sided infective endocarditis and patent foramen ovale in a pregnant woman. BMJ Case Rep 2023; 16(3): e254403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rigau PV, Moral S, Bosch D, et al. Clinical prognosis of right-sided infective endocarditis not associated with cardiac devices or intravenous drug use: a cohort study and meta-analysis. Sci Rep 2020; 10(1): 7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Center for Medicare and Medicaid Services. Search the physician fee schedule, https://www.cms.gov/medicare/physician-fee-schedule/search?Y=0&T=4&HT=0&CT=3&H1=99367&M=5 (2023, accessed 2 May 2024).
- 58. El-Dalati S, Yoon P, Deeb GM. Is it time to rethink public reporting of surgical endocarditis outcomes in patients who inject drugs? J Am Heart Assoc 2021; 10(10): e020090. [DOI] [PMC free article] [PubMed] [Google Scholar]


