Abstract
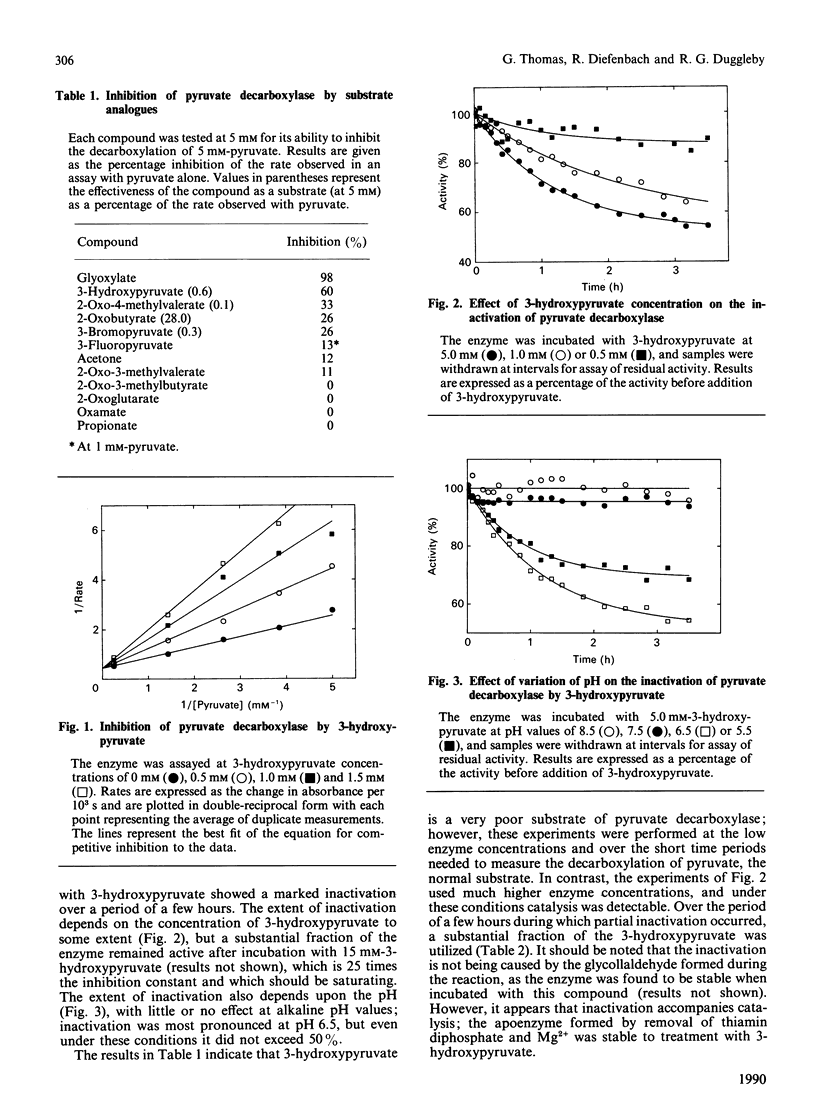
Pyruvate decarboxylase from Zymomonas mobilis is inhibited by 3-hydroxypyruvate, which can also act as a poor substrate. While catalysing the decarboxylation of this alternative substrate, the enzyme undergoes a progressive but partial inactivation over several hours. The extent of inactivation depends upon the pH and upon the concentration of 3-hydroxypyruvate. After partial inactivation and removal of unchanged 3-hydroxypyruvate, enzymic activity recovers slowly. We suggest that inactivation results from accumulation of enzyme-bound glycollaldehyde, which is relatively stable, possibly because it is dehydrated to form an acetyl group.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apfel M. A., Ikeda B. H., Speckhard D. C., Frey P. A. Escherichia coli pyruvate dehydrogenase complex. Thiamin pyrophosphate-dependent inactivation by 3-bromopyruvate. J Biol Chem. 1984 Mar 10;259(5):2905–2909. [PubMed] [Google Scholar]
- Duggleby R. G. Regression analysis of nonlinear Arrhenius plots: an empirical model and a computer program. Comput Biol Med. 1984;14(4):447–455. doi: 10.1016/0010-4825(84)90045-3. [DOI] [PubMed] [Google Scholar]
- Kuo D. J., Dikdan G., Jordan F. Resolution of brewers' yeast pyruvate decarboxylase into two isozymes. J Biol Chem. 1986 Mar 5;261(7):3316–3319. [PubMed] [Google Scholar]
- Lowe P. N., Perham R. N. Bromopyruvate as an active-site-directed inhibitor of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry. 1984 Jan 3;23(1):91–97. doi: 10.1021/bi00296a015. [DOI] [PubMed] [Google Scholar]
- Neale A. D., Scopes R. K., Wettenhall R. E., Hoogenraad N. J. Pyruvate decarboxylase of Zymomonas mobilis: isolation, properties, and genetic expression in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1024–1028. doi: 10.1128/jb.169.3.1024-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlemann H., Schellenberger A. Glyoxylic acid as an active site marker of yeast pyruvate decarboxylase. FEBS Lett. 1976 Mar 15;63(1):37–39. doi: 10.1016/0014-5793(76)80189-5. [DOI] [PubMed] [Google Scholar]
- Ullrich J. Structure-function relationships in pyruvate decarboxylase of yeast and wheat germ. Ann N Y Acad Sci. 1982;378:287–305. doi: 10.1111/j.1749-6632.1982.tb31203.x. [DOI] [PubMed] [Google Scholar]
- Zehender H., Trescher D., Ullrich J. Improved purification of pyruvate decarboxylase from wheat germ. Its partial characterisation and comparison with the yeast enzyme. Eur J Biochem. 1987 Aug 17;167(1):149–154. doi: 10.1111/j.1432-1033.1987.tb13316.x. [DOI] [PubMed] [Google Scholar]


