Abstract
Demodex blepharitis, a chronic lid margin disease, is caused by an infestation of Demodex mites, the most common ectoparasites in human skin and eyelids. Lotilaner ophthalmic solution, 0.25% (Xdemvy, Tarsus Pharmaceuticals), is the first therapy approved to treat Demodex blepharitis. This narrative review characterizes lotilaner ophthalmic solution, 0.25%, and describes its efficacy, safety, and tolerability. The safety and efficacy of lotilaner ophthalmic solution, 0.25%, for treating Demodex blepharitis was evaluated in four phase 2 and two phase 3 trials. The data of 980 patients included in these phase 2 and 3 clinical trials revealed that the proportion of eyes with a clinically meaningful reduction to 10 or fewer collarettes (the cylindrical, waxy debris found at the base of the eyelashes) ranged from 81 to 93%. The mite eradication rate confirmed by a microscopy of epilated lashes ranged from 52 to 78%. No serious treatment-related adverse events were reported in any of these clinical studies. As high as 92% of the patients receiving lotilaner eyedrops in the phase 3 trials found it to be neutral to very comfortable. Given the positive safety and efficacy outcomes, the drug is likely to become the standard of care in the treatment of Demodex blepharitis.
Keywords: Demodex, blepharitis, dry eye, lotilaner
1. Introduction
Lotilaner ophthalmic solution, 0.25% (Xdemvy, Tarsus Pharmaceuticals), is the first treatment approved by the FDA for the treatment of Demodex blepharitis, a condition that affects about 58% of all eye care patients, or about 25 million U.S. adults [1,2,3]. Demodex blepharitis, a chronic lid margin disease, is caused by an infestation of Demodex mites, the most common ectoparasites in human skin and eyelids. When residing in the hair follicles of the eyelids, the mites cause direct damage through mechanical burrowing and laying eggs, irritation from released chemicals, including digestive enzymes and waste products, and bacterial buildup from harboring and acting as vectors for bacteria in the eye [1,4,5]. Clinical manifestations of Demodex blepharitis include collarettes, lid margin erythema, lid and lash abnormalities, chalazia, pterygia, and contact lens intolerance [4,5,6,7,8]. The disease has significant clinical, functional, and psychosocial effects on patients, with more than 77% of patients with confirmed Demodex blepharitis indicating that the disease has negatively affected their daily lives [9]. It is prevalent in both sexes and across racial groups [10,11,12].
Demodex blepharitis is often intertwined with dry eye disease. A poor-quality tear film due to dry eye disease could make conditions more hospitable for Demodex mites [13]. Conversely, the overgrowth of Demodex mites can complicate dry eye disease and exacerbate symptoms [14]. Patients using prescription eyedrops for dry eyes are just as likely as those not using such medications to have signs of Demodex blepharitis, suggesting that dry eye treatments alone do not mitigate Demodex blepharitis [12]. Although there is a significant overlap in symptoms between dry eye disease and Demodex blepharitis, dry eye treatments may not provide any relief for the patient’s symptomatic burden [9,13].
Recent studies suggest a costly and substantial burden of illness in patients with Demodex blepharitis and highlight the unmet needs in diagnosing and managing Demodex blepharitis [9,15]. Nearly all patients (99.2%) with confirmed Demodex blepharitis have at least one symptom, and a large majority (96.9%) report three or more symptoms [9]. The results of a patient survey of 113 patients with Demodex blepharitis in the U.S. demonstrated that patients have often experienced delays in diagnosis, multiple healthcare provider (HCP) visits, unresolved symptoms, and high costs of disease management [16]. Eye redness, dryness, and itchy eyes/eyelids were some of the chief symptoms that prompted patients to visit their HCP prior to their Demodex blepharitis diagnosis [16]. Despite using several management options like warm compresses, artificial tears, and tea-tree-oil-based lid hygiene products, most patients reported unresolved Demodex blepharitis [16]. Healthcare resource utilization was also reported to be burdensome in this patient survey. Before diagnosis, patients reported an average of 3.5 HCP visits and a mean of 1.2 years between the appearance of symptoms and diagnosis [16]. After diagnosis with Demodex blepharitis, patients continued to visit HCPs multiple times a year due to unresolved disease [16]. More than 50% of Demodex blepharitis patients reported visiting an urgent care facility in the previous 12 months due to their Demodex blepharitis [16]. Prior to the availability of lotilaner ophthalmic solution, 0.25%, patients reported spending USD 192, on average, for doctor visits, USD 115 for over-the-counter products, USD 175 for prescription medications, and USD 368 for in-office procedures to address their Demodex blepharitis symptoms. Additionally, previous management strategies have been shown to provide only limited relief [17,18,19,20,21].
This review characterizes the new agent, lotilaner ophthalmic solution, 0.25%, by its chemical structure and describes the efficacy, safety, and tolerability outcomes seen in the formal clinical trial program for the drug.
2. Lotilaner: The First-in-Class Treatment for Demodex Blepharitis
Isoxazolines are a family of compounds shown to have acaricidal effects against fleas and ticks [22,23,24]. Isoxazolines are potent inhibitors of gamma-aminobutyric acid (GABA)-gated chloride channels, blocking the transport of chloride ions across cell membranes [25]. Ectoparasites exposed to isoxazolines exhibit spastic paralysis, leading to their starvation and death [26].
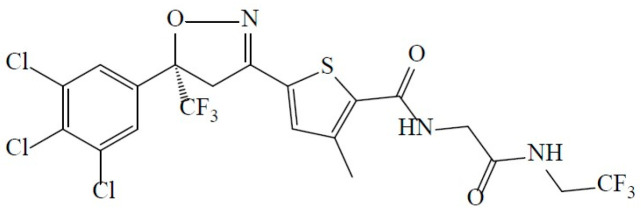
Lotilaner, i.e., 2-Thiophenecarboxamide, 5-[(5S)-4,5-dihydro-5-(3,4,5-trichlorophenyl)-5-(trifluoromethyl)-3-isoxazolyl]-3-methyl-N-[2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl]-2-thiophenecarboxamide (Figure 1), is a member of the isoxazoline class with a molecular weight of 596.76 g/mol and a measured log Pow (octanol/water partition coefficient) of 5.3 [26]. The lotilaner molecule has a high logP value, making it more soluble in lipophilic, organic solvents than in an aqueous solution. The activity of lotilaner is specific to insect and acari neuroreceptors; its best-in-class lack of effect on the mammalian nervous system at clinically relevant doses has been confirmed in numerous laboratory and target animal safety studies [26]. The lipophilic nature of the lotilaner molecule is thought to promote its preferential uptake into the lid margin and specifically the oily sebum of the eyelash follicles where the Demodex mites reside [27].
Figure 1.
Chemical structure of lotilaner.
In well-controlled laboratory and field studies, an oral veterinary formulation of lotilaner was shown to be >98% effective against fleas and ticks in dogs and cats [28,29]. Efficacy in dogs was demonstrated as soon as 2 h after administration, with the ability to maintain efficacy against subsequent re-infestations for at least 35 days after initial administration [30,31]. Likewise, in cats, oral administration of lotilaner resulted in rapid onset of flea- and tick-killing activity with consistent and sustained efficacy for at least one month [32].
Lotilaner has been marketed since 2018 as Credelio chewable tablets (Elanco). Both the U.S. FDA [33], and the European Medicines Agency (EMA) have concluded that isoxazoline veterinary products are safe and effective [34].
3. Lotilaner in Humans for the Treatment of Demodex Blepharitis
Demodex mites are microscopic ectoparasites of the phylum Arthropoda with a semi-transparent, elongated body and four pairs of legs [1,3,35]. The mites consume epithelial cells at the eyelash follicle and induce epithelial hyperplasia and hyperkeratinization, subsequently leading to the formation of collarettes (also known as cylindrical dandruff) [1,5,18]. Collarettes are the pathognomonic sign of Demodex blepharitis and can be readily diagnosed by having the subject look down during a routine slit-lamp examination.
An innovative multidose eyedrop formulation of lotilaner (Xdemvy, lotilaner ophthalmic solution, 0.25%, Tarsus Pharmaceuticals) was approved by the U.S. FDA in July 2023 for the treatment of Demodex blepharitis, following the successful conclusion of an extensive clinical trial program. Prior to the clinical trials leading to the approval of Xdemvy (formerly known as TP-03), lotilaner had not been used in humans, let alone for any ophthalmic indications.
The recommended human ophthalmic dose is one drop in each eye twice daily for 6 weeks (42 days). Assuming a 35 μL drop size, four drops per day (two per eye) of 0.25% lotilaner would provide a dose of 0.35 mg/day, or a total dose of 0.005 mg/kg/day for a 75 kg person, well below the preclinical NOAEL [36]. In vitro testing demonstrated that lotilaner did not inhibit mammalian GABA-Cl channels at concentrations up to 30 µM (~1100 times the recommended human ophthalmic dose) [36]. The 42-day course of treatment is intended to provide acaricidal dosing across at least two full life cycles of Demodex mites. The life cycle of a mite, from egg through larva, nymph, and adult mite, is estimated to be from 14 to 23 days [37,38].
4. Clinical Trial Program
Lotilaner ophthalmic solution, 0.25%, for the treatment of Demodex blepharitis has been evaluated in four phase 2 trials [39,40,41,42], conducted at Asociación para Evitar la Ceguera en México I.A.P in Mexico City, Mexico, followed by two large, randomized, double-masked, vehicle-controlled U.S. trials, phase 2b/3 Saturn-1 and phase 3 Saturn-2 [43,44]. Two of the early clinical studies evaluated treatment with lotilaner for 28 days [40,41], while all subsequent studies involved a 6-week (42 days) course of twice-daily treatment. In all, 147 patients participated in the phase 2 studies, and another 833 patients participated in the Saturn-1 and Saturn-2 pivotal trials. The design and outcomes of these studies are summarized in Table 1. A pooled analysis of the Saturn-1 and Saturn-2 studies has also been conducted and submitted for publication. In all these studies, subjects were confirmed to have Demodex blepharitis prior to enrollment by meeting all of the following criteria in at least one eye: more than 10 collarettes present on the upper eyelid; at least mild lid margin erythema of the upper eyelid, and average mite density of ≥1.5 mites per lash (upper and lower eyelids combined).
Table 1.
Summary of clinical trial outcomes for lotilaner ophthalmic solution, 0.25%.
| Publication | Study | Sample Size (N) | # of Sites | Country | Drug/Dose | Outcome Measures | Safety |
|---|---|---|---|---|---|---|---|
| Gonzalez-Salinas R et al. (MARS) 2021 [42] |
Single-arm, prospective | 15 | 1 | Mexico | Lotilaner 0.25% 1 drop OU BID for 28 days |
|
No AEs |
| Gonzalez-Salinas R et al. (IO) 2021 [40] |
Single-arm, prospective | 18 | 1 | Mexico | Lotilaner 0.25% 1 drop OU BID for 42 days |
|
5 drug-related TEAEs: mild blurriness (n = 1); mild burning (n = 4) |
| Gonzalez-Salinas R et al. (JUPITER) 2022 [41] |
Randomized controlled trial (Phase 2b) |
60 | 1 | Mexico | Lotilaner 0.25% 1 drop OU BID for 28 days |
|
No SAEs; no drug-related AEs |
| Yeu E et al. (EUROPA) 2023 [43] |
Randomized controlled trial (Phase 2b) |
54 | 1 | Mexico | Lotilaner 0.25% 1 drop OU BID for 42 days |
|
No SAEs; 4 drug-related mild AEs: burning (n = 2); burning with red eyes/blurriness (n = 1); change in taste sensation for a few hours (n = 1) |
| Yeu E et al. (SATURN-1) 2023 [45] |
Randomized controlled trial (Phase 2b/3) |
421 | 15 | U.S. | Lotilaner 0.25% 1 drop OU BID for 42 days |
|
No related SAEs; most commonly reported drug-related AE: instillation site pain: 11.8% (lotilaner) vs. 7.7% (vehicle) |
| Gaddie IB et al. (SATURN-2) 2023 [44] |
Randomized controlled trial (Phase 3) |
412 | 21 | U.S. | Lotilaner 0.25% 1 drop OU BID for 42 days |
|
No related SAEs; most commonly reported drug-related AE: instillation site pain: 7.9% (lotilaner) vs. 6.7% (vehicle) |
AE: adverse event; SAE: serious adverse event; TEAE: treatment-emergent adverse event; OU: both eyes; BID: twice daily.
The efficacy of lotilaner ophthalmic solution, 0.25%, for the treatment of Demodex blepharitis has been evaluated based on the reduction in collarettes at the base of the eyelashes, mite eradication confirmed by microscopy of epilated lashes, and cure of lid margin erythema. Safety was rigorously evaluated in each of the phase 2–3 trials. Additionally, a 1-year follow-up of patients from Saturn-1 has also been reported [45].
Following the publication of data from the clinical trials, four independent systematic reviews and meta-analyses were conducted, with all four concluding that lotilaner ophthalmic solution, 0.25%, is a promising treatment option for patients with Demodex blepharitis, with good evidence of safety and efficacy [46,47,48,49].
5. Clinical Trial Outcomes
5.1. Efficacy
Lotilaner ophthalmic solution, 0.25%, has demonstrated an early onset of action, with highly statistically significant changes compared to the vehicle group as early as day 15 [43,44]. In the Saturn-1 and Saturn-2 pivotal clinical trials, all pre-specified primary and secondary endpoints were met, with statistically significant differences (p < 0.0001) between lotilaner ophthalmic solution 0.25% and the vehicle group [43,44].
5.2. Reduction in Collarettes
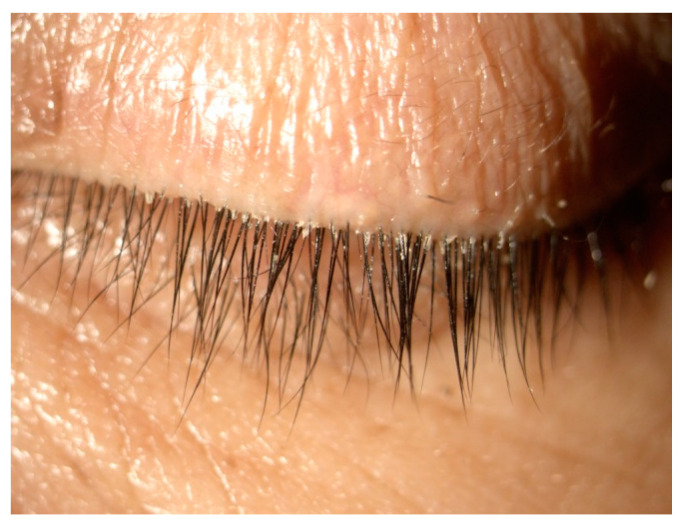
The primary endpoint in the Saturn-1 and Saturn-2 pivotal clinical trials was reduction in collarettes to 0–2 collarettes. These cylindrical, waxy debris found at the base of the eyelashes (Figure 2) are pathognomonic for Demodex blepharitis [4,5,21,50]. Collarettes consist of undigested material, keratinized cells, dead or living mites, and eggs/egg casings [3,39,51]. Because they are easy to visualize under slit-lamp magnification, the presence of collarettes represents the best way to diagnose Demodex blepharitis and follow the effects of treatment in routine clinical practice [52].
Figure 2.
Collarettes are sleeve-like exudative excretions that adhere to the base of the eyelashes.
A grade 0-to-4 collarette scale based on the groupings and/or scales used by Gao et al. and Hosseini et al. [51,53] was first described in a phase 2b study [40] and has been used in all subsequent clinical trials of lotilaner ophthalmic solution, 0.25%. This grading scale is non-linear, with grades 2 to 4 representing much higher levels of Demodex infestation (Table 2). A 2-collarette grade improvement—for example, from grade 3 to grade 1—can reflect a 90% reduction in the number of collarettes per eyelid. As shown previously by Gao et al. [51], a reduction in collarettes to ≤10 is associated with reduced mite density and a reduction in the severity of Demodex blepharitis. Reducing the number of collarettes to this level, which corresponds to Grade 0–1 on the current grading scale, is considered a clinically meaningful improvement, while a reduction to a collarette grade of 0 (≤2 collarettes) has been considered the most effective measure of success in lotilaner clinical trials.
Table 2.
Grading scales used in pivotal Saturn-1 and Saturn-2 clinical trials.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Collarettes | 0–2 lashes per eyelid with collarettes | 3–10 lashes per eyelid with collarettes | >10 but <1/3 of the lashes per eyelid with collarettes | ≥1/3 but <2/3 of the lashes per eyelid with collarettes | ≥2/3 of the lashes per eyelid with collarettes |
| Erythema | None Normal age-related lid coloration |
Mild Pink capillary involvement along the lid edge, no patches of confluent capillary redness throughout the lid edge |
Moderate Deep pink or red confluent capillary redness present locally along the lid edge |
Severe Deep red, diffuse confluent capillary redness present along the lid edge |
N/A |
In the first pivotal study, Saturn-1 [44], lotilaner ophthalmic solution, 0.25%, demonstrated a statistically significantly greater collarette reduction effect compared to the vehicle group. The proportion of patients achieving collarette grade 0 (0–2 collarettes) at day 43 was significantly higher in the lotilaner-treated study group compared to the vehicle control group (44.0% vs. 7.4%, p < 0.0001). The proportion of eyes with collarette grade 0–1 (≤10 collarettes) for the upper eyelid of the analysis eye was 81.3% in the study group versus 23.0% in the control group at day 43 (p < 0.0001). The mean collarette grade in the study group improved from 2.8 to 0.8 over the course of the study.
Similarly, in the second pivotal study, Saturn-2 [43], the proportion of patients achieving collarette grade 0 (0–2 collarettes) at day 43 was statistically significantly higher in the lotilaner-treated study group compared to the vehicle control group (56.0% vs. 12.5%, p < 0.0001). The proportion of eyes with collarette grade 0–1 (≤10 collarettes) for the upper eyelid of the analysis eye was 89.1% in the study group versus 33.0% in the control group at day 43 (p < 0.0001). Additionally, 96.4% of lotilaner-treated eyes had at least a 1-grade improvement in collarettes after 6 weeks of treatment.
5.3. Mite Eradication
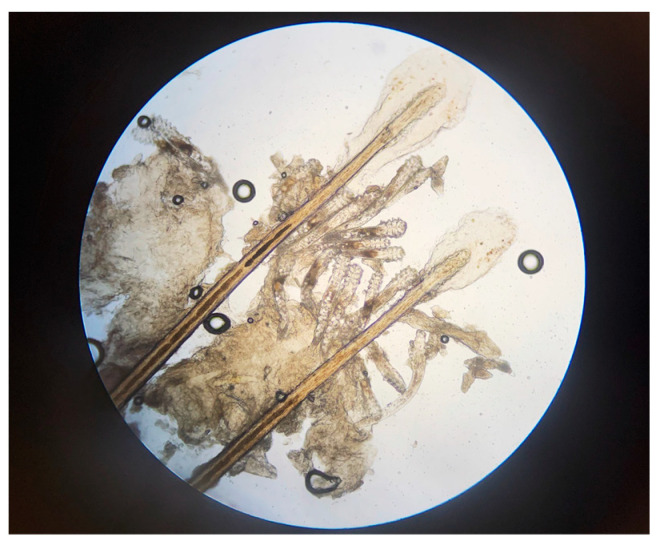
Demodex mites (Figure 3) can be identified by epilating eyelashes and viewing them under a microscope, although this is impractical in routine clinical practice and not required, as collarettes are a pathognomonic sign of Demodex blepharitis [4,5,21,50], as discussed above. In all phase 2–3 studies of lotilaner ophthalmic solution, 0.25%, microscopy confirmation of at least 1.5 mites per lash was required for study inclusion, and mite eradication was an important efficacy measure [40,42,43,44]. In the pivotal Saturn-1 and Saturn-2 trials [43,44], mites were counted at screening and on days 15, 22, and 43 using a slit-lamp biomicroscope to select two or more lashes from the upper and lower eyelids of each eye. Lashes with visible collarettes, if present, were targeted for epilation. The epilated lashes were examined, and mite density was calculated as the number of mites per lash. Eradication was defined as a mite density of 0 mites/eyelash [43,44].
Figure 3.
Demodex mites shown on epilated eyelashes under magnification (Image courtesy of Dr. Patrick Vollmer, Vita Eye Clinic, Shelby, North Carolina).
Reductions in mite density occurred early in the course of treatment. By day 43 in the Saturn-1 study, mites had been eradicated in 67.9% of study patients vs. 17.6% of control patients (p < 0.0001). Similarly, in the Saturn-2 study, the mite eradication rate was 51.8% in the study group vs. 14.6% in the control group (p < 0.0001) at day 43. When complete eradication was not achieved, 94.7% of the study group in Saturn-1 and 86.5% in Saturn-2 had a reduction in mite density to ≤0.5 mites/lash at day 43, compared to 35.8% and 34.7%, respectively, of the control groups in the two studies. The differences between the study and control groups were highly statistically significant [43,44].
5.4. Erythema Cure
Erythema of the eyelid margin, caused by chronic inflammation, is a common clinical sign of blepharitis [54,55]. Erythema may also be noted as a symptom by patients, who complain that it negatively affects their physical appearance, potentially influencing social and professional interactions [9].
An erythema grading scale of 0 to 3 (Table 2) was used in the phase 2 Europa clinical trial and all subsequent clinical trials of lotilaner ophthalmic solution, 0.25% [42]. The rates of erythema cure (reduction in redness to grade 0 in the upper eyelid of the analysis eye), as well as a composite grade of 0 for both erythema and collarettes, were evaluated in both pivotal Saturn clinical trials.
In the Saturn-1 trial, the erythema cure rate at day 43 was 19.1% in the study group vs. 6.9% in the vehicle group (p = 0.0001). The composite grade 0 rate for both collarettes and erythema was 13.9%, which was significantly higher than in the control group (1.0%, p < 0.0001). In Saturn-2, researchers again found statistically significant differences (p < 0.0001) between lotilaner ophthalmic solution and vehicle groups in erythema cure (31.1% vs. 9.0%) and a composite grade of 0 (19.2% vs. 4.0%) at day 43.
5.5. Safety and Tolerability
5.5.1. Adverse Events
No serious treatment-related adverse events have occurred in any of the human clinical studies of lotilaner ophthalmic solution, 0.25%, to date [39,40,41,42,43,44].
Most ocular treatment-emergent adverse events (TEAEs) in the Saturn pivotal trials were mild, and none were considered serious; the most common was instillation site pain (11.8% in Saturn-1 and 7.9% in Saturn-2) [43,44]. Other ocular adverse reactions worth noting included chalazion/hordeolum (stye) and punctate keratitis, both reported in less than 2% of patients [36]. There were no adverse safety signals on multiple safety measures evaluated in the Saturn-1 and Saturn-2 clinical trials, including intraocular pressure, endothelial cell density, corneal staining, slit-lamp biomicroscopy, fundus examination, or distance visual acuity. Additionally, there were no clinically relevant changes from baseline in median values for systemic clinical laboratory values, including hematology, clinical chemistry, and urinalysis [43,44].
5.5.2. Drop Comfort
Drop comfort on instillation can affect patient compliance and, therefore, the efficacy of treatment. In Saturn-1 and Saturn-2, the drop comfort of lotilaner ophthalmic solution, 0.25%, was assessed at all visits. Patients rated the comfort of the study medications as very comfortable, slightly comfortable, neither comfortable nor uncomfortable, slightly uncomfortable, or very uncomfortable. Most patients (91.9% in Saturn-1 and 90.7% in Saturn-2) in the study group found the drops to be neutral to very comfortable at day 43 [43,44]. There was no significant difference in drop comfort between the study group and vehicle control.
5.5.3. Drug Compliance
Patient compliance with eyedrop administration was verified through an in-office review of patients’ daily diaries at each study visit. Noncompliance or overcompliance with the drug administration schedule was defined as having less than 80% or more than 125% of the expected number of eyedrop administrations [42]. Patients reported high compliance with the drop regimen (mean of 98.6%), which may have been due in part to good tolerability of the drops and their negligible effect on vision.
5.5.4. Long-Term Safety and Duration of Response
The long-term safety and duration of response beyond the recommended 42-day treatment period are of interest to prescribing clinicians. Following the successful completion of the Saturn-1 study, participants who completed that study were invited to participate in an extension study in which they would be monitored for an additional 46 weeks, or 1 year from the initiation of treatment with lotilaner ophthalmic solution, 0.25%, to observe any incidence of long-term safety issues [45]. The duration of response out to 1 year after the 6-week treatment was also observed and evaluated. In all, 239 patients participated in the extension study, during which no additional intervention was administered, and no restriction related to the use of concomitant medications or therapies was enforced.
Only one treatment-related ocular adverse event (blurred vision) occurred in the study group (0.8%) during the 1-year duration of the study, and it was not considered serious. While no ocular serious adverse events (SAEs) were observed in the study group, the two non-ocular SAEs (hip fracture and hematuria) that occurred in the study group were determined to be not related to the study drug. As such, no long-term safety concerns for the study drug were observed in the 1-year extension study [45].
In addition, a statistically significantly (p < 0.0001) higher proportion of patients treated with lotilaner ophthalmic solution, 0.25%, for 6 weeks had 0–2 collarettes (grade 0) and ≤10 collarettes (collarette grade of 0 or 1) at every assessment timepoint throughout the 1-year extension study compared to patients who received vehicle control [45].
Furthermore, a continuous improvement in lid margin erythema was observed in the extension study. The proportion of patients in the study group with erythema cure (grade 0) was 21% at day 180 and 29% at day 365, compared to 19% at day 43 in the precursor study. The continuous improvement in erythema after treatment cessation suggested that it may take more time for inflammation to resolve or improve once there is a substantial reduction in mite infestation and collarettes [45].
6. Conclusions
To date, lotilaner ophthalmic solution, 0.25%, dosed twice daily as an eye drop, is the only formulation of lotilaner that has been extensively studied in humans. A topical aqueous gel formulation (TP-04, Tarsus Pharmaceuticals) for the potential treatment of papulopustular rosacea is currently being studied in a Phase 2a trial. As the first FDA-approved treatment for Demodex blepharitis, lotilaner ophthalmic solution, 0.25%, offers patients an alternative to previous symptom management options that have not been proven safe and effective. Lotilaner ophthalmic solution, 0.25%, was subjected to a robust clinical trial program and demonstrated safety and efficacy, including in the two successful randomized, double-masked, vehicle-controlled pivotal clinical trials enrolling more than 800 patients. Given the positive safety and efficacy outcomes reported in the published literature to date, it is expected that the drug is likely to become the standard of care in the treatment of Demodex blepharitis.
Acknowledgments
Editorial assistance was provided by Jan Beiting (Wordsmith Consulting, Cary, NC, USA) and Raman Bedi (IrisARC—Analytics, Research & Consulting, Chandigarh, India). Funding for their assistance was provided by Tarsus Pharmaceuticals, Inc.
Author Contributions
Conceptualization: P.G.D., A.C., S.N. and E.Y.; investigation: P.K., I.B.G., A.C., J.M., S.N. and E.Y.; writing—original draft preparation: P.G.D., A.C. and J.M.; writing—review and editing: P.G.D., M.F., P.K., I.B.G., A.C., J.M., S.N. and E.Y.; visualization: P.G.D. and J.M.; supervision: P.G.D., M.F., J.M. and E.Y.; project administration: J.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
A.C., J.M., and S.N. are employees and equity stakeholders of Tarsus Pharmaceuticals. E.Y. has received consulting fees from, serves on the board of directors of, and is an equity stakeholder in Tarsus Pharmaceuticals. P.K. is a consultant and equity stakeholder in Tarsus Pharmaceuticals. I.B.G. and M.F. are consultants for Tarsus Pharmaceuticals. P.G.D. has no financial disclosure related to the products or materials discussed.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fromstein S.R., Harthan J.S., Patel J., Opitz D.L. Demodex blepharitis: Clinical perspectives. Clin. Optom. 2018;10:57–63. doi: 10.2147/OPTO.S142708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teo A., Rosenberg E., Jacobson A. Prevalence of Demodex colonization in patients presenting to an outpatient clinic. Investig. Ophthalmol. Vis. Sci. 2021;62:1236. [Google Scholar]
- 3.Zhang A.C., Muntz A., Wang M.T.M., Craig J.P., Downie L.E. Ocular Demodex: A systematic review of the clinical literature. Ophthalmic Physiol. Opt. 2020;40:389–432. doi: 10.1111/opo.12691. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Sheha H., Tseng S.C. Pathogenic role of Demodex mites in blepharitis. Curr. Opin. Allergy Clin. Immunol. 2010;10:505–510. doi: 10.1097/ACI.0b013e32833df9f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X., Li J., Chen C., Tseng S., Liang L. Ocular Demodicosis as a Potential Cause of Ocular Surface Inflammation. Cornea. 2017;36((Suppl. 1)):S9–S14. doi: 10.1097/ICO.0000000000001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putnam C.M. Diagnosis and management of blepharitis: An optometrist’s perspective. Clin. Optom. 2016;8:71–78. doi: 10.2147/OPTO.S84795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarkowski W., Moneta-Wielgoś J., Młocicki D. Demodex sp. as a potential cause of the abandonment of soft contact lenses by their existing users. BioMed Res. Int. 2015;2015:259109. doi: 10.1155/2015/259109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X.-B., Ding Y.-H., He W. The association between demodex infestation and ocular surface manifestations in meibomian gland dysfunction. Int. J. Ophthalmol. 2018;11:589. doi: 10.18240/ijo.2018.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett M., Simmons B., Vollmer P., Patel A., Whitson W.E., Berdy G.J., Karpecki P., Periman L.M., Holdbrook M., Baba S.N., et al. The impact of Demodex blepharitis on patient symptoms and daily life. Optom. Vis. Sci. 2024;101:151–156. doi: 10.1097/OPX.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 10.Biernat M.M., Rusiecka-Ziółkowska J., Piątkowska E., Helemejko I., Biernat P., Gościniak G. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: A 10-year observational study. Jpn. J. Ophthalmol. 2018;62:628–633. doi: 10.1007/s10384-018-0624-3. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A.M., Hwang J., Dermer H., Galor A. Prevalence of ocular demodicosis in an older population and its association with symptoms and signs of dry eye. Cornea. 2021;40:995–1001. doi: 10.1097/ICO.0000000000002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trattler W., Karpecki P., Rapoport Y., Sadri E., Schachter S., Whitley W.O., Yeu E. The Prevalence of Demodex Blepharitis in US Eye Care Clinic Patients as Determined by Collarettes: A Pathognomonic Sign. Clin. Ophthalmol. 2022;16:1153–1164. doi: 10.2147/OPTH.S354692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee M.K., Yeu E., Barnett M., Rapuano C.J., Dhaliwal D.K., Nichols K.K., Karpecki P., Mah F.S., Chan A., Mun J., et al. Demodex Blepharitis: A Comprehensive Review of the Disease, Current Management, and Emerging Therapies. Eye Contact Lens. 2023;49:311–318. doi: 10.1097/ICL.0000000000001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziaja-Sołtys M., Kołodziejczyk M., Rymgayłło-Jankowska B., Wróbel-Dudzińska D., Suchodoła-Ratajewicz E., Szlonzak D., Żarnowski T., Bogucka-Kocka A. Massive demodicosis of the eyes in a patient with Sjögren syndrome: A case report. Acta Parasitol. 2021;66:677–681. doi: 10.1007/s11686-020-00297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Dell L., Dierker D.S., Devries D.K., Garlich J., Whitley W.O., Holdbrook M., Baba S.N., Yeu E. Psychosocial impact of Demodex blepharitis. Clin. Ophthalmol. 2022;16:2979. doi: 10.2147/OPTH.S374530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho A., Mun J., Chan A., Doshi R., Gupta P., O’Dell L., Thompson V., Skaar J., Hadker N., Green O. Characteristics, Healthcare Resource Utilization, and Costs among Patients with Demodex Blepharitis: Results of a Patient Survey in the United States. Academy of Managed Care Pharmacy; San Antonio, TX, USA: 2023. [Google Scholar]
- 17.Holzchuh F.G., Hida R.Y., Moscovici B.K., Villa Albers M.B., Santo R.M., Kara-Jose N., Holzchuh R. Clinical treatment of ocular Demodex folliculorum by systemic ivermectin. Am. J. Ophthalmol. 2011;151:1030–1034.e1. doi: 10.1016/j.ajo.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Messaoud R., El Fekih L., Mahmoud A., Ben Amor H., Bannour R., Doan S., Khairallah M. Improvement in ocular symptoms and signs in patients with Demodex anterior blepharitis using a novel terpinen-4-ol (2.5%) and hyaluronic acid (0.2%) cleansing wipe. Clin. Ophthalmol. 2019;13:1043–1054. doi: 10.2147/OPTH.S198585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira Filho P.A., Hazarbassanov R.M., Grisolia A.B.D., Pazos H.B., Kaiserman I., Gomes J.Á.P. The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex spp. Br. J. Ophthalmol. 2011;95:893–895. doi: 10.1136/bjo.2010.201194. [DOI] [PubMed] [Google Scholar]
- 20.Salem D.A.-B., El-Shazly A., Nabih N., El-Bayoumy Y., Saleh S. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin–metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int. J. Infect. Dis. 2013;17:e343–e347. doi: 10.1016/j.ijid.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Savla K., Le J.T., Pucker A.D. Tea tree oil for Demodex blepharitis. Cochrane Database Syst. Rev. 2020;6:CD013333. doi: 10.1002/14651858.CD013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gassel M., Wolf C., Noack S., Williams H., Ilg T. The novel isoxazoline ectoparasiticide fluralaner: Selective inhibition of arthropod gamma-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem. Mol. Biol. 2014;45:111–124. doi: 10.1016/j.ibmb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 23.McTier T.L., Chubb N., Curtis M.P., Hedges L., Inskeep G.A., Knauer C.S., Menon S., Mills B., Pullins A., Zinser E., et al. Discovery of sarolaner: A novel, orally administered, broad-spectrum, isoxazoline ectoparasiticide for dogs. Vet. Parasitol. 2016;222:3–11. doi: 10.1016/j.vetpar.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Shoop W.L., Hartline E.J., Gould B.R., Waddell M.E., McDowell R.G., Kinney J.B., Lahm G.P., Long J.K., Xu M., Wagerle T., et al. Discovery and mode of action of afoxolaner, a new isoxazoline parasiticide for dogs. Vet. Parasitol. 2014;201:179–189. doi: 10.1016/j.vetpar.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Rufener L., Danelli V., Bertrand D., Sager H. The novel isoxazoline ectoparasiticide lotilaner (Credelio™): A non-competitive antagonist specific to invertebrates γ-aminobutyric acid-gated chloride channels (GABACls) Parasites Vectors. 2017;10:530. doi: 10.1186/s13071-017-2470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toutain C.E., Seewald W., Jung M. The intravenous and oral pharmacokinetics of lotilaner in dogs. Parasites Vectors. 2017;10:522. doi: 10.1186/s13071-017-2475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiti S., Crean C., Neervannan S. Pharmacokinetics of Lotilaner Ophthalmic Solution, 0.25% in Rabbits. American Academy of Optometry; New Orleans, LA, USA: 2023. [Google Scholar]
- 28.Cavalleri D., Murphy M., Seewald W., Nanchen S. A randomized, controlled field study to assess the efficacy and safety of lotilaner (Credelio™) in controlling fleas in client-owned cats in Europe. Parasites Vectors. 2018;11:410. doi: 10.1186/s13071-018-2971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karadzovska D., Chappell K., Coble S., Murphy M., Cavalleri D., Wiseman S., Drake J., Nanchen S. A randomized, controlled field study to assess the efficacy and safety of lotilaner flavored chewable tablets (Credelio™) in eliminating fleas in client-owned dogs in the USA. Parasites Vectors. 2017;10:528. doi: 10.1186/s13071-017-2469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalleri D., Murphy M., Seewald W., Drake J., Nanchen S. Assessment of the speed of flea kill of lotilaner (Credelio™) throughout the month following oral administration to dogs. Parasites Vectors. 2017;10:529. doi: 10.1186/s13071-017-2466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalleri D., Murphy M., Seewald W., Drake J., Nanchen S. Assessment of the onset of lotilaner (Credelio™) speed of kill of fleas on dogs. Parasites Vectors. 2017;10:521. doi: 10.1186/s13071-017-2474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toutain C.E., Seewald W., Jung M. Pharmacokinetics of lotilaner following a single oral or intravenous administration in cats. Parasites Vectors. 2018;11:412. doi: 10.1186/s13071-018-2966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Food & Drug Administration Fact Sheet for Pet Owners and Veterinarians about Potential Adverse Events Associated with Isoxazoline Flea and Tick Products. [(accessed on 15 June 2024)]; Available online: https://www.fda.gov/animal-veterinary/animal-health-literacy/fact-sheet-pet-owners-and-veterinarians-about-potential-adverse-events-associated-isoxazoline-flea.
- 34.European Medicines Agency: Credelio Summary of Product Characteristics. [(accessed on 15 June 2024)]. Available online: https://www.ema.europa.eu/en/documents/product-information/credelio-epar-product-information_en.pdf.
- 35.Suresha A., Sadhwini M. Role of demodex infestation in blepharitis and coconut oil as a treatment option. Indian J. Clin. Exp. Ophthalmol. 2020;6:270–275. [Google Scholar]
- 36.Tarsus Pharmaceuticals, Inc. XDEMVY [Prescribing Information] 2023. [(accessed on 1 February 2024)]. Available online: https://tarsusrx.com/wp-content/uploads/XDEMVY-Prescribing-Information-24JUL23.pdf.
- 37.Lam N.S.K., Long X.X., Li X., Yang L., Griffin R.C., Doery J.C. Comparison of the efficacy of tea tree (Melaleuca alternifolia) oil with other current pharmacological management in human demodicosis: A Systematic Review. Parasitology. 2020;147:1587–1613. doi: 10.1017/S003118202000150X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls S.G., Oakley C.L., Tan A., Vote B.J. Demodex species in human ocular disease: New clinicopathological aspects. Int. Ophthalmol. 2017;37:303–312. doi: 10.1007/s10792-016-0249-9. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Salinas R., Yeu E., Holdbrook M., Baba S.N., Ceballos J.C., Massaro-Corredor M., Corredor-Ortega C., Ramos-Betancourt N., Quiroz-Mercado H. Collarette Elimination and Demodex Mite Eradication with Topical Lotilaner Ophthalmic Solution, 0.25. J. Ocul. Pharmacol. Ther. 2021;37:479–484. doi: 10.1089/jop.2021.0011. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Salinas R., Karpecki P., Yeu E., Holdbrook M., Baba S.N., Ceballos J.C., Massaro-Corredor M., Corredor-Ortega C., Ramos-Betancourt N., Quiroz-Mercado H. Safety and efficacy of lotilaner ophthalmic solution, 0.25% for the treatment of blepharitis due to demodex infestation: A randomized, controlled, double-masked clinical trial. Contact Lens Anterior Eye. 2022;45:101492. doi: 10.1016/j.clae.2021.101492. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Salinas R., Yeu E., Holdbrook M., Baba S.N., Ceballos J.C., Massaro-Corredor M., Corredor-Ortega C., Ramos-Betancourt N., Quiroz-Mercado H. Safety and Efficacy of Topical Lotilaner Ophthalmic Solution 0.25% for the Treatment of Demodex Blepharitis: A Pilot Study. J. Ophthalmol. 2021;2021:3862684. doi: 10.1155/2021/3862684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeu E., Holdbrook M., Baba S.N., Ceballos J.C., Massaro-Corredor M., Corredor-Ortega C., Ramos-Betancourt N., Quiroz-Mercado H., Gonzalez-Salinas R. Treatment of Demodex Blepharitis: A Prospective, Randomized, Controlled, Double-Masked Clinical Trial Comparing Topical Lotilaner Ophthalmic Solution, 0.25% Eyedrops to Vehicle. Ocul. Immunol. Inflamm. 2023;31:1653–1661. doi: 10.1080/09273948.2022.2093755. [DOI] [PubMed] [Google Scholar]
- 43.Gaddie I.B., Donnenfeld E.D., Karpecki P., Vollmer P., Berdy G.J., Peterson J.D., Simmons B., Edell A.R.P., Whitson W.E., Ciolino J.B., et al. Lotilaner Ophthalmic Solution 0.25% for Demodex Blepharitis: Randomized, Vehicle-Controlled, Multicenter, Phase 3 Trial (Saturn-2) Ophthalmology. 2023;130:1015–1023. doi: 10.1016/j.ophtha.2023.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Yeu E., Wirta D.L., Karpecki P., Baba S.N., Holdbrook M., Saturn I.S.G. Lotilaner Ophthalmic Solution, 0.25%, for the Treatment of Demodex Blepharitis: Results of a Prospective, Randomized, Vehicle-Controlled, Double-Masked, Pivotal Trial (Saturn-1) Cornea. 2023;42:435–443. doi: 10.1097/ICO.0000000000003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadri E., Paauw J.D., Ciolino J.B., Nijm L., Simmons B., Meyer J., Gaddie I.B., Berdy G.J., Holdbrook M., Baba S.N., et al. Long-Term Outcomes of 6-Week Treatment of Lotilaner Ophthalmic Solution, 0.25%, for Demodex Blepharitis: A Noninterventional Extension Study. Cornea. 2024 doi: 10.1097/ICO.0000000000003484. [DOI] [PubMed] [Google Scholar]
- 46.Abo Zeid M., Elrosasy A., Abbas A.W., Elganady A., Rhab A.E., Abu Serhan H. Efficacy and Safety of Lotilaner Ophthalmic Solution 0.25% in the Treatment of Demodex Blepharitis: A Systematic Review and Meta-Analysis. Ocul. Immunol. Inflamm. 2024:1–12. doi: 10.1080/09273948.2024.2309552. [DOI] [PubMed] [Google Scholar]
- 47.Akhtar S.M.M., Fareed A., Asghar M.S., Mumtaz M., Kaur S. Efficacy and Safety of Lotilaner Ophthalmic Solution 0.25% for the Treatment of Demodex Blepharitis: A Meta-Analysis of Randomized Controlled Trials. Contact Lens Anterior Eye. 2024;47:102148. doi: 10.1016/j.clae.2024.102148. [DOI] [PubMed] [Google Scholar]
- 48.Awan B., Elsaigh M., Tariq A., Badee M., Loomba A., Khedr Y., Abdelmaksoud A. A Systematic Review and Meta-Analysis of the Safety and Efficacy of 0.25% Lotilaner Ophthalmic Solution in the Treatment of Demodex Blepharitis. Cureus. 2024;16:e52664. doi: 10.7759/cureus.52664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talha M., Haris Ali M., Fatima E., Nadeem A., Ahmed A., Nashwan A.J. Efficacy and Safety of Lotilaner Ophthalmic Solution (0.25%) for the Treatment of Demodex Blepharitis: A GRADE Assessed Systematic Review and Meta-Analysis of Observational & Experimental Studies. Am. J. Ophthalmol. 2024;264:8–16. doi: 10.1016/j.ajo.2024.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Rabensteiner D.F., Aminfar H., Boldin I., Nitsche-Resch M., Berisha B., Schwantzer G., Horwath-Winter J. Demodex mite infestation and its associations with tear film and ocular surface parameters in patients with ocular discomfort. Am. J. Ophthalmol. 2019;204:7–12. doi: 10.1016/j.ajo.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y.Y., Di Pascuale M.A., Li W., Liu D.T., Baradaran-Rafii A., Elizondo A., Kawakita T., Raju V.K., Tseng S.C. High prevalence of Demodex in eyelashes with cylindrical dandruff. Investig. Ophthalmol. Vis. Sci. 2005;46:3089–3094. doi: 10.1167/iovs.05-0275. [DOI] [PubMed] [Google Scholar]
- 52.Aumond S., Bitton E. Palpebral and facial skin infestation by Demodex folliculorum. Contact Lens Anterior Eye. 2020;43:115–122. doi: 10.1016/j.clae.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Hosseini K., Bourque L.B., Hays R.D. Development and evaluation of a measure of patient-reported symptoms of Blepharitis. Health Qual. Life Outcomes. 2018;16:11. doi: 10.1186/s12955-018-0839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milner M.S., Beckman K.A., Luchs J.I., Allen Q.B., Awdeh R.M., Berdahl J., Boland T.S., Buznego C., Gira J.P., Goldberg D.F., et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders—new strategies for diagnosis and treatment. Curr. Opin. Ophthalmol. 2017;27((Suppl. 1)):3–47. doi: 10.1097/01.icu.0000512373.81749.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raval Y., Gomes P.J., Abelson M.B. Standardized lid margin redness scale for blepharitis. Investig. Ophthalmol. Vis. Sci. 2019;60:6270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.