Abstract
GB virus B (GBV-B) is a virus of the family Flaviviridae that infects small primates (Saguinus sp. [tamarins]) and shows similarities to hepatitis C virus (HCV) in genome organization, protein function, tissue tropism, and pathogenicity. This suggests the possibility of using tamarins infected by GBV-B or GBV-B/HCV chimeric viruses as a surrogate animal model of HCV infection. To achieve the construction of such chimeric viruses, it is essential to produce a complete and infectious GBV-B genomic RNA. We have identified a novel sequence at the 3′ end of the GBV-B genome and show that it can be arranged in a secondary structure resembling that of the 3′ end of the HCV genome, which is known to be essential for infectivity.
Hepatitis C virus (HCV) (22) is responsible for a widespread form of hepatitis (1, 4, 38) that often evolves into cirrhosis and hepatocarcinoma (23), for which neither a long-term effective therapy nor a vaccine exists (19). The study of HCV infection is also hampered by the lack of suitable experimental models. The only animal model is the chimpanzee (7, 8, 14, 17, 37, 39), a protected species whose use is inconvenient for pharmacological studies requiring large numbers of small animals. To circumvent this problem, we are exploring the possibility of establishing a surrogate animal model for HCV by infecting tamarins (monkeys of the genus Saguinus) with GB virus B (GBV-B) or with recombinant viruses in which the GBV-B genome is used as a scaffold for insertion of HCV targets of interest.
GBV-B belongs to a small group of flaviviruses that also includes GBV-A and GBV-C/HGV. GBV-A and GBV-B were recently shown to be associated with GB agent hepatitis (18, 31), originally described by Deinhardt and coworkers (6). It was subsequently shown that only GBV-B causes hepatitis (28, 29). In spite of the human origin of the initial inoculum, it is not yet clear whether GBV-B is indeed a human pathogen. Though immune reactivity against GBV-B recombinant proteins in humans has been detected (21), probably due to cross-reactivity to antigens of the related human virus GBV-C/HGV (16, 30), attempts to detect GBV-B sequences in human reactive plasma have been unsuccessful (30). Moreover, in contrast to GBV-A (3), no GBV-B isolate has ever been identified in wild tamarins. In summary, there is a lack of information about the host range of GBV-B in nonexperimental infections, and only one genomic sequence for this virus is available (GenBank accession no. U22304).
In spite of the limited information in the literature, GBV-B appears to be particularly interesting as a candidate for establishing an animal model for HCV infection, since it not only causes hepatitis in small monkeys (32, 41) but also shows a genome organization that actually parallels that of HCV (18, 20). A colinearity between the polyprotein coding regions of the two viruses exists, and amino acid residues of catalytic sites and of other functional motifs are conserved in individual proteins. Beyond the structural similarity, the two viruses share functional characteristics, as we have observed for key enzymes: the NS3 protease (26), which is essential for processing of the viral polyprotein; the NS3 helicase (9); and the NS5B polymerase (35), which is involved in replication of the viral genome. The 5′ untranslated region (UTR) of HCV, including an internal ribosomal entry site, is shorter than the corresponding region of GBV-B; nonetheless, the nucleotide homology is higher than in the rest of the viral genome, reaching about 70% in the 100 nucleotides (nt) preceding the open reading frame (ORF). Furthermore, the 5′ UTRs of the two viruses can be modelled in very similar secondary and tertiary structures, including the presence of peculiar features such as a pseudoknot immediately upstream of the ATG translation start codon (10, 15), which suggests a functional similarity.
While the ORFs of HCV and GBV-B are colinear and the 5′ UTRs of the two viruses can be arranged in strikingly similar secondary structures, the published GBV-B 3′ UTR is surprisingly different from that of HCV, not only in length and primary sequence but also at the level of potential secondary structures (13, 18). These observations led us to consider the possibility that part of the information about the GBV-B genome could be missing in the published genomic sequence. With the aim of constructing a complete GBV-B genomic molecule capable of sustaining infection in animals, we decided to determine the sequence of the very 3′ end of the viral genome. We found that the GBV-B genome includes an extension of 259 nt, which we named 3′Y. In addition, the insertion of a C base after position 9137 of the published sequence was found. Together, our results indicate that the GBV-B genome is 260 nt longer than originally determined.
Animals.
Captive-outbred Saguinus fuscicollis and Saguinus oedipus tamarins were housed at the Max-von-Pettenkofer-Institut of Munich University, Munich, Germany, and at the Biomedical Primate Research Centre, Rijswik, The Netherlands, respectively. Animals were maintained under conditions that fulfilled all ethical and scientific requirements for animal use.
Identification of a novel sequence in the GBV-B genome.
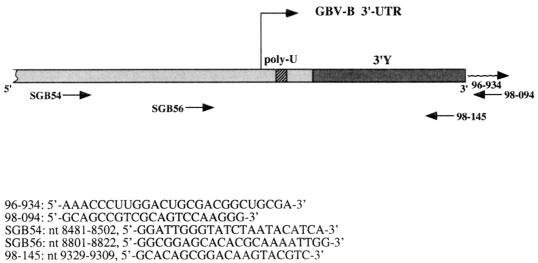
To identify the 3′ end of the GBV-B genome, RNA was prepared from the serum of an infected Saguinus oedipus tamarin and subjected to the procedure described below. Figure 1 shows the GBV-B 3′ UTR organization and the oligonucleotides used in this study. RNA (10 μl) extracted from 28 μl of serum of a GBV-B-infected tamarin, was mixed with 10 μl of 5′-phosphorylated, 3′-blocked (NaIO4-oxidized) RNA oligonucleotide 96-934 and with 6.5 μl of sterile water. After 3 min at 90°C, the mix was quickly chilled by immersion in an ice-water bath and briefly centrifuged at 4°C. The following components of the ligation reaction mixture were then added in amounts suitable to reach the indicated concentrations in a final volume of 30 μl: 10% dimethyl sulfoxide, 50 mM HEPES (pH 7.5), 20 mM MgCl2, 3 mM dithiothreitol, 10 μg of bovine serum albumin per ml, 20 units of RNasin (Promega), 0.1 mM ATP, and 23.8 units of RNA ligase (Gibco-BRL). The reaction mixture was prepared in a cold room (4°C) with the sample on ice. The ligation reaction was performed by incubating the mixture at 4°C for 24 h. The reaction was stopped by incubation at 75°C for 10 min, extracted once with phenol-chloroform and once with chloroform, and ethanol precipitated in the presence of 0.3 M Na acetate (pH 5) and glycogen as a carrier. After a wash with 70% ethanol, the sample was dried and resuspended in 20 μl of sterile water. Ten microliters of this sample was used for subsequent first-strand cDNA synthesis by using as an antisense primer the DNA oligonucleotide 98-094, which is complementary to part of the sequence of the RNA oligonucleotide 96-934. cDNA was amplified by PCR with primer 98-094 in combination with primer SGB56, of opposite polarity. PCR products were analyzed on an agarose gel, and a faint but sharp band was visualized (data not shown). The band was eluted and cloned, and the DNA from nine colonies was sequenced (25) with primers annealing to the vector. In all clones, the sequence of primer SGB56 was followed by the published GBV-B genome sequence (accession no. U22304) showing an extra C after position 9137. This sequence was followed by a new segment of 259 nt, which we named 3′Y, and by the sequence of the RNA oligonucleotide 96-934. In Fig. 2, the GBV-B genome sequence is shown spanning the portion downstream of the poly(U) tract and including the whole novel sequence. The GBV-B 3′Y sequence was used to search the most recent available release of the nucleotide sequences in the GenBank and EMBL databases, by using the FASTA and BLAST programs, without finding any significant homology to known sequences (data not shown). All potential translation products of the six frames in the novel sequence were used to search the protein databases PIR-Protein and SWISS-PROT. Moreover, the ORFs obtained by translating the nucleotide sequences databases named above were compared to all of the amino acid sequences that can be deduced from the nucleotide novel sequence. In none of these cases was significant homology found (data not shown).
FIG. 1.
Oligonucleotides used in this study. (Top) Schematic representation of the GBV-B genome showing the orientations and approximate positions of the primers. (Bottom) Locations and sequences of the primers. Numbering refers to the GBV-B genomic sequence, including the C at position 9138, the 3′Y (259 nt), and a poly(U) tract of 27 nt, as in the U22304 sequence.
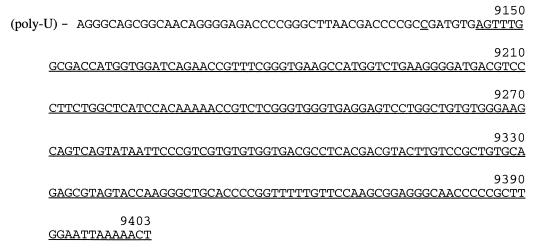
FIG. 2.
Sequence of the 3′ UTR of the GBV-B genome following the poly(U) tract. nt 9138 and 9145 to 9403 (3′Y), not present in the published sequence of the GBV-B genome (GenBank accession no. U22304), are underlined. For numbering, see the legend to Fig. 1.
Rescue of GBV-B genomic sequences by using a primer in the 3′Y sequence.
To confirm that the newly discovered sequence is actually part of the GBV-B genome, we performed reverse transcription-PCR experiments with one of the primers annealing in the new sequence. The sequences and positions of the primers are shown in Fig. 1. An antisense primer (98-145) complementary to the 3′Y sequence was used to produce cDNA from RNA of the liver and serum of infected tamarins. PCR was performed with this primer together with the sense primer SGB54, annealing in the published GBV-B sequence. RNAs from noninfected animals and from human serum were used to perform negative control reactions. Negative controls were also run with the sense primer SBG54 in combination with the antisense primer complementary to the RNA oligonucleotide originally used in the RNA ligation experiment (primer 98-094). Sequencing of a number of clones obtained from independent amplification products invariably confirmed the presence of the 3′Y fragment and of the C at position 9138 (data not shown). Analogous results were obtained by synthesizing cDNA from the negative strand of the GBV-B RNA genome with primer SGB54 and then performing PCR with primers SGB54 and 98-145. The poly(U) tract was heterogeneous in length, as in HCV genome (13), varying from 9 to 30 nt.
Detection of the 3′Y sequence in GBV-B-infected tamarin RNA by Northern blotting.
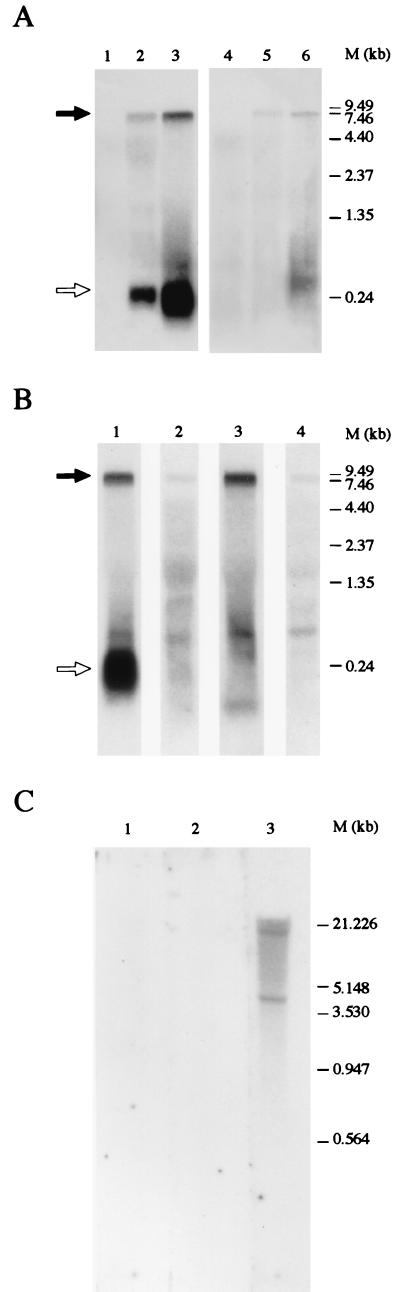
To obtain a PCR-independent confirmation of the presence of the novel sequence in the GBV-B genome, we performed Northern blot experiments. RNA extracted from the liver of two animals infected with GBV-B was subjected to electrophoresis, blotted, and probed with DNA corresponding to the 3′Y fragment or, as a positive control probe, to a fragment of the published sequence (nt 8800 to 9068). As a negative control, RNA from a noninfected animal liver was used. The results (Fig. 3A) showed that the 3′Y probe hybridizes specifically to an RNA of the size expected for the GBV-B genome. To evaluate whether the novel sequence was also present in the negative strand of the GBV-B RNA, RNA from the liver of an infected animal was probed with strand-specific RNA probes, 3′Y-antisense and 3′Y-sense, of opposite and identical polarities, respectively, compared to the strand of the 3′Y sequence shown in Fig. 2. Positive controls were run with probes, control-antisense and control-sense, annealing in the published sequence (nt 8800 to 9068) and complementary to positive and negative GBV-B RNA strands, respectively. A band coincident with the viral RNA size was visualized by each of the probes (Fig. 3B), thus demonstrating that the novel 3′Y is located in both the positive and negative strands of GBV-B RNA. The signal was stronger when the RNA probes 3′Y-antisense and control-antisense were used. The ratio of the intensity of the signals obtained with the two 3′Y probes of opposite polarity was the same as that obtained with the two control probes, hybridizing to the positive and negative strands of GBV-B RNA, respectively. This result is a confirmation of the specificity of the radioactive signal and a strong indication that the bands visualized with the 3′Y-antisense and control-antisense probes correspond to the same molecular species, that is, GBV-B positive-strand RNA.
FIG. 3.
Detection of the 3′Y sequence in GBV-B-infected tamarin liver RNA and lack of detection of the 3′Y sequence in noninfected tamarin genomic DNA. (A) Northern blot of tamarin liver RNA. RNA samples: lanes 1 and 4, uninfected tamarin RNA; lanes 2 and 5, GBV-B-infected S. fuscicollis tamarin RNA; lanes 3 and 6, GBV-B-infected S. oedipus tamarin RNA. DNA probes: lanes 1 to 3, 3′Y probe; lanes 4 to 6, control probe. (B) Northern blot of GBV-B-infected S. oedipus tamarin RNA. RNA probes: lane 1, 3′Y-antisense; lane 2, 3′Y-sense; lane 3, control-antisense; lane 4, control-sense. The solid arrows indicate the viral genomic RNA band; the open arrows indicate the low-molecular-weight RNA species. (C) Southern blot of noninfected S. oedipus tamarin genomic DNA. Lane 1, GBV-B 3′Y probe; lane 2, GBV-B control probe; lane 3, tamarin gene probe.
In addition to the viral genome band, the probes specific for the 3′Y region annealed to a low-molecular-weight RNA not detected by the control probes when infected tamarin liver RNA was blotted (Fig. 3A and B). The size of this RNA molecular species is similar to that of the 3′Y region itself. The abundance of this low-molecular-weight species with respect to the genomic RNA may be apparent only because of the higher blotting efficiency of molecules of that size, which are about 40 times shorter than those of the viral genome. This RNA is probably encoded by the GBV-B genome and generated as an endonucleolytic processing product or a degradation product. It could not, however, be excluded a priori that this RNA is a mobile element encoded by the tamarin genome and transcribed upon infection with GBV-B. In both cases, GBV-B genomic RNA would exist in two forms, with and without this element. Unfortunately, the resolution of the gel does not allow discrimination between two molecular species of more than 9,000 nt that differ by only 200 to 300 nt.
Search for the 3′Y sequence in tamarin genomic DNA.
In order to ascertain that the low-molecular-weight band identified with 3′Y probes in Northern blots of tamarin liver RNA from infected animals was not the transcript of a host gene induced by GBV-B infection, we performed a Southern blotting experiment (Fig. 3C). Genomic DNA was prepared from whole blood (24) of a noninfected S. oedipus tamarin and digested with BglII. Digested DNA (10 μg) was separated on a 0.8% agarose gel, blotted, and hybridized with the 3′Y DNA fragment used in Northern blotting. As a negative control, the DNA fragment of the published GBV-B sequence (nt 8800 to 9068), corresponding to the positive control probe of the Northern blots, was used. A positive control for hybridization was performed with a 300-nt DNA probe corresponding to a gene fragment from S. oedipus (27). The stringency of all hybridization steps was comparable to that of the Northern blot experiments. The results of this experiment (Fig. 3C) showed that hybridization was obtained only with the tamarin gene probe; even with an exposure time much longer than that shown, neither GBV-B probe give any signal, indicating that the 3′Y sequence is not encoded by tamarin DNA. Whether the low-molecular-weight species of RNA is only a degradation product of the GBV-B genome or plays some role in the GBV-B life cycle is an interesting issue which, at the moment, is only a matter of speculation.
Predicted secondary structure of the 3′Y sequence.
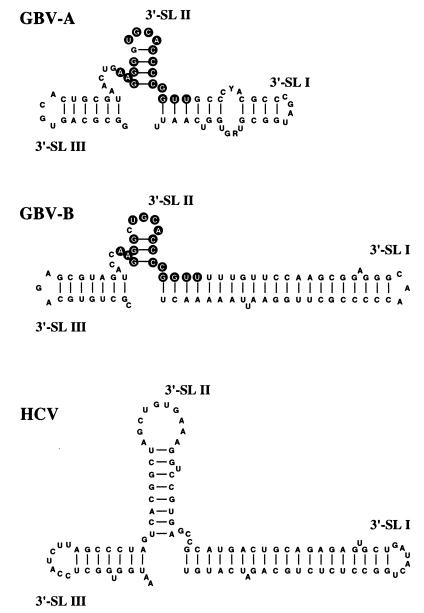
A prevision of the secondary structure of the GBV-B 3′Y novel sequence, showing a potential complex stem-loop structure, was performed (data not shown). The 3′-terminal part of this structure (corresponding to nt 9320 to 9403 of the complete genome sequence) is presented in Fig. 4, where the stem-loop structures are designated according to the nomenclature of the HCV 3′X region. While the search in nucleic acid databases with the whole GBV-B 3′Y sequence did not give significant homology results, by running comparison analyses on portions of the 3′Y sequence, an identity of 80% in a 39-nt segment was found with the sequence of an isolate of GBV-A (GenBank accession no. U22303), as well as of several isolates of GBV-C/HGV (data not shown). The sequences of the 3′-terminal 17 nt in this stretch are identical between the GBV-A and GBV-C/HGV genomes, with only one difference with GBV-B sequence. The 39-nt homology region spans the 3′-terminal stem-loop structures 3′-SL III and 3′-SL II in the GBV-B model; in particular, the stretch of 17 nt is arranged to form the 3′-SL II structure of all three GB viruses, as shown in Fig. 4 for GBV-A and GBV-B. This observation would suggest that similar roles are played by this structure in all GB viruses, including interaction with host proteins or viral factors necessary for viral replication, polyprotein translation, or viral genome encapsidation, as has been proposed for the 3′X region of HCV (5, 12–14, 36). Even more interesting is the observation that, in spite of the lack of sequence homology, the 3′-SL I of the GBV-B structure is remarkably similar to the analogous structure in the HCV genome (2, 11, 13, 33, 34), particularly for features such as the distance between the two unpaired nucleotides in the stem and the lack of unpaired nucleotides at the stem terminus. This finding may suggest that the 3′-SL I structures are functionally equivalent in the two viruses. In experimental infections of chimpanzees with HCV RNA, infectivity has been obtained only with RNA genomic molecules transcribed from clones including the 3′X fragment (14, 39), whose sequence is extremely conserved among different HCV genotypes. Recent mutagenesis experiments (40) have also demonstrated that the presence of 3′-SL I is indispensable for obtaining an infectious HCV RNA. The difference in the putative secondary structures of 3′-SL I between HCV and GBV-A might either reflect a real difference or indicate that the available GBV-A sequences are incomplete.
FIG. 4.
Comparison of the putative secondary structures of the 3′-end regions of GBV-A, GBV-B, and HCV encompassing the 3′ stem-loop structures (3′-SL I, 3′-SL II, and 3′-SL III). In 3′-SL II of GBV-A and GBV-B, identical nucleotides are indicated in white on circular black backgrounds. GBV-B and HCV structures start at the nucleotide following poly(U); GBV-A, lacking a poly(U) tract, starts at a position chosen to highlight the similarity with the GBV-B structure. RNA secondary structure prediction was performed by running the program Mfold of the Wisconsin Package, version 9.1 (Genetics Computer Group [GCG], Madison, Wis.). The output was produced by the program Plotfold of the GCG package.
Conclusions.
We have identified a novel sequence in the GBV-B genome located at its 3′ end. The sequence is present in GBV-B RNA extracted from the liver and serum of infected tamarins of different species, both in the genomic positive-strand RNA and in the replicative intermediate negative-strand RNA. This novel sequence might play roles analogous to those of the HCV 3′-end sequence as a region that is indispensable for viral replication and/or assembly processes. Based on this hypothesis, the inclusion of this sequence in a GBV-B genomic cDNA molecule might be essential for obtaining in vitro-transcribed RNA capable of infecting tamarins and for constructing viable chimeras between the HCV and GBV-B genomes. Experiments to assess these points are in progress.
Nucleotide sequence accession number.
The nucleotide sequence of the 3′ UTR of the GBV-B genome following the poly(U) tract has been deposited in the DDBJ/EMBL/GenBank database under accession no. Y18973.
Acknowledgments
We thank Ernst Verschoor for help with S. oedipus tissue samples. We are grateful to Riccardo Cortese, Raffaele De Francesco, Giovanni Galfré, Armin Lahm, Nicola La Monica, Giovanni Migliaccio, Giacomo Paonessa, and Alessandra Vitelli for helpful suggestions. We also thank Manuela Emili for artwork and Brenda McManus for revision of the manuscript.
REFERENCES
- 1.Alter H J. To C or not to C: these are the questions. Blood. 1995;85:1681–1695. [PubMed] [Google Scholar]
- 2.Blight K J, Rice C M. Secondary structure determination of the conserved 98-base sequence at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1997;71:7345–7352. doi: 10.1128/jvi.71.10.7345-7352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukh J, Apgar C L. Five new or recently discovered (GBV-A) virus species are indigenous to new world monkeys and may constitute a separate genus of the Flaviviridae. Virology. 1997;229:429–436. doi: 10.1006/viro.1997.8461. [DOI] [PubMed] [Google Scholar]
- 4.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 5.Chung R T, Kaplan L M. Heterogeneous nuclear ribonucleoprotein I (hnRNP-I/PTB) selectively binds the conserved 3′ terminus of hepatitis C viral RNA. Biochem Biophys Res Commun. 1999;254:351–362. doi: 10.1006/bbrc.1998.9949. [DOI] [PubMed] [Google Scholar]
- 6.Deinhardt F, Holmes A W, Capps R B, Popper H. Studies on the transmission of human viral hepatitis to marmoset monkeys. Transmission of disease, serial passages, and description of liver lesion. J Exp Med. 1967;125:673–687. doi: 10.1084/jem.125.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farci P, Alter H J, Govindarajan S, Wong D C, Engle R, Lesniewski R R, Mushahwar I K, Desai S M, Miller R H, Ogata N, et al. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 8.Farci P, Munoz S J, Shimoda A, Govindarajan S, Wong D C, Coiana A, Peddis G, Rubin R, Purcell R H. Experimental transmission of hepatitis C virus-associated fulminant hepatitis to a chimpanzee. J Infect Dis. 1999;179:1007–1011. doi: 10.1086/314653. [DOI] [PubMed] [Google Scholar]
- 9.Gallinari, P., et al. Unpublished data.
- 10.Honda M, Beard M R, Ping L-H, Lemon S. A phylogenetically conserved stem-loop structure at the 5′ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J Virol. 1999;73:1165–1174. doi: 10.1128/jvi.73.2.1165-1174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Lai M M. Determination of the secondary structure and of cellular protein binding to the 3′ untranslated region of the hepatitis C virus RNA genome. J Virol. 1997;71:8698–8706. doi: 10.1128/jvi.71.11.8698-8706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Tahara S M, Lai M M C. The 3′ untranslated region of hepatitis C virus RNA enhances translation from an internal ribosomal entry site. J Virol. 1998;72:8788–8796. doi: 10.1128/jvi.72.11.8789-8796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolykhalov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolykhalov A A, Agapov E V, Blight K J, Mihalik K, Feinstone S M, Rice C M. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 15.Lemon S M, Honda M. Internal ribosome entry sites within the RNA genomes of hepatitis C virus and other flaviviruses. Semin Virol. 1997;8:274–288. [Google Scholar]
- 16.Linnen J, Wages J, Zhang-Keck Z-Y, Fry K, Krawczynski K Z, Alter H, Koonin E, Gallacher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih J, Young L, Piatak M, Hoover C, Fernandez J, Chen S, Zou J C, Morris T, Hyams K C, Ismay S, Lifson J D, Hess G, Foung S K H, Thomas H, Bradley D, Margolis H, Kim J P. Molecular cloning and disease association of hepatitis G virus: a new transfusion transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 17.Major M E, Mihalik K, Fernandez J, Seidman J, Kleiner D, Kolykhalov A A, Rice C M, Feinstone S M. Long-term follow-up of chimpanzees inoculated with the first infectious clone for hepatitis C virus. J Virol. 1999;73:3317–3325. doi: 10.1128/jvi.73.4.3317-3325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muerhoff A S, Leary T P, Simons J N, Pilot-Matias T J, Dawson G J, Erker J C, Chalmers M L, Schlauder G G, Desai S M, Mushahwar I K. Genomic organization of GB viruses A and B: two new members of the Flaviviridae associated with GB agent hepatitis. J Virol. 1995;69:5621–5630. doi: 10.1128/jvi.69.9.5621-5630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health Consensus Panel. National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997;26(Suppl. 1):2S–10S. doi: 10.1002/hep.510260701. [DOI] [PubMed] [Google Scholar]
- 20.Ohba K, Mizokami M, Lau J Y N, Orito E, Ikeo K, Gojobori T. Evolutionary relationship of hepatitis C, pesti-, flavi-, plantiviruses, and newly discovered GB hepatitis agent. FEBS Lett. 1996;378:232–234. doi: 10.1016/0014-5793(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 21.Pilot-Matias T J, Muerhoff A S, Simons J N, Leary T P, Buijk S L, Chalmers M L, Erker J C, Dawson G J, Desai S M, Mushahwar I K. Identification of antigenic regions in the GB hepatitis viruses GBV-A, GBV-B, and GBV-C. J Med Virol. 1996;48:329–338. doi: 10.1002/(SICI)1096-9071(199604)48:4<329::AID-JMV6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. New York, N.Y: Raven Press; 1996. pp. 931–960. [Google Scholar]
- 23.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, Choo Q L, Houghton M, Kuo G. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarselli E, Urbani A, Sbardellati A, Tomei L, De Francesco R, Traboni C. GB virus B and hepatitis C virus NS3 serine proteases share substrate specificity. J Virol. 1997;71:4985–4989. doi: 10.1128/jvi.71.7.4985-4989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarselli, E., et al. Unpublished data.
- 28.Schlauder G G, Dawson G J, Simons J N, Pilot-Matias T J, Gutierrez R A, Heynen C A, Knigge M F, Kurpiewsky G S, Buijk S L, Leary T P, Muerhoff A S, Desai S M, Mushahwar I K. Molecular and serological analysis in the transmission of the GB hepatitis agents. J Med Virol. 1995;46:81–90. doi: 10.1002/jmv.1890460117. [DOI] [PubMed] [Google Scholar]
- 29.Schlauder G G, Pilot-Matias T J, Gabriel G S, Simons J N, Muerhoff A S, Dawson G J, Mushahwar I K. Origin of GB-hepatitis viruses. Lancet. 1995;346:447–448. doi: 10.1016/s0140-6736(95)92821-9. [DOI] [PubMed] [Google Scholar]
- 30.Simons J N, Leary T P, Dawson G J, Pilot-Matias T J, Muerhoff A S, Schlauder G G, Desai S M, Mushahwar I K. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 31.Simons J N, Pilot-Matias T J, Leary T P, Dawson G J, Desai S M, Schlauder G G, Muerhoff A S, Erker J C, Buijk S L, Chalmers M L, Van Sant C L, Mushahwar I K. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci USA. 1995;92:3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons J N, Desai S M, Mushahwar I K. The GB viruses: isolation, characterization, diagnosis and epidemiology. Viral Hepatitis Rev. 1996;2:229–246. [Google Scholar]
- 33.Tanaka T, Kato N, Cho M-J, Shimotohno K. A novel sequence found at 3′ terminus of hepatitis C virus genome. Biochem Biophys Res Commun. 1995;215:744–749. doi: 10.1006/bbrc.1995.2526. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka T, Kato N, Cho M J, Sugiyama K, Shimotohno K. Structure of the 3′ terminus of the hepatitis C virus genome. J Virol. 1996;70:3307–3312. doi: 10.1128/jvi.70.5.3307-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomei, L., I. Incitti, R. De Francesco, and C. Traboni. Unpublished data.
- 36.Tsuchihara K, Tanaka T, Hijikata M, Kuge S, Toyoda H, Nomoto A, Yamamoto N, Shimotohno K. Specific interaction of polypyrimidine tract-binding protein with the extreme 3′-terminal structure of the hepatitis C virus genome, the 3′X. J Virol. 1997;71:6720–6726. doi: 10.1128/jvi.71.9.6720-6726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner A, Erickson A L, Kansopon J, Crawford K, Muchmore E, Hughes A L, Houghton M, Walker C M. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci USA. 1995;92:2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Hepatitis C: global prevalence. Weekly Epidemiol Rec. 1997;72:341–344. [PubMed] [Google Scholar]
- 39.Yanagi M, Purcell R H, Emerson S U, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanagi M, St. Claire M, Emerson S U, Purcell R H, Bukh J. In vivo analysis of the 3′ untranslated region of the hepatitis C virus after in vitro mutagenesis of an infectious cDNA clone. Proc Natl Acad Sci USA. 1999;96:2291–2295. doi: 10.1073/pnas.96.5.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuckerman A J. The new GB hepatitis viruses. Lancet. 1995;345:1453–1454. doi: 10.1016/s0140-6736(95)91032-8. [DOI] [PubMed] [Google Scholar]