Abstract
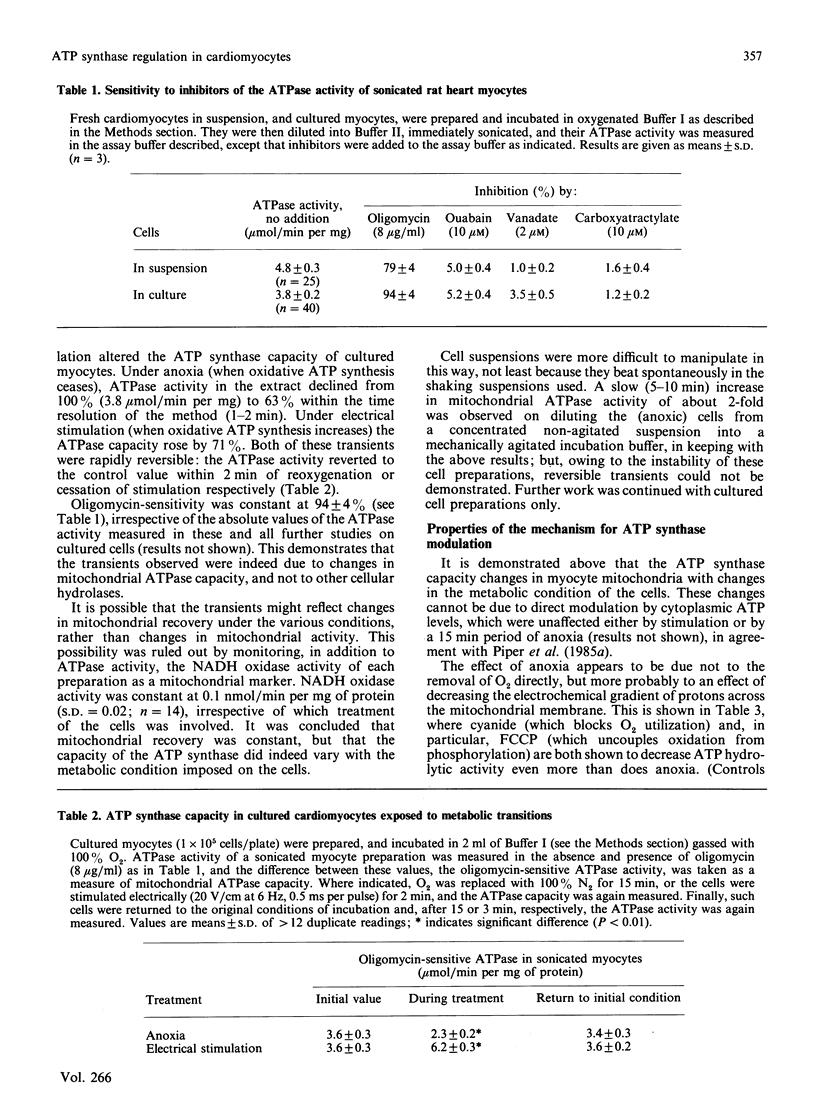
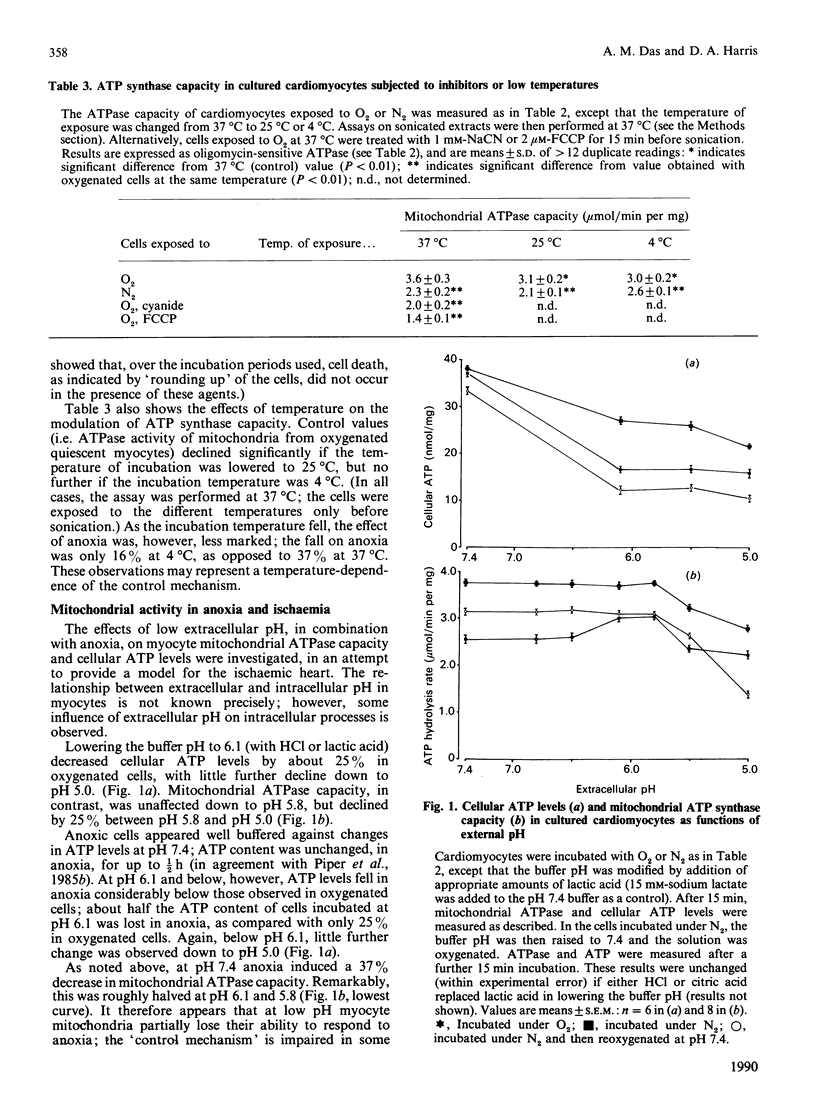
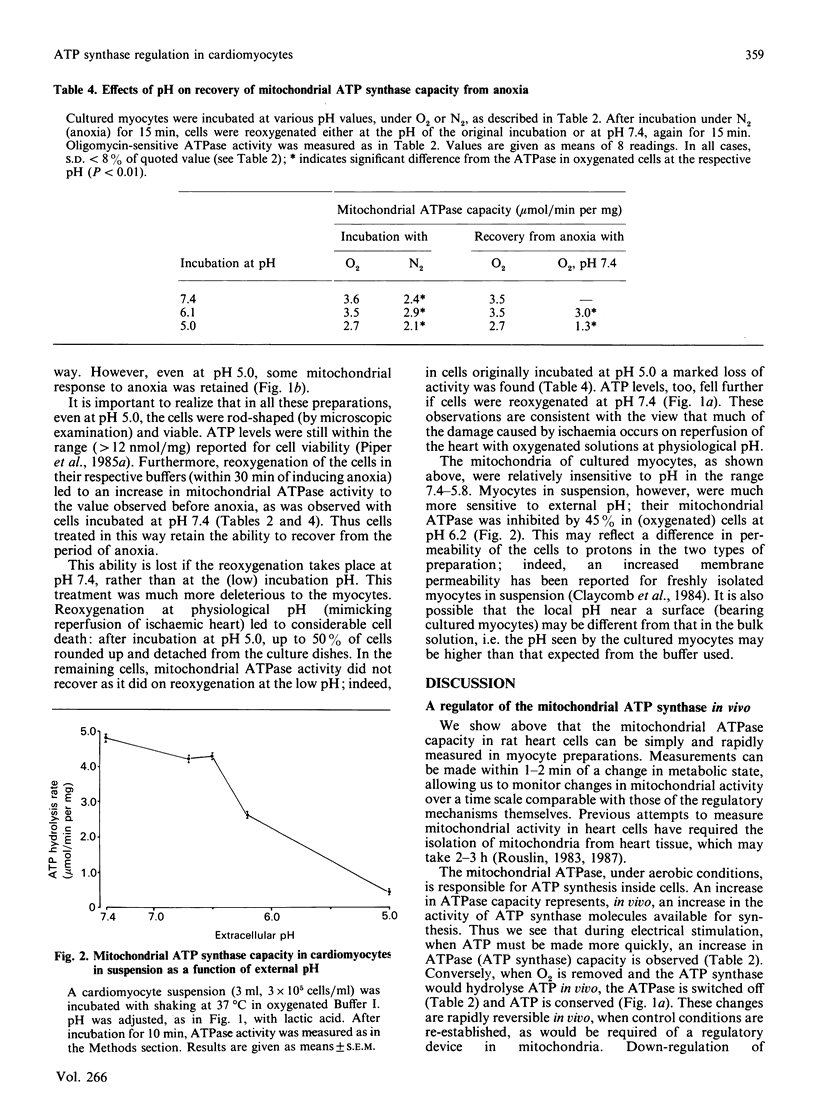
The ATP synthase capacity of rat heart myocytes can be measured in sonicated cell suspensions and in sonicated preparations of cultured cardiomyocytes. This procedure allows the rapid measurement of mitochondrial function in response to changes in the metabolic status of the cell. In cultured myocytes, transitions in ATP synthase capacity (with no detectable change in cellular ATP concentration) accompany a change to anoxia or electrically stimulated contraction (rise of 70%). These changes are reversed on returning to the original conditions. Exposure of myocytes to low pH has little effect on basal ATP synthase capacity (down to values less than pH 6), but markedly affects cellular ATP levels and the response of the cells to anoxia and reoxygenation, possibly mimicking changes seen in ischaemic heart. Similar effects are seen in suspensions of freshly prepared myocytes, but these preparations are less stable and more pH-sensitive than are cells in culture. It is proposed that mitochondria in vivo are directly regulated at the level of the ATP synthase, and that a regulator protein, the naturally occurring inhibitor protein from mitochondria, may be responsible for this regulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta D., Li C. P. Actions of extracellular acidosis on primary cultures of rat myocardial cells deprived of oxygen and glucose. J Mol Cell Cardiol. 1980 Dec;12(12):1459–1463. doi: 10.1016/0022-2828(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bing O. H., Brooks W. W., Messer J. V. Heart muscle viability following hypoxia: protective effect of acidosis. Science. 1973 Jun 22;180(4092):1297–1298. doi: 10.1126/science.180.4092.1297. [DOI] [PubMed] [Google Scholar]
- Cintrón N. M., Pedersen P. L. A protein inhibitor of the mitochondrial adenosine triphosphatase complex of rat liver. Purification and characterization. J Biol Chem. 1979 May 10;254(9):3439–3443. [PubMed] [Google Scholar]
- Claycomb W. C., Burns A. H., Shepherd R. E. Culture of the terminally differentiated ventricular cardiac muscle cell. Characterization of exogenous substrate oxidation and the adenylate cyclase system. FEBS Lett. 1984 Apr 24;169(2):261–266. doi: 10.1016/0014-5793(84)80330-0. [DOI] [PubMed] [Google Scholar]
- Cobbe S. M., Poole-Wilson P. A. The time of onset and severity of acidosis in myocardial ischaemia. J Mol Cell Cardiol. 1980 Aug;12(8):745–760. doi: 10.1016/0022-2828(80)90077-2. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Kaltenbach J. P. Oxygen-induced enzyme release: early events and a proposed mechanism. J Mol Cell Cardiol. 1979 Apr;11(4):389–406. doi: 10.1016/0022-2828(79)90425-5. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Slater E. D. Tightly bound nucleotides of the energy-transducing ATPase of chloroplasts and their role in photophosphorylation. Biochim Biophys Acta. 1975 May 15;387(2):335–348. doi: 10.1016/0005-2728(75)90114-0. [DOI] [PubMed] [Google Scholar]
- Harris D. A., von Tscharner V., Radda G. K. The ATPase inhibitor protein in oxidative phosphorylation. The rate-limiting factor to phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1979 Oct 10;548(1):72–84. doi: 10.1016/0005-2728(79)90188-9. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. Contracture in isolated adult rat heart cells. Role of Ca2+, ATP, and compartmentation. Circ Res. 1981 Nov;49(5):1119–1128. doi: 10.1161/01.res.49.5.1119. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger J. A., Grotyohann L. W., Neely J. R. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978 Jun 25;253(12):4310–4318. [PubMed] [Google Scholar]
- Kübler W., Spieckermann P. G. Regulation of glycolysis in the ischemic and the anoxic myocardium. J Mol Cell Cardiol. 1970 Dec;1(4):351–377. doi: 10.1016/0022-2828(70)90034-9. [DOI] [PubMed] [Google Scholar]
- Lippe G., Sorgato M. C., Harris D. A. The binding and release of the inhibitor protein are governed independently by ATP and membrane potential in ox-heart submitochondrial vesicles. Biochim Biophys Acta. 1988 Mar 30;933(1):12–21. doi: 10.1016/0005-2728(88)90051-5. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+. Biochem J. 1984 Feb 15;218(1):235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Ferrari R., Poole-Wilson P. A., Yepez C. E. A protective effect of a mild acidosis on hypoxic heart muscle. J Mol Cell Cardiol. 1979 Oct;11(10):1053–1071. doi: 10.1016/0022-2828(79)90394-8. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Das A. The role of fatty acids in ischemic tissue injury: difference between oleic and palmitic acid. Basic Res Cardiol. 1986 Jul-Aug;81(4):373–383. doi: 10.1007/BF01907458. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Schwartz P., Spahr R., Hütter J. F., Spieckermann P. G. Absence of reoxygenation damage in isolated heart cells after anoxic injury. Pflugers Arch. 1984 May;401(1):71–76. doi: 10.1007/BF00581535. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosing J., Harris D. A., Kemp A., Jr, Slater E. C. Nucleotide-binding properties of native and cold-treated mitochondrial ATPase. Biochim Biophys Acta. 1975 Jan 31;376(1):13–26. doi: 10.1016/0005-2728(75)90201-7. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Factors affecting the loss of mitochondrial function during zero-flow ischemia (autolysis) in slow and fast heart-rate hearts. J Mol Cell Cardiol. 1988 Nov;20(11):999–1007. doi: 10.1016/0022-2828(88)90577-9. [DOI] [PubMed] [Google Scholar]
- Rouslin W. Protonic inhibition of the mitochondrial oligomycin-sensitive adenosine 5'-triphosphatase in ischemic and autolyzing cardiac muscle. Possible mechanism for the mitigation of ATP hydrolysis under nonenergizing conditions. J Biol Chem. 1983 Aug 25;258(16):9657–9661. [PubMed] [Google Scholar]
- Rouslin W. The mitochondrial adenosine 5'-triphosphatase in slow and fast heart rate hearts. Am J Physiol. 1987 Mar;252(3 Pt 2):H622–H627. doi: 10.1152/ajpheart.1987.252.3.H622. [DOI] [PubMed] [Google Scholar]
- Senior A. E. ATP synthesis by oxidative phosphorylation. Physiol Rev. 1988 Jan;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]


