Abstract
Pancreatic cystic lesions (PCLs) pose a diagnostic challenge due to their increasing incidence and the limitations of cross-sectional imaging and endoscopic-ultrasound-guided fine-needle aspiration (EUS-FNA). EUS-guided through the needle biopsy (EUS-TTNB) has emerged as a promising tool for improving the accuracy of cyst type determination and neoplastic risk stratification. EUS-TTNB demonstrates superior diagnostic performance over EUS-FNA, providing critical preoperative information that can significantly influence patient management and reduce unnecessary surgeries. However, the procedure has risks, with an overall adverse event rate of approximately 9%. Preventive measures and further prospective studies are essential to optimize its safety and efficacy. This review highlights the potential of EUS-TTNB to enhance the diagnostic and management approaches for patients with PCLs. It examines the current state of EUS-TTNB, including available devices, indications, procedural techniques, specimen handling, diagnostic yield, clinical impact, and associated adverse events.
Keywords: mucinous cystic neoplasm, EUS-FNA, pancreatic cancer, cystic fluid analysis, intraductal papillary mucinous neoplasm (IPMN), neuroendocrine tumor
1. Introduction
Pancreatic cystic lesions (PCLs) represent a significant burden for health care systems. Their diagnosis is increasing, with a pooled prevalence of 8% in a recent meta-analysis [1] and an incidence of 12.9% in a population-based study over a period of 5-year follow-up [2], explained by incidental detection in cross-sectional imaging [3,4]. Other drivers may also have a role, as the growth in new diagnoses far outpaces the growth of imaging use [5].
The spectrum of PCLs comprises lesions with no malignant potential, like inflammatory pseudocysts and serous cystadenoma (SCA) that need no resection or surveillance (unless symptomatic) to malignant lesions, like solid pseudopapillary neoplasm and cystic neuroendocrine tumors (cNETs), that benefit from surgery. Between these categories lies the group of mucin-producing lesions like intraductal papillary mucinous neoplasm (IPMNs) and mucinous cystic neoplasm (MCN) with different grades of dysplasia that require surveillance or resection. The majority of incidental PCLs are neoplastic, mostly IPMNs that have risk of malignant transformation [2].
International guidelines [6,7,8] are based on knowledge of specific cyst types, but cross-sectional imaging alone has limited accuracy for PCLs subtyping and risk stratification [9,10]. Moreover, despite the use of endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (EUS-FNA) with cyst fluid analysis and cytology [11,12,13], misdiagnosis is still common among resected lesions [14]. As a consequence, unnecessary surgery rate is still high, carrying a significant risk of morbidity and mortality, even in expert centers [15].
New EUS-based innovations have been developed to overcome these limitations. In particular, EUS-guided through needle biopsy (EUS-TTNB) has gained interest in the past few years as an important tool for cyst type determination and neoplastic cyst risk stratification [16,17,18]. Several systematic reviews and meta-analyses revealed higher diagnostic performance of EUS-TTNB when compared with EUS-FNA [19,20,21,22,23]. However, safety concerns [24] and questions regarding patient selection [25,26], as well as doubts about the procedure’s clinical impact [27,28], have prevented its widespread adoption among endoscopists.
In this review, we aimed to summarize several aspects regarding using EUS-TTNB to evaluate PCLs, namely the available devices, indications, technical tips, specimen handling, and adverse events. The literature search was conducted using Medline and Embase electronic databases until April 2024. Text words and MeSH terms (pancreatic cyst, pancreatic intraductal neoplasm, cystadenoma, mucinous, endoscopic-ultrasound-guided fine needle aspiration) were used in combination with through-the-needle biopsy, EUS-guided microforceps biopsy, and Moray microforceps. Cross-references were identified manually through the citation list of selected articles to capture additional sources.
2. Devices
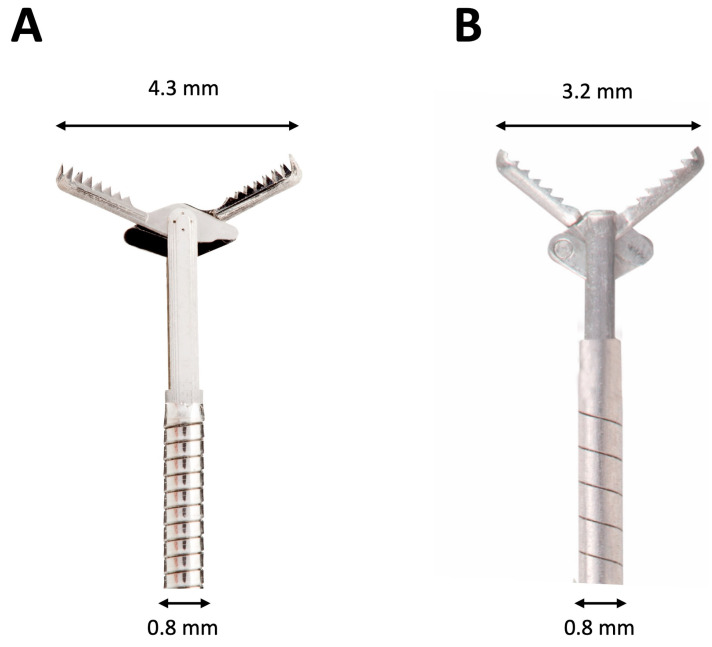
The first two cases of EUS-TTNB of PCLs were described in 2010 by Aparicio et al. in a pilot study using a 0.8 mm diameter forceps (Lumenis Surgical, Santa Clara, CA, USA) [29]. More recently, the Moray™ microforceps (Steris, Mentor, OH, USA) was developed specifically to target pancreatic cysts [30]. The sheath diameter is 0.8 mm, and the opening width of its toothed jaws is 4.3 mm. Its use has been described in several, mostly retrospective, observational studies [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. A third new microforceps, the Micro Bite™ (MTW Endoskopie Manufakture, Wesel, Germany), was developed and used in 25 EUS-TTNB procedures by Stigliano et al. in a retrospective study published in 2021 [46]. The shape of the forceps is oval and toothed with a spoon-shaped mouth and a diameter of 0.8 mm (Figure 1).
Figure 1.
Image depicting key differences between the two currently available microforceps for endoscopic ultrasound through-the-needle biopsy. (A) Moray™ microforceps (Steris, Mentor, OH, USA); (B) Micro Bite™ microforceps (MTW Endoskopie Manufakture, Wesel, Germany).
These devices are passed through the lumen of a standard 19-gauge FNA needle, allowing the sampling of cyst wall, septa, or mural nodules for histologic analysis of cyst epithelium and stroma [16,38,47].
3. Indications to EUS-TTNB and Patient Selection
Computed tomography and magnetic resonance imaging are imperfect for cyst-type determination and differentiation [9,10,48]. Moreover, EUS-FNA cytology is hindered by the low cellularity of the samples [49,50,51], and cyst fluid CEA levels were reported to have sub-optimal accuracy for mucinous cyst diagnosis and do not correlate with the grade of dysplasia [52,53].
EUS-TTNB adoption is far from widespread [54], and indications of its use are being refined [25,27]. The most consensual indication is for the morphologically indeterminate cystic lesion when its nature cannot be determined by imaging and EUS-FNA (cytology and cyst fluid chemistry). Determining specific cyst types frequently has important implications for patient management. Indeed, the treatment and follow-up of many different cysts are based on the histologic subtype, regardless of the presence of worrisome features. EUS-TTNB has been shown to allow accurate preoperative diagnosis of SCA, solid pseudopapillary neoplasia, cNETs [55], MCN [40,56], and other rare cysts [57,58].
As per international evidence-based guidelines, IPMNs with worrisome features indicate EUS with or without cyst sampling [6,7,8]. For mucinous cysts, especially IPMNs, which comprise the majority of incidental pancreatic cysts, EUS-TTNB may be used for risk stratification, as clinical and morphological risk factors in the form of worrisome features, are associated with unnecessary surgery in a significant number of patients [59,60]. In fact, EUS-TTNB allows the determination of the grade of dysplasia in mucinous lesions with improved detection of advanced neoplasia [40,42] and good concordance with surgical specimens for the histologic grade [20,21]. However, clinicians must be aware of the possibility of inhomogeneous distribution of dysplasia within the same lesion, which may result in sampling error. Moreover, the EUS-TTNB trajectory is limited by the FNA needle entry point in the cyst [34], and sometimes, it is impossible to target the most suspected region of the cysts.
EUS-TTNB has also shown in several studies to allow preoperative IPMN subtyping based on histologic morphology and mucin expression [32,42,45]. This issue could be very important for decision-making and risk stratification [25] since phenotype was recently associated with the natural history and malignancy risk in IPMNs [61]. However, considering the intracystic variability of IPMN subtypes [62], further studies should assess the reliability of IPMN subtyping and expression of mucins on TTNB samples compared with surgical pathology as well as the interobserver agreement among pathologists was found to be moderate for IPMN subtyping on surgical specimens [63].
Several authors reporting on the molecular analysis of pancreatic cyst fluid identified distinct mutational profiles for different PCLs, and recently, Paniccia et al. also shown prospectively that for mucinous cysts, next-generation sequencing analysis (NGS) allows the determination of risk for advanced neoplasia [64].
In addition to cyst fluid molecular analysis, it has also been demonstrated that EUS-TTNB specimens are also suitable for NGS in a small series of 23 specimens published in 2019 [65]. The same group recently published a larger study, including 91 specimens submitted to NGS. Adequacy for NGS was 82.4%. Sensitivity and specificity values of 83.7% and 81.8%, respectively, were demonstrated as for diagnosing a mucinous cyst and 87.2% and 84.6% for diagnosing an IPMN. Therefore, these preliminary data suggest that EUS-TTNB could be used for combined histologic and molecular diagnosis, with a potential advantage for risk stratification [66].
Overall, EUS-TTNB is especially useful when a definitive diagnosis of the nature of the cyst is critical to determine the patient’s management, with the ultimate goal of reducing the rate of inappropriate resections or modifying the follow-up protocol [25]. However, to obtain a satisfying result, patient selection is crucial. In fact, Kovacevic et al. performed a prospective study on 101 unselected patients (all patients with a cyst larger than 15 mm were enrolled) [42]. They reported a clinical impact of 12% (10 follow-up discontinuation and two mucinous cyst diagnoses) at the cost of a 10% adverse event rate [42]. EUS-TTNB offers the chance to evaluate a micro histological sample of the cyst wall. Therefore, the greater advantage of the procedure is gained when the histotype of the cyst is unknown, and the “name” of the cyst changes its management. For example, MCN or cNET is almost always indicated for resection, whereas SCA is not. This situation typically happens in the case of unilocular/oligocystic PCL. This cyst “morphology” encompasses numerous cysts, including IPMN, MCN, SCA, or rare conditions, such as cNET [55] or schwannomas [67,68]. Therefore, EUS-TTNB should be used to answer the question “What is this cyst?”, but only if the answer can modify the decision-making process [25]. Indications and contraindications of the technique are summarized in Table 1.
Table 1.
Main indications and contraindications to endoscopic ultrasound through-the-needle biopsy.
| EUS-TTNB | Main | Secondary |
|---|---|---|
| Indications | Morphologically indeterminate lesion after MRI and EUS-FNA, when knowledge of cyst type may change the management | IPMN risk stratification (grading of dysplasia) |
| IPMN subtyping * | ||
| Molecular analysis | ||
| Contraindications | When the results will not change patient management | Cyst size < 15 mm Cyst in communication with the main pancreatic duct (e.g., IPMN **) |
* Based on morphology and mucin expression. ** Needs proper patient selection as IPMN is associated with a higher adverse event rate. EUS-TTNB, endoscopic-ultrasound-guided through the needle biopsy; MRI, magnetic resonance imaging; EUS-FNA, endoscopic-ultrasound-guided fine-needle aspiration; IPMN, intraductal papillary mucinous neoplasm.
4. Technique
The EUS-TTNB procedure is not standardized, contributing to the high heterogeneity among the included studies in two recently published meta-analyses [19,20]. Unfortunately, a few studies have included a detailed description of the technical steps of the procedure, and several technique variations have been described [69]. In general, the cyst is punctured with the needle, the microforceps are advanced into the cyst, and the jaws are opened by the assistant in a similar fashion to standard endoscopic forceps. Then, the forceps are gently pushed against the cyst wall and closed. It is suggested to wait a few seconds before retrieving the forceps to guarantee complete closure of the jaws. If the cyst wall has been properly secured, the wall is retracted toward the needle tip, and the so-called “tent sign” is observed [34]. Observed “tenting” of the cyst wall is a good predictor of an adequate sample. In most cases, it is possible to withdraw the micro forceps from the needle, keeping the latter in the cyst. However, if the volume of the grabbed specimen is too large, passing the forceps jaws backward into the needle is impossible. Therefore, retrieving the needle and the micro forceps together may be necessary. Alternatively, the cyst wall may be released, and another bite is performed (Figure 2).
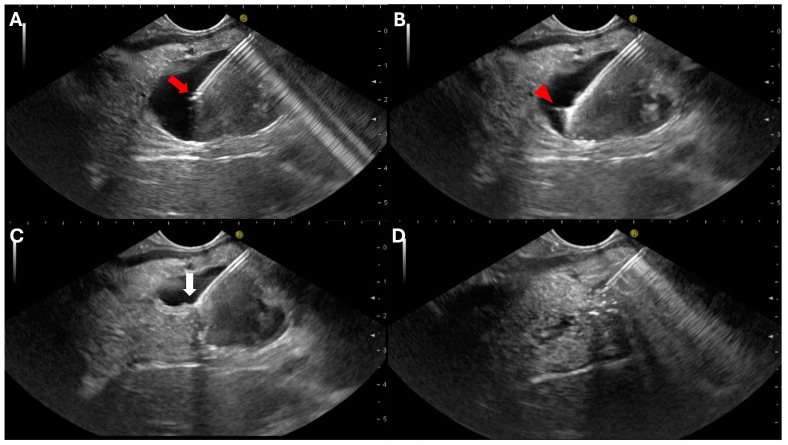
Figure 2.
Endoscopic-ultrasound-guided through-the-needle biopsy technique. (A) A large unilocular pancreatic cyst is punctured using a 19 G needle (red arrow: needle tip); (B) The microforceps is advanced through the needle into the cyst (red arrowhead: microforceps jaws; (C) The cyst wall is grabbed by the microforceps jaws and pulled until the “tent sign” (white arrow) is observed; (D) The cyst is completely aspirated.
The technical success, measured by the ability to obtain a macroscopically visible specimen, is reported to be very high [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. The small number of technical failures was associated with unfavorable scope positions that may be overcome by using more flexible needles made of nitinol [69].
Most of the variations in relation to the technique concern timing for cyst fluid aspiration, forceps preloading, number of passes, bites per pass, number of collected specimens, and specimen handling [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
The majority of the authors describe performing TTNB before cyst fluid aspiration [40,42,45,46], and some reports describe the possibility of performing biopsies with a collapsed cyst after aspiration [30]. A third possibility is to partially aspirate the cyst, perform TTNB, and finally, completely aspirate the cyst [36,38]. To safely perform TTNB, one should ensure enough space for forceps manipulation inside the cyst and that the accessory is visible on the ultrasound image throughout the procedure. This may justify the fact that most experts choose to perform cyst fluid aspiration after biopsies. In fact, the complete aspiration of the cyst before TTNB may require saline injection through the needle for cyst re-expansion. A potential but unexplored advantage of performing TTNB before fluid aspiration is the chance that forceps manipulation results in cell exfoliation into the fluid, leading to a higher sensitivity of fluid cytology [45]. On the other hand, TTNB often causes intracystic bleeding that could contaminate the fluid for biochemical and molecular diagnostics or even result in the impossibility of aspirating the cyst. Therefore, it seems that the initial partial aspiration of the cyst can ensure some “clean” fluid to analyze while completing the aspiration of the cyst after TTNB provides fluid for cytology. Another advantage of performing partial aspiration of the cyst before TTNB is that it reduces the tension of the cystic wall, favoring the grab of the tissue by the forceps’ jaws (Figure 3).
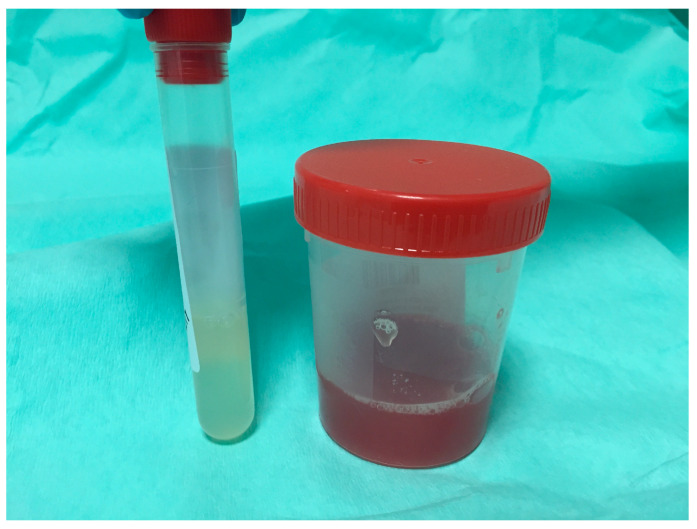
Figure 3.
The appearance of cyst fluid aspirated from the same mucinous cyst before (small vial on the left) and after (larger container on the right) endoscopic-ultrasound-guided through the needle biopsy (EUS-TTNB). The fluid aspirated before EUS-TTNB is clean and suitable for biochemical and molecular analyses. After EUS-TTNB, the intracystic bleeding contaminates the fluid that is, however, sent for cytological examination.
Forceps preloading in the needle, keeping the distal end of the forceps a few mm inside the needle tip, may avoid cyst wall collapse after the puncture, which would reduce the space for its manipulation inside the lesion, especially in smaller lesions, due to the fluid suction into the needle lumen after stylet removal [32,41,45,46]. A possible disadvantage of forceps preloading is that it reduces the needle/forceps bundle flexibility, which could make the cyst puncture more difficult. On the other hand, when the scope is torqued, as occurs in the second part of the duodenum, it may be difficult to advance the micro forceps through the needle due to resistance from within a bent needle. If resistance is experienced, a slightly gentle movement of opening and closing the forceps may reduce the friction and help overcome this issue. Still, this issue needs further evaluation in future studies.
Regarding the number of passes, a systematic review described that a mean of 3.1 forceps passes is needed to obtain adequate histologic samples [19]. Still, more importantly, we should guarantee the acquisition of two fragments with a minimum number of forceps passes since, as described in the study by Crinò et al., two macroscopically visible specimens are enough to guarantee an adequate histological evaluation and a greater number of passes may increase the risk of adverse events [38]. Nevertheless, this topic should be further explored because a more recent study found that a smaller (<4 vs. ≥4) number of visible specimens is associated with diagnostic failure [44]. In contrast, other reports failed to show a significant correlation between the number of specimens and histological diagnosis adequacy [45,46]. These data should also be interpreted taking into account specimen handling and processing and pathologist experience, which may impact diagnostic yield [70]. Additionally, denuded epithelium in the cyst wall may justify difficulties in obtaining adequate samples even with many passes or fragments [47,56].
The number of forceps passes and acquired specimens should be systematically included in the EUS report for future studies to further explore the correlation between specimen number and diagnostic accuracy and between the number of passes and adverse event risk.
Finally, the number of bites per pass remains unstandardized. Most authors performed one bite for each pass [33,34,38,39,41,44,46], whereas others performed two to three bites [36,40,43]. In our experience, the most important predictor of an adequate specimen is the “tent” sign described above. Table 2 summarizes the technical aspects of the TTNB procedure described in the most relevant studies.
Table 2.
Technical variations in published studies concerning endoscopic-ultrasound-guided through-the-needle biopsy.
| First Author, Year | Forceps Preloading | Passes | Bite per Pass | Timing for Cyst Fluid Aspiration | Target Number of Specimen | Antibiotic Prophylaxis |
|---|---|---|---|---|---|---|
| Basar et al., 2018 [31] | No | Until seemed adequate for histologic analysis | N/A | Before | N/A | Yes (broad-spectrum antibiotics, prior to the procedure) |
| Kovacevic et al., 2018 [32] | No | N/A | N/A | After | N/A | N/A |
| Mittal et al., 2018 [33] | No | 3–4 passes (until 3–4 macroscopically visible specimens) | One | After | 3–4 macroscopically visible specimens | Yes (ampicillin-sulbactam or ciprofloxacin, prior to the procedure, continued 3–5 days) |
| Barresi et al., 2018 [34] | No | At the endoscopist’s discretion | One | Before + after full aspiration | N/A | Yes (piperacillin-tazobactam or ceftriaxone or ciprofloxacin, prior to the procedure, continued 3–5 days post-procedure #) |
| Zhang et al., 2018 [35] | No | Until seemed macroscopically adequate | N/A | Before | N/A | N/A |
| Yang et al., 2018 [36] | No | At the endoscopist’s discretion | 2–3 bites per pass | Before + after full aspiration | N/A | Yes (following ASGE guidelines) |
| Cheesman et al., 2019 [37] | No | At the endoscopist’s discretion | At the endoscopist’s discretion | After | N/A | Yes (quinolones *, during the procedure, continued 3–5 days) |
| Crinò et al., 2019 [38] | No | Until 3 visible specimens are obtained | One | Before | 3 visible specimens (each in a different vial) | Yes (ciprofloxacin or piperacillin-tazobactam, prior to the procedure, continued 5 days if intracystic bleeding or cyst not totally drained |
| Samarasena et al., 2019 [39] | Yes | 3–4 passes | One | After | 1 adequate specimen | N/A |
| Yang et al., 2019 [40] | No | 3 passes | 2–3 bites per pass | Before | 1 macroscopically visible specimen | Yes (following ASGE guidelines) |
| Hashimoto et al., 2019 [41] | No | 3–4 passes | One bite per pass | After | N/A | Yes (Cephazolin, prior to the procedure) |
| Kovacevic et al., 2021 [42] | At the endoscopist’s discretion | Maximum 4 passes | N/A | After | 2 macroscopically visible specimens | Yes (Cefuroxime 1500 mg) |
| Stigliano et al., 2021 [46] | Yes | As required to visible fragment | One bite per pass | After | A visible fragment | Yes (quinolones) |
| Robles-Medranda et al., 2022 [43] | No | N/A | 2–3 bites per pass | N/A | N/A | Yes |
| Cho et al., 2022 [44] | No | As required to obtain 3–4 visible specimen | One bite per pass | After | 3–4 visible fragments | Yes (3rd generation cephalosporins, prior to the procedure) |
| Vilas-Boas et al., 2022 [45] | Only for lesions ≤ 20 mm | As required to obtain 4 visible specimen | One bite per pass | After | 4 visible fragments | Yes (intravenous ciprofloxacin 200 mg, during the procedure) |
* Except for allergy (alternative gentamicin). # Except for one center. N/A, not disclosed.
5. Specimen Handling and Processing
The specimens obtained during TTNB are much smaller than fragments gathered using standard endoscopy forceps, thus challenging technicians and pathologists to find the best specimen handling and processing method [70,71]. Most authors describe the TNNB sample processing as routine histologic specimens (formalin fixation, paraffin embedding, section, and staining) [31,38,42,44,71]. Still, it is also possible to prepare a cell block [72,73] similar to cytology samples [34,45].
After the biopsy, the specimen is extracted from the jaws of the forceps using the included extraction pick or a needle with minimal manipulation and fixated in formalin. All the collected specimens are usually put in the same container, but the group of Verona and the Pathologists from Copenhagen suggest handling each specimen individually [70,74]. Crinò et al. also described using a customized paper–tissue complex. Each sample is embedded and trimmed separately, preventing tissue loss during handling and resulting in more homogenous tissue sections (Figure 4) [70]. This suggestion should be useful when handling all microfragments [74], even gathered during other procedures (e.g., cholangioscopy-guided biopsies). Table 3 summarizes the available tissue handling and processing possibilities.
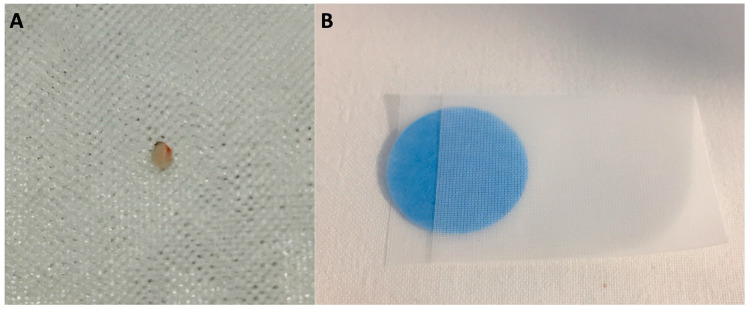
Figure 4.
Explanation of the customized handling for specimens collected with endoscopic-ultrasound-guided through-the-needle biopsy. (A) The sample is extracted from the microforceps’ jaws. (B) The specimen is enclosed between two colored discs of paper, thus creating a “sandwiched” paper–tissue complex that is secured into a gauze envelope and then placed into the formalin container.
Table 3.
EUS-TTNB specimen handling and processing.
| Advantages/Disadvantages | |||
|---|---|---|---|
| TTNB | Specimen handling | Each specimen in individual container and one paraffin block per specimen [70,74] | Avoids tissue loss when cutting because of difference in level between tissue fragments [70]. |
| All obtained specimens in the same jar. | Reduced processing time. | ||
| Customized paper–tissue complex. | Can be embedded in paraffin to minimize specimen handling; May become the standard for handling all types of small fragments. | ||
| Specimen processing | Same as routine histology specimens [31,38,42,71] | Formalin fixation, paraffin embedding, section and staining. Obtention of several sections for histology and immunohistochemical analysis [71]. | |
| Cell block [34,45] | Similarly to cytology samples. May minimize tissue loss when dealing with microfragments [45,73]. | ||
TTNB, through-the-needle biopsy.
Apart from establishing the number of specimens needed for an adequate diagnosis, in a paper published in 2019, Crinò et al. propose the assessment of the samples for the fulfillment of 4 histologic criteria (presence of cyst lining epithelium, differentiating mucinous from non-mucinous cysts, defining dysplasia grade, provide a specific cyst diagnosis) [38]. The grade of dysplasia is one of the most important features to report as it will impact management decisions [71]. Still, we must acknowledge that the degree of epithelial atypia may vary along the cyst epithelium, and TTNB may result in an underestimation of dysplasia [34,45,46]. Another important feature worth exploring is the fact that TTNB samples are suitable for immunohistochemical staining in the majority of cases [71] and allow for IPMN subclassification based on morphology and mucin expression [42,45]. There are important differences in invasive progression, recurrence risk, and prognosis between IPMN subtypes, so this information could affect decision-making [75,76,77,78].
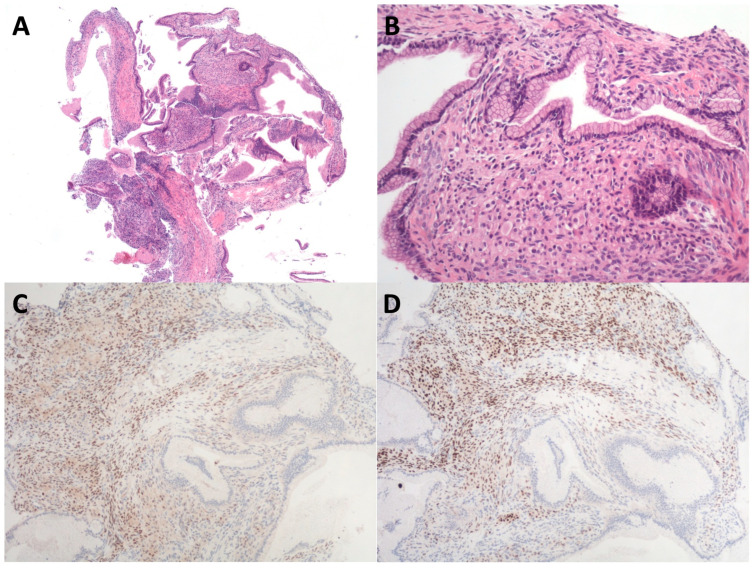
The material acquired with EUS-TTNB is not easy to retrieve and should be handled carefully to avoid wasting it. As reported by Rift et al., after specimen sections have been obtained from the paraffin block, the first and the last slides should be stained with hematoxylin and eosin [71]. According to the presence of epithelium, stroma, or both, and in relation to the specimen’s morphology, additional immunohistochemical analyses are performed to assess the final diagnosis [71]. A positive stain in the ovarian-like stroma of estrogen and progesterone receptors indicates the diagnosis of MCN. Positive periodic acid–Schiff (PAS) and inhibin are used to establish the diagnosis of SCA. Furthermore, the subepithelial capillary network can be visualized by staining for CD34 [71]. Importantly, the interobserver agreement among pathologists when describing TTNB specimens was found to be substantial in a study by Larghi et al. regarding specimen adequacy, presence of epithelium and grade of dysplasia, presence of ovarian stroma, and specific diagnosis [79]. Figure 5 shows an example of EUS-TTNB specimen.
Figure 5.
A typical example of histological evaluation of a specimen gathered with endoscopic-ultrasound-guided through-the-needle biopsy. (A) The sample is initially stained with hematoxylin and eosin and contains both epithelium and stroma. (B) At higher magnification, a single layer of mucinous epithelium covers the highly cellular stroma. (C) Stromal cells were estrogen- and (D) progesterone-receptor-positive, conclusive for the diagnosis of mucinous cystic neoplasm.
6. Diagnostic Yield and Clinical Impact
EUS-FNA has a disappointing performance in cyst type determination and malignancy risk stratification in the case of mucinous lesions [51,80], even if fine-needle biopsy needles are used [81,82]. EUS-TTNB revealed a higher diagnostic yield than EUS-FNA for identifying mucinous cysts in several cohort series [31,40,42], confirmed in several systematic reviews and meta-analyses [19,20,21,22,23]. Moreover, the diagnostic performance is not affected by cyst morphology, size, or location [19]. Table 4 summarizes the findings of available meta-analyses [19,20,21,22,23,83,84,85,86,87].
Table 4.
Main results on diagnostic yield of published meta-analyses.
| First Author, Year | Studies Included | Number of Patients | Technical Success (95%CI) | Diagnostic Outcomes (95%CI) | Comparison with Surgical Pathology |
|---|---|---|---|---|---|
| Faias et al., 2019 [83] | TTNB: 4 retrospective Molecular analysis: 2 retrospective 2 prospective |
TTNB: 148 Molecular analysis: 1058 |
TTNB: 93.2% Molecular analysis: 94.9% |
Diagnostic yield TTNB: 73.1% (61.4–82.2) Molecular analyses: 54.3% (49.8–58.7) Diagnosis of specific cyst type TTNB: 70.7% (49.4–85.6) Molecular analyses: 73.1% (61.6–82.2) |
NA |
| Facciorusso et al., 2020 [19] | 9 retrospective 2 prospective |
490 | NA | Sample adequacy 85.3% (78.2–92.5) OR vs. FNA cytology: 4.83 (1.63–14.31) Diagnostic accuracy 78.8% (73.4–84.2) OR vs. FNA cytology: 3.44 (1.32–8.96) |
Accuracy of 88.3% (80.1–96.5) in patients who underwent resection |
| Tacelli et al., 2020 [20] | 8 retrospective 1 prospective |
463 | 98.5% (97.3–99.6) | Histologic adequacy 86.7% (80.1–93.4) Diagnostic yield TTNB: 69.5% (59.2–79.7) FNA cytology: 28.7% (15.7–41.6) |
Concordance with surgical pathology: TTNB: 87.0% FNA cytology: 37.1% |
| Westerveld et al., 2020 [21] | 7 retrospective 1 prospective |
426 | 98.2% (96.8–99.3) | Diagnosis of specific cyst type TTNB: 72.5% (60.6–83.0) FNA cytology: 38.1% (18.0–60.5) OR: 9.37 (5.69–15.42) Diagnosis of mucinous cyst TTNB: 56.2% (45.1–67.0) FNA cytology: 29.5% (15.5– 45.9) OR: 3.86 (2.0–7.44) |
Concordance with surgical pathology: TTNB: 82.3% (71.9–90.7) FNA cytology: 26.8% (17.0–37.8) OR: 13.49 (3.49–52.29) |
| McCarty et al., 2020 [22] | 10 retrospective 1 prospective |
518 | 97.1% (93.7–98.7) | Diagnostic yield 79.6% (72.6–85.1) OR vs. FNA cytology: 4.79 (1.52–15.06) Diagnostic accuracy 82.8% (77.8–86.8) OR vs. FNA cytology: 8.69 (1.12–67.1) |
NA |
| Balaban et al., 2020 [84] | 8 retrospective 1 prospective |
463 | 98.5% (89–100) | Tissue acquisition 88.2% (79–97) Diagnostic accuracy 68.6% (61%–76) |
NA |
| Guzmán-Calderón et al., 2020 [85] | 7 retrospective 1 prospective |
423 | 95.6% (93.2–97.3) | Diagnosis of specific cyst type 74.6% (70.2–78.7) |
NA |
| Rift et al., 2021 [23] | 8 retrospective 2 prospective |
99 | NA | Sensitivity for detection of mucinous cysts TTNB: 86% (62–96) FNA cytology: 46% (35–57) Sensitivity for detection of high-risk cysts TTNB: 78% (61–89) FNA cytology: 38% (23–55) Sensitivity for a specific diagnosis TTNB: 69% (50–83) FNA cytology: 29% (21–39) |
All included patients underwent resection |
| Kovacevic et al., 2021 [86] | TTNB: 9 retrospective 2 prospective nCLE: 2 retrospective 7 prospective |
TTNB: 533 nCLE: 557 |
TTNB: 94% (94–98) nCLE: 99% (97–100) |
Diagnostic yield TTNB: 74% (69–78) nCLE: 85% (82–88) Sensitivity for detection of mucinous cysts TTNB: 80% (65–89) nCLE: 86% (69–94) |
Concordance with surgical pathology: TTNB: 82% (72–91) nCLE: 65% (36–91) |
| Gopakumar et al., 2024 [87] | 9 retrospective 2 prospective |
575 | 98.6% (97.5–99.4) | Sensitivity 76.6% (72.6–80.3) Specificity 98.9% (93.8–100) Diagnosis of malignant/pre-malignant cyst OR vs. non-malignant: 41.3 (17.4–98.1) |
NA |
TTNB: through-the-needle biopsy; OR: odds ratio; FNA: fine-needle biopsy; nCLE: needle-based confocal laser endomicroscopy; N/A, not disclosed.
Clinically, the ability to differentiate between specific cyst types has important implications for management. In fact, the diagnosis of SCA avoids the need for surgery or surveillance, except if the lesion is causing symptoms [42], and in the case of MCN, initial surveillance may be acceptable under certain circumstances [88]. EUS provides high-quality images and allows differential diagnosis in case of typical cyst morphology, such as SCA with honeycomb appearance or extrapancreatic lesions mimicking PCLs, such as gastric duplication cyst [89]. However, for MCN, the rate of preoperative misdiagnosis is around 20% based on clinical and imaging modalities [14,48]. Its distinction from IPMNs may be difficult as the connection between the cyst and the main pancreatic duct is sometimes difficult to determine. EUS-TTNB was reported to have a significant clinical impact, allowing the diagnosis of SCA (especially in the case of the diagnostically challenging oligocystic variant), leading to follow-up discontinuation and preoperative MCN detection by documentation of ovarian-type stroma [42,45,47,56].
Recently, in a study published by Chessman et al. that included 44 patients, the authors reported that the diagnostic yield for the combination of the current “composite standard” (morphology, cyst fluid cytology, and chemical analysis) and TTNB was higher (but not statistically significant) than that for each individual modality, which may prove to be clinically useful. The authors concluded that TTNB led to an overall change in clinical management in 39% of cases [37]. These changes included an increase in surveillance discontinuation, a reduction in the number of follow-up imaging and endoscopic studies, and the referral for surgery in 2 out of 28 patients who would have undergone further surveillance as per standard of care [37]. The clinical impact of EUS-TTNB on the management of PCLs should be further evaluated in future prospective studies.
Moreover, the possibility of combining EUS-TTNB with another through-the-needle technique, i.e., needle-based confocal laser endomicroscopy (nCLE) [90], should be considered. In fact, in the above-mentioned study by Cheesman et al. [37], adding EUS-TTNB and nCLE to standard evaluation based on cyst morphology and EUS-FNA increased the diagnostic yield by up to 93% compared to 75% and 84% for EUS-TTNB and nCLE alone, respectively [37]. This increased diagnostic yield reflected a higher clinical impact. Compared to standard evaluation, an overall change in clinical management in 38.6%, 43.2%, and 52.3% of cases was obtained by EUS-TTNB, nCLE, and their combination, respectively [37].
A meta-analysis compared EUS-TTNB with nCLE [86]. Diagnostic yield (defined as the possibility of obtaining a diagnosis) was higher with nCLE (85% vs. 74%, p < 0.0001). Still, the sensitivity and the specificity were similar (pooled sensitivity: 80% vs. 86% and pooled specificity: 80% vs. 83% for EUS-TTNB and nCLE, respectively), as were technical success and adverse event rates [86].
Furthermore, a meta-analysis [83] compared EUS-TTNB to molecular analyses of cyst fluid [91]. The diagnostic yield was higher with EUS-TTNB than with molecular analyses (73.1% vs. 54.3%, respectively), but the rates of correctly identified cysts were similar (70.7% vs. 73.1%, respectively). Similarly, the sensitivities and specificities of differentiating benign from low- or high-risk cysts were comparable (sensitivity: 73% vs. 75%; specificity: 88% vs. 72% for EUS-TTNB and molecular analyses, respectively) [83].
Finally, a network meta-analysis compared several EUS-based techniques for diagnosing PCLs, including contrast-enhanced harmonic EUS, fluid biochemical and molecular analyses, nCLE, and EUS-TTNB [92]. nCLE and EUS-TTNB had a significantly higher network ranking of the superiority index than other techniques for differentiating mucinous PCLs. EUS-TTNB also ranked first in identifying malignant PCLs [92].
7. Adverse Events
The overall adverse events rate ranges between 8.6% and 10.1% in different meta-analyses [19,20,21,22,23,83,84,85,86,87]. In the meta-analysis of Tacelli et al., the adverse event rate ranged widely from 1% to 23% [20]. In another meta-analysis, the pooled occurrence for intracystic bleeding was 5% (95% CI, 1.2–11.2), and that for acute pancreatitis was 2.3% (95% CI, 0.5–5.3) [21].
McCarty et al. reported a rate of severe adverse events of 1.08% (95% CI, 0.43–2.69) [22].
The most frequent adverse events are acute pancreatitis, bleeding (mainly intracystic), abdominal pain, and infection (Table 5).
Table 5.
Description of adverse events related to EUS-guided through-the-needle biopsy.
| First Author, Year | Overall AEs Rate | Bleeding | Acute Pancreatitis | Infection | Other |
|---|---|---|---|---|---|
| Basar et al., 2018 [31] | 4.8% | Self-limited intracystic bleeding (n = 1, 2.4%) | - | - | Mild abdominal pain (n = 1, 2.4%) |
| Kovacevic et al., 2018 [32] | 10.7% | - | Mild (n = 2, 7.1%) | - | Non-specific abdominal pain (n = 1, 3.6%) |
| Mittal et al., 2018 [33] | No AEs | - | - | - | - |
| Barresi et al., 2018 [34] | 16.1% | Self-limited intracystic bleeding (n = 7, 12.5%) | - | - | Mild abdominal pain (n = 2, 3.6%) Moderate-to-severe abdominal pain (n = 1, 1.8%) |
| Yang et al., 2018 [36] | 4.2% | Self-limited intracystic bleeding (n = 1, 2.1%) | Mild (n = 1, 2.1%) | - | - |
| Cheesman et al., 2020 [37] | 9.1% | Self-limited intracystic bleeding (n = 1, 2.3%) | - | Moderate-to-severe pseudocyst infection (n= 1, 2.3%) * | Mild abdominal pain (n = 2, 4.5%) |
| Crinò et al., 2019 [38] | 22.9% | Self-limited intracystic bleeding (n = 10, 16.4%) Self-limited peripancreatic bleeding (n = 1, 1.6%) |
Mild (n = 2, 3.3%) | Fever without evidence of infection (n = 1, 1.6%) | - |
| Samarasena et al., 2019 [39] | 6.7% | Self-limited intracystic bleeding (n = 1, 6.7%) | - | - | - |
| Yang et al., 2019 [40] | 12.3% | Self-limited intracystic bleeding (n = 7, 6.1%) | Mild (n = 5, 3.5%) Moderate-to-severe (n = 1, 0.7%) |
- | - |
| Hashimoto et al., 2019 [41] | 3.6% | - | Mild (n = 2, 3.6%) | - | - |
| Kovacevic et al., 2021 [42] | 9.9% | Self-limited intracystic on intraductal bleeding (n = 4, 4%) ° Mild bleeding in the lesser sac (n = 1, 1%) |
Mild-to-severe (n = 8, 8%) Fatal (n = 1, 1%) |
- | - |
| Robles-Medranda et al., 2022 [43] | No AEs | - | - | - | - |
| Cho et al., 2022 [44] | 6.7% | Self-limited intracystic bleeding (n = 1, 2.2%) | Mild acute pancreatitis (n = 2, 4.4%) | - | - |
| Vilas-Boas et al., 2022 [45] | 7.5% | Mild, self-limited intracystic bleeding (n = 2, 5%) | - | - | Mild abdominal pain (n = 1, 2.5%) |
| Stigliano et al., 2021 [46] | 10.2% | - |
n = 3 (6.1%) Mild acute pancreatitis (n = 2, 4.1%) Moderate-to-severe acute pancreatitis (n = 1, 2.0%) |
Infection (n = 2, 4.1%) | - |
| Facciorrusso et al., 2022 [24] | 11.5% | Self-limited intracystic bleeding (n = 10, 2.0%) | Mild acute pancreatitis (n = 9, 1.8%) Moderate-to-severe acute pancreatitis (n = 18, 3.6%), Fatal acute pancreatitis (n = 2, 0.4%) |
Mild Infection (n = 5, 1.0%) Moderate-to-severe Infection (n = 4, 0.8%) Fatal Infection (n = 1, 0.2%) |
Mild abdominal pain (n = 6, 1.2%) Peripancreatic collection without evidence of pancreatitis (n = 1, 0.2%) Hypotension (n = 1 (0.2%) Mass forming Xanthogranuloma (n = 1, 0.2%) |
The severity of adverse events (AEs) was graded according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon [93]. * Post-procedural admission; ° considered as incident according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon.
In a more recent multicenter retrospective study including more than 500 procedures, the reported overall AE rate was 11.5%, and a model to predict post-TTNB AE was created that included age, number of forceps passes, inability to completely aspirate the cyst, and diagnosis of IPMN [24]. In this study, 15 adverse events were moderate, 9 were severe, and there were 3 fatalities related to 2 acute pancreatitis and 1 sepsis with organ failure [24]. These fatalities were reported in the context of broadened inclusion criteria [25]. Intra-cystic bleeding is the most frequent event, but it is almost always self-limited, requiring no intervention, and should be considered an “incident” according to the ASGE lexicon [93]. As in ERCP [94], periprocedural hydration with Ringer’s lactate and rectal non-steroid anti-inflammatory drugs (NSAIDs) have been suggested to prevent acute pancreatitis, but the multicenter study by Facciorusso et al. [24] and the prospective study by Kovacevic et al. [42] failed to demonstrate a protective effect of rectal NSAIDs or intravenous hydration. However, despite not being statistically significant, Kovacevic at al. observed a reduction in adverse events rate (17.6% vs. 8.3%) after the implementation of perioperative hydration and rectal NSAID administration [24].
Regarding antibiotic prophylaxis, despite the recommendation of current guidelines [95], more recent evidence showed the lack of benefit from antibiotic prophylaxis after EUS-FNA [96,97] and EUS-TTNB [98]. However, the previously cited multicenter study concluded that it might be useful in the subgroup of patients submitted to TTNB with cysts that cannot be completely aspirated [24].
8. Limitations of the Study
This review has several limitations that result from the heterogeneity of EUS-TTNB studies, especially regarding the lack of standardization of the technique, the retrospective nature of most of the cited works, as well as their small sample size and follow-up duration. These issues limit the ability to assess long-term outcomes and the true impact of the technique on patient management and prognosis. Addressing these limitations through large-scale prospective studies would enhance the applicability of the findings in future research.
9. Conclusions
EUS-TTNB represents a significant advancement in the diagnostic evaluation of PCLs. By providing superior diagnostic accuracy compared to EUS-FNA, it has the potential to reduce unnecessary surgeries and optimize patient management. Despite its promise, EUS-TTNB is associated with a higher rate of adverse events than EUS-FNA. Continued refinement of the procedure and standardizing technique steps are essential to maximizing its benefits and safety. Future studies should focus on investigating technical aspects and on the evaluation of prophylactic measures.
Author Contributions
Conceptualization, F.V.-B. and S.F.C. writing—original draft preparation, F.V.-B. and S.F.C.; writing—review and editing, F.V.-B., T.R., M.C.C.B., N.D.P., J.D., J.S., S.S., E.M. and S.F.C.; supervision, G.M., A.F. and L.F. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zerboni G., Signoretti M., Crippa S., Falconi M., Arcidiacono P.G., Capurso G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19:2–9. doi: 10.1016/j.pan.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Kromrey M.L., Bülow R., Hübner J., Paperlein C., Lerch M.M., Ittermann T., Völzke H., Mayerle J., Kühn J.P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138–145. doi: 10.1136/gutjnl-2016-313127. [DOI] [PubMed] [Google Scholar]
- 3.Moris M., Bridges M.D., Pooley R.A., Raimondo M., Woodward T.A., Stauffer J.A., Asbun H.J., Wallace M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol. Hepatol. 2016;14:585–593.e3. doi: 10.1016/j.cgh.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Soroida Y., Sato M., Hikita H., Hagiwara S., Sato M., Gotoh H., Kato S., Iwai T., Yamazaki T., Yatomi Y., et al. Pancreatic cysts in general population on ultrasonography: Prevalence and development of risk score. J. Gastroenterol. 2016;51:1133–1140. doi: 10.1007/s00535-016-1196-y. [DOI] [PubMed] [Google Scholar]
- 5.Schweber A.B., Agarunov E., Brooks C., Hur C., Gonda T.A. Prevalence, Incidence, and Risk of Progression of Asymptomatic Pancreatic Cysts in Large Sample Real-world Data. Pancreas. 2021;50:1287–1292. doi: 10.1097/MPA.0000000000001918. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka T., Fernandez-Del Castillo C., Furukawa T., Hijioka S., Jang J.Y., Lennon A.M., Miyasaka Y., Ohno E., Salvia R., Wolfgang C.L., et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2024;24:255–270. doi: 10.1016/j.pan.2023.12.009. [DOI] [PubMed] [Google Scholar]
- 7.European Study Group on Cystic Tumours of the Pancreas European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elta G.H., Enestvedt B.K., Sauer B.G., Lennon A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018;113:464–479. doi: 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 9.Megibow A.J. Pancreatic Cysts: Radiology. Gastrointest. Endosc. Clin. N. Am. 2023;33:519–531. doi: 10.1016/j.giec.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Chu L.C., Singhi A.D., Haroun R.R., Hruban R.H., Fishman E.K. The many faces of pancreatic serous cystadenoma: Radiologic and pathologic correlation. Diagn. Interv. Imaging. 2017;98:191–202. doi: 10.1016/j.diii.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan J.H., Gonda T.A. The use of biomarkers in the risk stratification of cystic neoplasms. Gastrointest. Endosc. Clin. N. Am. 2018;28:549–568. doi: 10.1016/j.giec.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 12.van der Waaij L.A., van Dullemen H.M., Porte R.J. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest. Endosc. 2005;62:383–389. doi: 10.1016/S0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 13.Oguz D., Öztaş E., Kalkan I.H., Tayfur O., Cicek B., Aydog G., Kurt M., Beyazit Y., Etik D., Nadir I., et al. Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: A single-center experience. J. Dig. Dis. 2013;14:132–139. doi: 10.1111/1751-2980.12014. [DOI] [PubMed] [Google Scholar]
- 14.Salvia R., Burelli A., Nepi A., Caravati A., Tomelleri C., Dall’Olio T., Casciani F., Crinò S.F., Perri G., Marchegiani G. Pancreatic cystic neoplasms: Still high rates of preoperative misdiagnosis in the guidelines and endoscopic ultrasound era. Surgery. 2023;174:1410–1415. doi: 10.1016/j.surg.2023.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Marchegiani G., Crippa S., Perri G., Rancoita P.M.V., Caravati A., Belfiori G., Dall’Olio T., Aleotti F., Partelli S., Bassi C., et al. Surgery for Intraductal Papillary Mucinous Neoplasms of the Pancreas: Preoperative Factors Tipping the Scale of Decision-Making. Ann. Surg. Oncol. 2022;29:3206–3214. doi: 10.1245/s10434-022-11326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Taee A.M., Taylor J.R. Endoscopic Imaging of Pancreatic Cysts. Gastrointest. Endosc. Clin. N. Am. 2023;33:583–598. doi: 10.1016/j.giec.2023.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Sanchez M.V., Napoleon B. New horizons in the endoscopic ultrasonography-based diagnosis of pancreatic cystic lesions. World J. Gastroenterol. 2018;24:2853–2866. doi: 10.3748/wjg.v24.i26.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerson A., Ho S. The use of EUS-microforceps biopsies to evaluate patients with pancreatic cystic lesions. Endosc. Ultrasound. 2020;9:209–210. doi: 10.4103/eus.eus_32_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facciorusso A., Del Prete V., Antonino M., Buccino V.R., Wani S. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: A meta-analysis. Gastrointest. Endosc. 2020;92:1–8.e3. doi: 10.1016/j.gie.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Tacelli M., Celsa C., Magro B., Barchiesi M., Barresi L., Capurso G., Arcidiacono P.G., Cammà C., Crinò S.F. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig. Endosc. 2020;32:1018–1030. doi: 10.1111/den.13626. [DOI] [PubMed] [Google Scholar]
- 21.Westerveld D.R., Ponniah S.A., Draganov P.V., Yang D. Diagnostic yield of EUS-guided through-the-needle microforceps biopsy versus EUS-FNA of pancreatic cystic lesions: A systematic review and meta-analysis. Endosc. Int. Open. 2020;8:E656–E667. doi: 10.1055/a-1119-6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarty T., Rustagi T. Endoscopic ultrasound-guided through-the-needle microforceps biopsy improves diagnostic yield for pancreatic cystic lesions: A systematic review and meta-analysis. Endosc. Int. Open. 2020;8:E1280–E1290. doi: 10.1055/a-1194-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rift C.V., Scheie D., Toxværd A., Kovacevic B., Klausen P., Vilmann P., Hansen C.P., Lund E.L., Hasselby J.P. Diagnostic accuracy of EUS-guided through-the-needle-biopsies and simultaneously obtained fine needle aspiration for cytology from pancreatic cysts: A systematic review and meta-analysis. Pathol. Res. Pract. 2021;220:153368. doi: 10.1016/j.prp.2021.153368. [DOI] [PubMed] [Google Scholar]
- 24.Facciorusso A., Kovacevic B., Yang D., Vilas-Boas F., Martinez-Moreno B., Stigliano S., Rizzatti G., Sacco M., Arevalo-Mora M., Villarreal-Sanchez L., et al. Predictors of adverse events after endoscopic ultrasound-guided through-the-needle biopsy of pancreatic cysts: A recursive partitioning analysis. Endoscopy. 2022;54:1158–1168. doi: 10.1055/a-1831-5385. [DOI] [PubMed] [Google Scholar]
- 25.Crinò S.F. Through-the-needle microforceps biopsy: A powerful tool but for selected patients. Endoscopy. 2021;53:53–54. doi: 10.1055/a-1243-0545. [DOI] [PubMed] [Google Scholar]
- 26.Nakai Y., Koike K. Pancreatic cysts: Is the answer on the wall? Dig. Endosc. 2018;30:739–740. doi: 10.1111/den.13223. [DOI] [PubMed] [Google Scholar]
- 27.Sahai A.V. Through-the-needle biopsy of pancreas cysts: Who, what, where, when, and mostly… why? Gastrointest. Endosc. 2020;92:9–10. doi: 10.1016/j.gie.2020.03.3752. [DOI] [PubMed] [Google Scholar]
- 28.Varadarajulu S. Let’s Start Looking at the Entire Patient Before Poking at Pancreatic Cysts. Clin. Gastroenterol. Hepatol. 2019;17:1444–1445. doi: 10.1016/j.cgh.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Aparicio J.R., Martinez J., Niveiro M., Cabezas A., Ruiz F., De Madaria E., Casellas J.A. Direct intracystic biopsy and pancreatic cystoscopy through a 19-gauge needle EUS (with videos) Gastrointest. Endosc. 2010;72:1285–1288. doi: 10.1016/j.gie.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Shakhatreh M.H., Naini S.R., Brijbassie A.A., Grider D.J., Shen P., Yeaton P. Use of a novel through-the-needle biopsy forceps in endoscopic ultrasound. Endosc. Int. Open. 2016;4:E439–E442. doi: 10.1055/s-0042-101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basar O., Yuksel O., Yang D.J., Samarasena J., Forcione D., DiMaio C.J., Wagh M.S., Chang K., Casey B., Fernandez-Del Castillo C., et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest. Endosc. 2018;88:79–86. doi: 10.1016/j.gie.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Kovacevic B., Klausen P., Hasselby J.P., Karstensen J.G., Rift C.V., Kalaitzakis E., Toxværd A., Hansen C.P., Storkholm J., Hassan H., et al. A novel endoscopic ultrasound-guided through-the-needle microbiopsy procedure improves diagnosis of pancreatic cystic lesions. Endoscopy. 2018;50:1105–1111. doi: 10.1055/a-0625-6440. [DOI] [PubMed] [Google Scholar]
- 33.Mittal C., Obuch J.C., Hammad H., Edmundowicz S.A., Wani S., Shah R.J., Brauer B.C., Attwell A.R., Kaplan J.B., Wagh M.S. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video) Gastrointest. Endosc. 2018;87:1263–1269. doi: 10.1016/j.gie.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Barresi L., Crinò S.F., Fabbri C., Attili F., Poley J.W., Carrara S., Tarantino I., Bernardoni L., Giovanelli S., Di Leo M., et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig. Endosc. 2018;30:760–770. doi: 10.1111/den.13197. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M.L., Arpin R.N., Brugge W.R., Forcione D.G., Basar O., Pitman M.B. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018;126:414–420. doi: 10.1002/cncy.21988. [DOI] [PubMed] [Google Scholar]
- 36.Yang D., Samarasena J.B., Jamil L.H., Chang K.J., Lee D., Ona M.A., Lo S.K., Gaddam S., Liu Q., Draganov P.V. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: A multicenter study. Endosc. Int. Open. 2018;6:E1423–E1430. doi: 10.1055/a-0770-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheesman A.R., Zhu H., Liao X., Szporn A.H., Kumta N.A., Nagula S., DiMaio C.J. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest. Endosc. 2020;91:1095–1104. doi: 10.1016/j.gie.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Crinò S.F., Bernardoni L., Brozzi L., Barresi L., Malleo G., Salvia R., Frulloni L., Sina S., Parisi A., Remo A., et al. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest. Endosc. 2019;90:933–943. doi: 10.1016/j.gie.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Samarasena J., Yu A., Lee D., Hashimoto R., Lu Y., Thieu D., Mai D., Lee J., Chang K. EUS-guided through-the-needle biopsy for pancreatic cystic lesions. VideoGIE. 2019;4:436–439. doi: 10.1016/j.vgie.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D., Trindade A.J., Yachimski P., Benias P., Nieto J., Manvar A., Ho S., Esnakula A., Gamboa A., Sethi A., et al. Histologic Analysis of Endoscopic Ultrasound-Guided Through the Needle Microforceps Biopsies Accurately Identifies Mucinous Pancreas Cysts. Clin. Gastroenterol. Hepatol. 2019;17:1587–1596. doi: 10.1016/j.cgh.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto R., Lee J.G., Chang K.J., Chehade N.E.H., Samarasena J.B. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A large single center experience. World J. Gastrointest. Endosc. 2019;11:531–540. doi: 10.4253/wjge.v11.i11.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacevic B., Klausen P., Rift C.V., Toxvaerd A., Grossjohann H., Karstensen J.G., Brink L., Hassan H., Kalaitzakis E., Storkholm J., et al. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy. 2021;53:44–52. doi: 10.1055/a-1214-6043. [DOI] [PubMed] [Google Scholar]
- 43.Robles-Medranda C., Olmos J.I., Puga-Tejada M., Oleas R., Baquerizo-Burgos J., Arevalo-Mora M., Del Valle Zavala R., Nebel J.A., Calle Loffredo D., Pitanga-Lukashok H. Endoscopic ultrasound-guided through-the-needle microforceps biopsy and needle-based confocal laser-endomicroscopy increase detection of potentially malignant pancreatic cystic lesions: A single-center study. World J. Gastrointest. Endosc. 2022;14:129–141. doi: 10.4253/wjge.v14.i3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho S.H., Song T.J., Seo D.W., Oh D., Park D.H., Lee S.S., Lee S.K., Kim M.H. Efficacy and safety of EUS-guided through-the-needle microforceps biopsy sampling in categorizing the type of pancreatic cystic lesions. Gastrointest. Endosc. 2022;95:299–309. doi: 10.1016/j.gie.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Vilas-Boas F., Ribeiro T., Costa-Moreira P., Barroca H., Lopes J., Martins D., Moutinho-Ribeiro P., Macedo G. Endoscopic Ultrasound Through-The-Needle Biopsy of Pancreatic Cysts: Toward Procedure Standardization. Dig. Dis. 2023;41:154–163. doi: 10.1159/000526332. [DOI] [PubMed] [Google Scholar]
- 46.Stigliano S., Covotta F., Di Matteo F.M. A new micro-forceps for endoscopic ultrasound-guided through-the-needle biopsy in the diagnosis of pancreatic cystic lesions: Single center experience. JGH Open. 2021;5:1004–1008. doi: 10.1002/jgh3.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crinò S.F., Bernardoni L., Gabbrielli A., Capelli P., Salvia R., Rusev B.C., Scarpa A., Manfrin E. Beyond Pancreatic Cyst Epithelium: Evidence of Ovarian-Like Stroma in EUS-Guided Through-the-Needle Micro-Forceps Biopsy Specimens. Am. J. Gastroenterol. 2018;113:1059–1060. doi: 10.1038/s41395-018-0124-6. [DOI] [PubMed] [Google Scholar]
- 48.de Pretis N., Mukewar S., Aryal-Khanal A., Bi Y., Takahashi N., Chari S. Pancreatic cysts: Diagnostic accuracy and risk of inappropriate resections. Pancreatology. 2017;17:267–272. doi: 10.1016/j.pan.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q.X., Xiao J., Orange M., Zhang H., Zhu Y.Q. EUS-Guided FNA for Diagnosis of Pancreatic Cystic Lesions: A Meta-Analysis. Cell. Physiol. Biochem. 2015;36:1197–1209. doi: 10.1159/000430290. [DOI] [PubMed] [Google Scholar]
- 50.Hong S.K., Loren D.E., Rogart J.N., Siddiqui A.A., Sendecki J.A., Bibbo M., Coben R.M., Meckes D.P., Kowalski T.E. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest. Endosc. 2012;75:775–782. doi: 10.1016/j.gie.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Barresi L., Tacelli M., Ligresti D., Traina M., Tarantino I. Tissue acquisition in pancreatic cystic lesions. Dig. Liver Dis. 2019;51:286–292. doi: 10.1016/j.dld.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Bhutani M.S., Gupta V., Guha S., Gheonea D.I., Saftoiu A. Pancreatic cyst fluid analysis—A review. J. Gastrointestin. Liver Dis. 2011;20:175–180. [PubMed] [Google Scholar]
- 53.Thornton G.D., McPhail M.J., Nayagam S., Hewitt M.J., Vlavianos P., Monahan K.J. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 54.Vázquez-Sequeiros E. Endoscopic ultrasound-guided through-the-needle biopsy for the diagnosis of cystic tumors of the pancreas: Not for all patients. Endoscopy. 2022;54:1169–1170. doi: 10.1055/a-1901-8718. [DOI] [PubMed] [Google Scholar]
- 55.Trikudanathan G., Snover D., Mallery S.J. Microforceps-Assisted Diagnosis of Cystic Pancreatic Neuroendocrine Tumor. Clin. Endosc. 2019;52:293–294. doi: 10.5946/ce.2018.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barresi L., Tacelli M., Chiarello G., Tarantino I., Traina M. Mucinous cystic neoplasia with denuded epithelium: EUS through-the-needle biopsy diagnosis. Gastrointest. Endosc. 2018;88:771–774. doi: 10.1016/j.gie.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Gong Z., Cheng G., Wu W., Hu D. Peritoneal adenocarcinoma diagnosed by endoscopic ultrasound-guided through-the-needle biopsy. Endoscopy. 2024;56:E460–E461. doi: 10.1055/a-2320-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barresi L., Tacelli M., Traina M. Endoscopic ultrasound through-the-needle biopsy diagnosis of a pancreatic lymphoepithelial cyst. Dig. Endosc. 2019;31:210. doi: 10.1111/den.13343. [DOI] [PubMed] [Google Scholar]
- 59.Marchegiani G., Pollini T., Andrianello S., Tomasoni G., Biancotto M., Javed A.A., Kinny-Köster B., Amini N., Han Y., Kim H., et al. Progression vs Cyst Stability of Branch-Duct Intraductal Papillary Mucinous Neoplasms After Observation and Surgery. JAMA Surg. 2021;156:654–661. doi: 10.1001/jamasurg.2021.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crippa S., Bassi C., Salvia R., Malleo G., Marchegiani G., Rebours V., Levy P., Partelli S., Suleiman S.L., Banks P.A., et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: A mid-term follow-up analysis. Gut. 2017;66:495–506. doi: 10.1136/gutjnl-2015-310162. [DOI] [PubMed] [Google Scholar]
- 61.Adsay N.V., Merati K., Basturk O., Iacobuzio-Donahue C., Levi E., Cheng J.D., Sarkar F.H., Hruban R.H., Klimstra D.S. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: Delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am. J. Surg. Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Schaberg K.B., DiMaio M.A., Longacre T.A. Intraductal Papillary Mucinous Neoplasms Often Contain Epi-thelium From Multiple Subtypes and/or Are Unclassifiable. Am. J. Surg. Pathol. 2016;40:44–50. doi: 10.1097/PAS.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 63.Kwak H.A., Liu X., Allende D.S., Pai R.K., Hart J., Xiao S.Y. Interobserver variability in intraductal papillary mucinous neoplasm subtypes and application of their mucin immunoprofiles. Mod. Pathol. 2016;29:977–984. doi: 10.1038/modpathol.2016.93. [DOI] [PubMed] [Google Scholar]
- 64.Paniccia A., Polanco P.M., Boone B.A., Wald A.I., McGrath K., Brand R.E., Khalid A., Kubiliun N., O’Broin-Lennon A.M., Park W.G., et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology. 2023;164:117–133.e7. doi: 10.1053/j.gastro.2022.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vestrup Rift C., Melchior L.C., Kovacevic B., Toxvaerd A., Klausen P., Karstensen J.G., Kalaitzakis E., Storkholm J., Palnaes Hansen C., Vilmann P., et al. Next-generation sequencing of endoscopic ultrasound guided microbiopsies from pancreatic cystic neoplasms. Histopathology. 2019;75:767–771. doi: 10.1111/his.13949. [DOI] [PubMed] [Google Scholar]
- 66.Rift C.V., Melchior L.C., Kovacevic B., Klausen P., Toxværd A., Grossjohann H., Karstensen J.G., Brink L., Hassan H., Kalaitzakis E., et al. Targeted next-generation sequencing of EUS-guided through-the-needle-biopsy sampling from pancreatic cystic lesions. Gastrointest. Endosc. 2023;97:50–58.e4. doi: 10.1016/j.gie.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Crinò S.F., Bernardoni L., Manfrin E., Parisi A., Gabbrielli A. Endoscopic ultrasound features of pancreatic schwannoma. Endosc. Ultrasound. 2016;5:396–398. doi: 10.4103/2303-9027.195873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takasumi M., Hikichi T., Takagi T., Suzuki R., Watanabe K., Nakamura J., Sugimoto M., Kikuchi H., Konno N., Waragai Y., et al. Efficacy of endoscopic ultrasound-guided fine-needle aspiration for schwannoma: Six cases of a retrospective study. Fukushima J. Med. Sci. 2017;63:75–80. doi: 10.5387/fms.2015-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kovacevic B., Kalaitzakis E., Klausen P., Brink L., Hassan H., Karstensen J.G., Vilmann P. EUS-guided through-the-needle microbiopsy of pancreatic cysts: Technical aspects (with video) Endosc. Ultrasound. 2020;9:220–224. doi: 10.4103/eus.eus_12_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crinò S.F., Ammendola S., Gabbrielli A., Manfrin E. Time to standardize through-the-needle biopsy specimen handling. Gastrointest. Endosc. 2020;92:981–983. doi: 10.1016/j.gie.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Rift C.V., Kovacevic B., Toxværd A., Klausen P., Hansen C.P., Vilmann P., Hasselby J.P. EUS-guided through-the-needle biopsy sampling of pancreatic cystic lesions: A pathologist’s guide for the endoscopist. Gastrointest. Endosc. 2020;92:252–258. doi: 10.1016/j.gie.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 72.Ieni A., Todaro P., Crino S.F., Barresi V., Tuccari G. Endoscopic ultrasound-guided fine-needle aspiration cytology in pancreaticobiliary carcinomas: Diagnostic efficacy of cell-block immunocytochemistry. Hepatobiliary Pancreat. Dis. Int. 2015;14:305–312. doi: 10.1016/S1499-3872(15)60367-8. [DOI] [PubMed] [Google Scholar]
- 73.Notohara K., Nakamura K. Tissue processing of endoscopic ultrasound-guided fine-needle aspiration specimens from solid pancreatic lesions. J. Med. Ultrason. 2024;51:261–274. doi: 10.1007/s10396-023-01387-8. [DOI] [PubMed] [Google Scholar]
- 74.Rift C.V., Kovacevic B., Toxvaerd A., Klausen P., Hansen C.P., Vilmann P., Hasselby J.P. Response. Gastrointest. Endosc. 2020;92:983–984. doi: 10.1016/j.gie.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Takasu N., Kimura W., Moriya T., Hirai I., Takeshita A., Kamio Y., Nomura T. Intraductal papillary-mucinous neoplasms of the gastric and intestinal types may have less malignant potential than the pancreatobiliary type. Pancreas. 2010;39:604–610. doi: 10.1097/MPA.0b013e3181c6947a. [DOI] [PubMed] [Google Scholar]
- 76.Furukawa T., Hatori T., Fujita I., Yamamoto M., Kobayashi M., Ohike N., Morohoshi T., Egawa S., Unno M., Takao S., et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–516. doi: 10.1136/gut.2010.210567. [DOI] [PubMed] [Google Scholar]
- 77.Vilas-Boas F., Costa-Moreira P., Moutinho-Ribeiro P., Martins D., Lopes J., Macedo G. Through-the-needle biopsy sampling may allow preoperative intraductal papillary mucinous neoplasia subtyping. Gastrointest. Endosc. 2020;92:235–236. doi: 10.1016/j.gie.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 78.Koh Y.X., Zheng H.L., Chok A.Y., Tan C.S., Wyone W., Lim T.K., Tan D.M., Goh B.K. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157:496–509. doi: 10.1016/j.surg.2014.08.098. [DOI] [PubMed] [Google Scholar]
- 79.Larghi A., Manfrin E., Fabbri C., Crinò S.F., Correale L., Chiarello G., Barresi L., Van Velthuysen M.L., Poley J.W., Rahal D., et al. Interobserver agreement among expert pathologists on through-the-needle microforceps biopsy samples for evaluation of pancreatic cystic lesions. Gastrointest. Endosc. 2019;90:784–792.e4. doi: 10.1016/j.gie.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki R., Thosani N., Annangi S., Guha S., Bhutani M.S. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: A systematic review and meta-analysis. Pancreatology. 2014;14:380–384. doi: 10.1016/j.pan.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Castro J.S.L., Taglieri E., Neto O.M., Guerra J.G., Venco F.E., Andrade E., Seraphim A.M., Romanini S.G., Torres I.T., Serrano J.P.R., et al. Clinical usefulness of tissue acquisition of pancreatic cystic lesions using an endoscopic ultrasound-guided needle for histological analysis. Endosc. Int. Open. 2024;30:E155–E163. doi: 10.1055/a-2240-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barresi L., Tarantino I., Traina M., Granata A., Curcio G., Azzopardi N., Baccarini P., Liotta R., Fornelli A., Maimone A., et al. Endoscopic ultrasound-guided fine needle aspiration and biopsy using a 22-gauge needle with side fenestration in pancreatic cystic lesions. Dig. Liver Dis. 2014;46:45–50. doi: 10.1016/j.dld.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Faias S., Pereira L., Luís Â., Chaves P., Cravo M. Genetic testing vs microforceps biopsy in pancreatic cysts: Systematic review and meta-analysis. World J. Gastroenterol. 2019;25:3450–3467. doi: 10.3748/wjg.v25.i26.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balaban V.D., Cazacu I.M., Pinte L., Jinga M., Bhutani M.S., Saftoiu A. EUS-through-the-needle microbiopsy forceps in pancreatic cystic lesions: A systematic review. Endosc. Ultrasound. 2021;10:19–24. doi: 10.4103/eus.eus_23_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guzmán-Calderón E., Martinez-Moreno B., Casellas J.A., de Madaria E., Aparicio J.R. Endoscopic ultra-sound-guided, through-the-needle forceps biopsy for diagnosis of pancreatic cystic lesions: A systematic review. Endosc. Int. Open. 2020;8:E1123–E1133. doi: 10.1055/a-1149-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovacevic B., Antonelli G., Klausen P., Hassan C., Larghi A., Vilmann P., Karstensen J.G. EUS-guided biopsy versus confocal laser endomicroscopy in patients with pancreatic cystic lesions: A systematic review and meta-analysis. Endosc. Ultrasound. 2021;10:270–279. doi: 10.4103/EUS-D-20-00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gopakumar H., Puli S.R. Value of Endoscopic Ultrasound-Guided Through-the-Needle Biopsy in Pancreatic Cystic Lesions. A Systematic Review and Meta-Analysis. J. Gastrointest. Cancer. 2024;55:15–25. doi: 10.1007/s12029-023-00949-w. [DOI] [PubMed] [Google Scholar]
- 88.Marchegiani G., Andrianello S., Crippa S., Pollini T., Belfiori G., Gozzini L., Cassalia F., Caravati A., Luchini C., Doglioni C., et al. Actual malignancy risk of either operated or non-operated presumed mucinous cystic neoplasms of the pancreas under surveillance. Br. J. Surg. 2021;108:1097–1104. doi: 10.1093/bjs/znab131. [DOI] [PubMed] [Google Scholar]
- 89.Boicean A., Prisca D., Bratu D.G., Bacila C.I., Tanasescu C., Chicea R., Fleaca S.R., Birsan S.A., Ichim C., Mohor C.I., et al. Uncommon Presentation of Gastric Duplication Cyst with Left-Sided Portal Hypertension: A Case Report and Literature Review. Diagnostics. 2024;14:675. doi: 10.3390/diagnostics14070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krishna S.G., Hart P.A., Malli A., Kruger A.J., McCarthy S.T., El-Dika S., Walker J.P., Dillhoff M.E., Manilchuk A., Schmidt C.R., et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin. Gastroenterol. Hepatol. 2020;18:432–440.e6. doi: 10.1016/j.cgh.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 91.Turner R.C., Melnychuk J.T., Chen W., Jones D., Krishna S.G. Molecular Analysis of Pancreatic Cyst Fluid for the Management of Intraductal Papillary Mucinous Neoplasms. Diagnostics. 2022;12:2573. doi: 10.3390/diagnostics12112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S.Y., Wang Z.J., Pan C.Y., Wu C., Li Z.S., Jin Z.D., Wang K.X. Comparative Performance of Endoscopic Ultrasound-Based Techniques in Patients With Pancreatic Cystic Lesions: A Network Meta-Analysis. Am. J. Gastroenterol. 2023;118:243–255. doi: 10.14309/ajg.0000000000002088. [DOI] [PubMed] [Google Scholar]
- 93.Cotton P.B., Eisen G.M., Aabakken L., Baron T.H., Hutter M.M., Jacobson B.C., Mergener K., Nemcek A., Jr., Petersen B.T., Petrini J.L., et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 94.Dumonceau J.M., Kapral C., Aabakken L., Papanikolaou I.S., Tringali A., Vanbiervliet G., Beyna T., Dinis-Ribeiro M., Hritz I., Mariani A., et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127–149. doi: 10.1055/a-1075-4080. [DOI] [PubMed] [Google Scholar]
- 95.Polkowski M., Jenssen C., Kaye P., Carrara S., Deprez P., Gines A., Fernández-Esparrach G., Eisendrath P., Aithal G.P., Arcidiacono P., et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline—March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 96.Colán-Hernández J., Sendino O., Loras C., Pardo A., Gornals J.B., Concepción M., Sánchez-Montes C., Murzi M., Andujar X., Velasquez-Rodriguez J., et al. Antibiotic Prophylaxis Is Not Required for Endoscopic Ultrasonography-Guided Fine-Needle Aspiration of Pancreatic Cystic Lesions, Based on a Randomized Trial. Gastroenterology. 2020;158:1642–1649.e1. doi: 10.1053/j.gastro.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 97.Facciorusso A., Mohan B.P., Tacelli M., Crinò S.F., Antonini F., Fantin A., Barresi L. Use of antibiotic prophylaxis is not needed for endoscopic ultrasound-guided fine-needle aspiration of pancreatic cysts: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020;14:999–1005. doi: 10.1080/17474124.2020.1797486. [DOI] [PubMed] [Google Scholar]
- 98.Facciorusso A., Arevalo-Mora M., Conti Bellocchi M.C., Bernardoni L., Ramai D., Gkolfakis P., Loizzi D., Muscatiello N., Ambrosi A., Tartaglia N., et al. Impact of Antibiotic Prophylaxis on Infection Rate after Endoscopic Ultrasound Through-the-Needle Biopsy of Pancreatic Cysts: A Propensity Score-Matched Study. Diagnostics. 2022;12:211. doi: 10.3390/diagnostics12010211. [DOI] [PMC free article] [PubMed] [Google Scholar]