Abstract
Transforming growth factor beta 1 (TGF beta 1) inhibits the proliferative response of mink lung epithelial cells (CCL64) to serum and to epidermal growth factor (EGF). This response to TGF beta 1 can be inhibited by prior exposure of the cells to nanogram concentrations of pertussis toxin (PT), suggesting the involvement of a guanine-nucleotide-binding regulatory protein (G-protein) in mediating TGF beta 1-induced growth inhibition. To characterize further this G-protein dependence, we have isolated, by chemical mutagenesis, a CCL64 variant (CCL64-D1) that is resistant to TGF beta 1. Whereas in the parental CCL64 cells TGF beta 1 stimulates both GTP[35S] (guanosine 5'-[gamma-[35S]thio]triphosphate) binding and GTPase activity, in the CCL64-D1 variants TGF beta 1 is without effect. Quantitative immunoblotting with antisera for G-protein alpha- and beta-subunits, as well as PT-catalysed ADP-ribosylation analyses, revealed no appreciable changes in the level of G-protein expression in the CCL64-D1 variants compared with parental cells. In contrast with another TGF beta-resistant clone, MLE-M, which we show lacks detectable type I receptor protein, the CCL64-D1 cells retain all three TGF beta cell-surface binding proteins. On the basis of these studies, we propose that a necessary component of TGF beta 1-mediated growth inhibition in CCL64 epithelial cells is the coupling of TGF beta 1 receptor binding to G-protein activation.
Full text
PDF

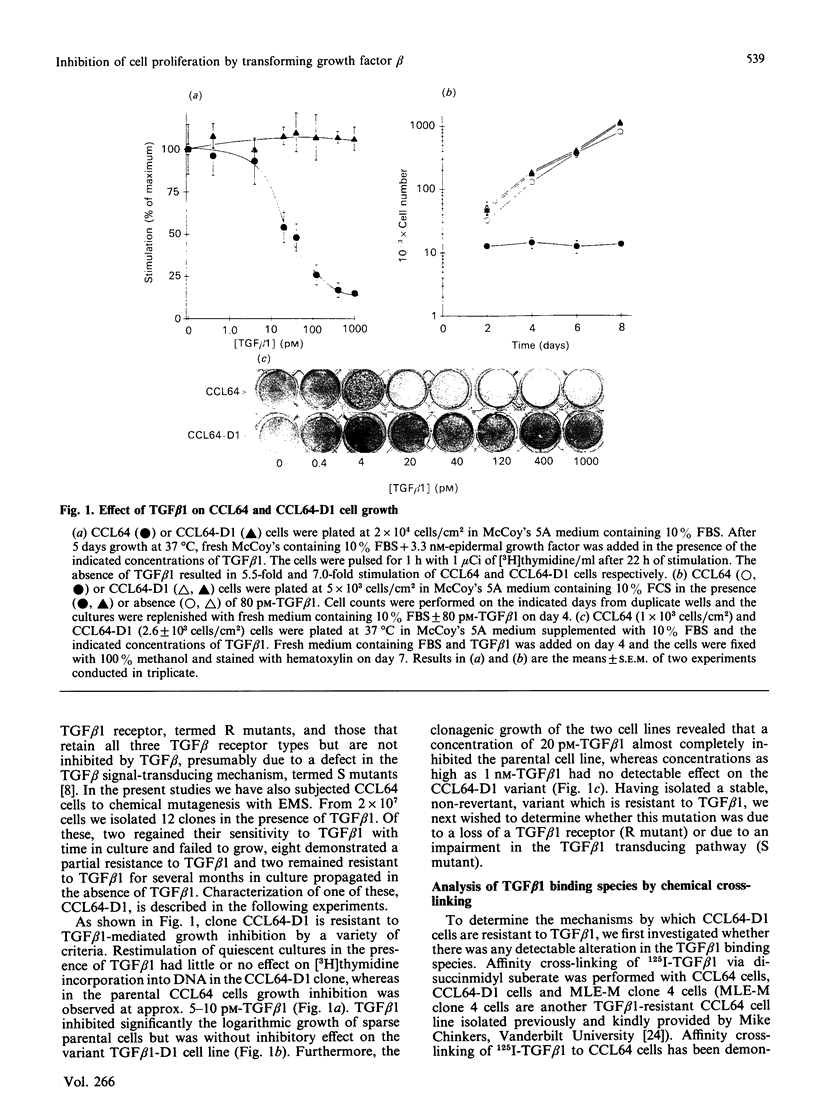
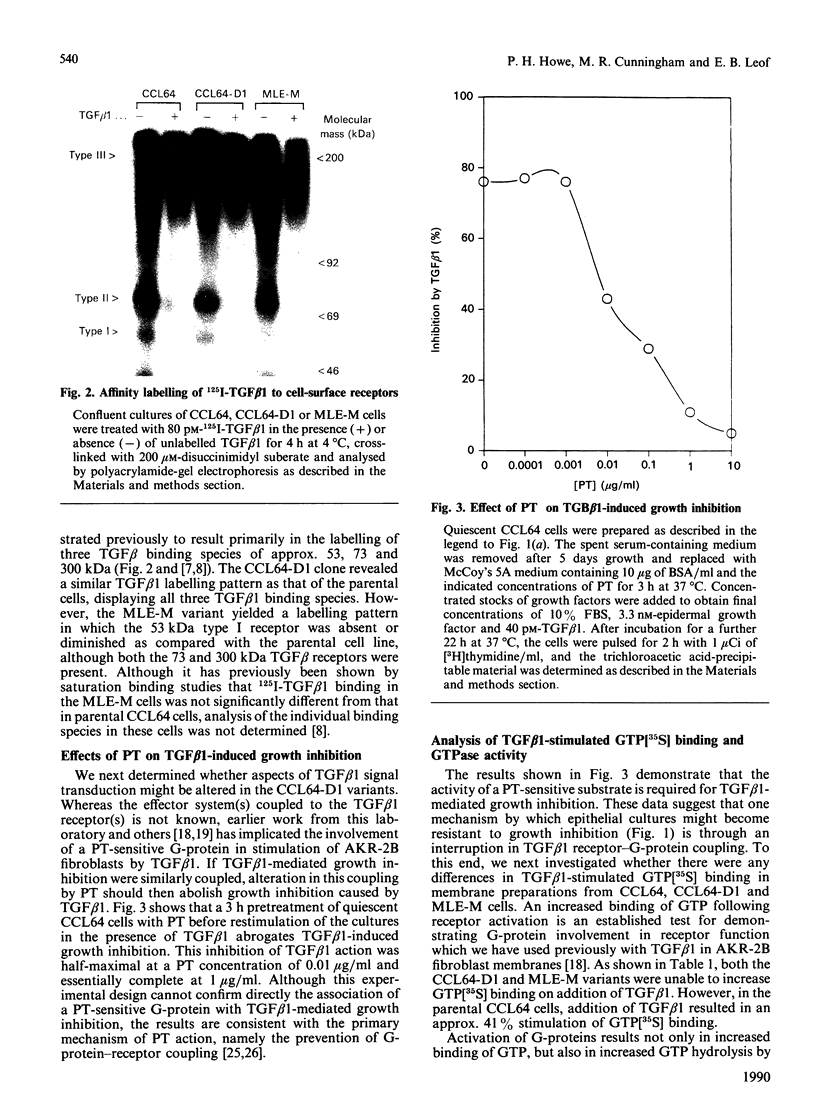


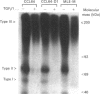
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Boyd F. T., Massagué J. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989 Feb 5;264(4):2272–2278. [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Chinkers M. Isolation and characterization of mink lung epithelial cell mutants resistant to transforming growth factor beta. J Cell Physiol. 1987 Jan;130(1):1–5. doi: 10.1002/jcp.1041300102. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Williams L. T. Agents that increase cAMP accumulation block endothelial c-sis induction by thrombin and transforming growth factor-beta. J Biol Chem. 1987 Sep 5;262(25):11893–11896. [PubMed] [Google Scholar]
- Fischer J. B., Schonbrunn A. The bombesin receptor is coupled to a guanine nucleotide-binding protein which is insensitive to pertussis and cholera toxins. J Biol Chem. 1988 Feb 25;263(6):2808–2816. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Howe P. H., Leof E. B. Transforming growth factor beta 1 treatment of AKR-2B cells is coupled through a pertussis-toxin-sensitive G-protein(s). Biochem J. 1989 Aug 1;261(3):879–886. doi: 10.1042/bj2610879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Imamura K., Kufe D. Colony-stimulating factor 1-induced Na+ influx into human monocytes involves activation of a pertussis toxin-sensitive GTP-binding protein. J Biol Chem. 1988 Oct 5;263(28):14093–14098. [PubMed] [Google Scholar]
- Imamura K., Sherman M. L., Spriggs D., Kufe D. Effect of tumor necrosis factor on GTP binding and GTPase activity in HL-60 and L929 cells. J Biol Chem. 1988 Jul 25;263(21):10247–10253. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L. M., Hewlett E. L., Romero G., Rogol A. D. Pertussis toxin treatment attenuates some effects of insulin in BC3H-1 murine myocytes. J Biol Chem. 1988 May 5;263(13):6134–6141. [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy U. S., Anzano M. A., Stadel J. M., Greig R. Coupling of TGF-beta-induced mitogenesis to G-protein activation in AKR-2B cells. Biochem Biophys Res Commun. 1988 May 16;152(3):1228–1235. doi: 10.1016/s0006-291x(88)80416-9. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Lefkowitz R. J. Activation and desensitization of beta-adrenergic receptor-coupled GTPase and adenylate cyclase of frog and turkey erythrocyte membranes. J Biol Chem. 1980 Jul 25;255(14):6860–6867. [PubMed] [Google Scholar]
- Segarini P. R., Rosen D. M., Seyedin S. M. Binding of transforming growth factor-beta to cell surface proteins varies with cell type. Mol Endocrinol. 1989 Feb;3(2):261–272. doi: 10.1210/mend-3-2-261. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Miller R. T., Masters S. B., Beiderman B., Heideman W., Bourne H. R. Identification of receptor contact site involved in receptor-G protein coupling. Nature. 1987 Dec 24;330(6150):758–760. doi: 10.1038/330758a0. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Codina J., Hamilton S. L., Brown A. M., Birnbaumer L. The stimulatory G protein of adenylyl cyclase, Gs, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem. 1988 Jul 15;263(20):9887–9895. [PubMed] [Google Scholar]