Abstract
Adult male rats were given [35S]sulphate intraperitoneally. Heparan [35S]sulphate (HS) chains were recovered from adipose tissue, brain, carcase, heart, intestine, kidneys, liver, lungs, skin and spleen by digestion with Pronase, precipitation with cetylpyridinium chloride, digestion with chondroitin ABC lyase and DNAase and gradient elution from DEAE-Sephacel. Purity was confirmed by agarose-gel electrophoresis and degradation with HNO2. Fractionation by gradient elution from antithrombin-agarose indicated that the proportion of HS with high binding affinity for antithrombin (HA-HS) ranged from 4.7% (kidneys) to 21.5% (brain). On a mass basis the major sources of HA-HS were carcase, skin and intestine. HA-HS from intestine was arbitrarily divided into subfractions I-VI, with anticoagulant activities ranging from 1 to 60 units/mg [by amidolytic anti-(Factor IIa) assay] and from 4 to 98 units/mg [by amidolytic anti-(Factor Xa) assay], indicating that the antithrombin-binding-site densities of HA-HS chains covered a wide range, as shown previously for rat HA-heparin chains [Horner, Kusche, Lindahl & Peterson (1988) Biochem. J. 251, 141-145]. HA-HS subfractions II, IV and VI were mixed with samples of HA-[3H]heparin chains and rechromatographed on antithrombin-agarose. Affinity for matrix-bound antithrombin did not correlate with anticoagulant activity, e.g. HA-HS subfraction IV [38 anti-(Factor Xa) units/mg] was co-eluted with HA-heparin chains [127 anti-(Factor Xa) units/mg].
Full text
PDF

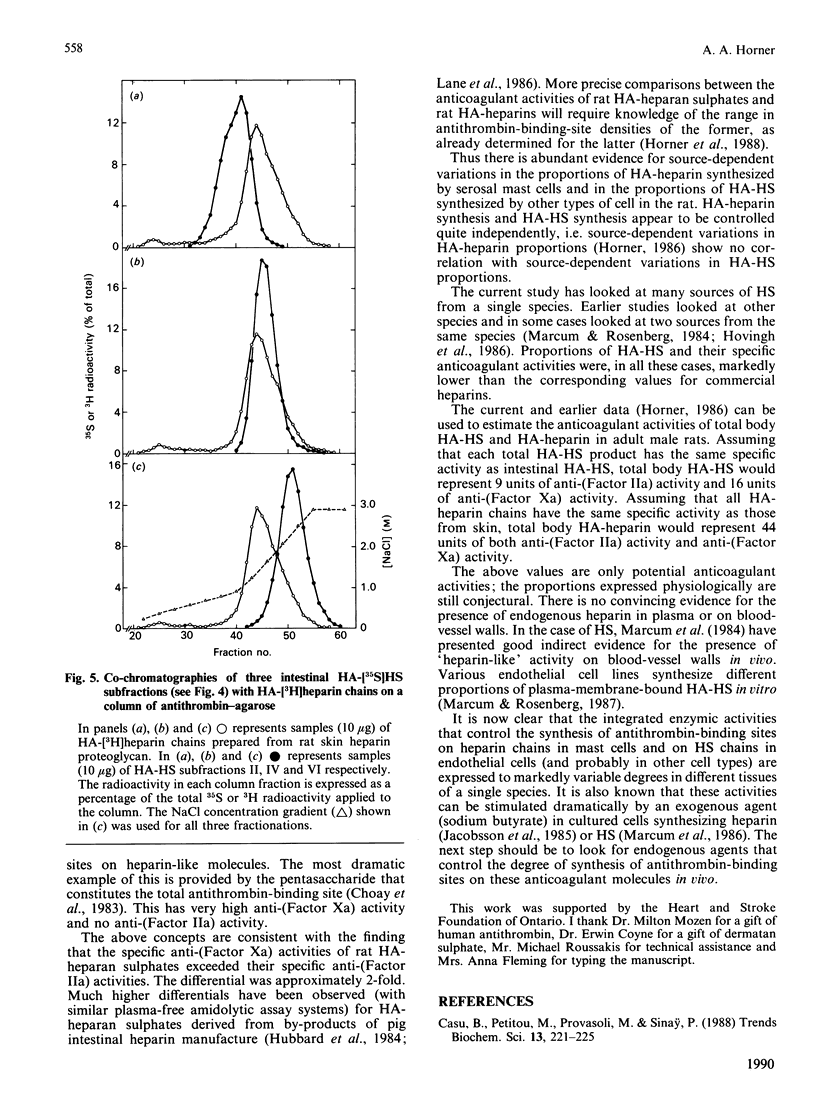

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casu B., Petitou M., Provasoli M., Sinaÿ P. Conformational flexibility: a new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem Sci. 1988 Jun;13(6):221–225. doi: 10.1016/0968-0004(88)90088-6. [DOI] [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- Danielsson A., Raub E., Lindahl U., Björk I. Role of ternary complexes, in which heparin binds both antithrombin and proteinase, in the acceleration of the reactions between antithrombin and thrombin or factor Xa. J Biol Chem. 1986 Nov 25;261(33):15467–15473. [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Heterogeneity of rat skin heparin chains with high affinity for antithrombin. Biochem J. 1987 Jun 15;244(3):693–698. doi: 10.1042/bj2440693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A., Kusche M., Lindahl U., Peterson C. B. Determination of the range in binding-site densities of rat skin heparin chains with high binding affinities for antithrombin. Biochem J. 1988 Apr 1;251(1):141–145. doi: 10.1042/bj2510141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Molecular-size-dependent variations in the proportions of chains with high binding affinities for antithrombin in rat skin heparin proteoglycans. Biochem J. 1989 Sep 15;262(3):953–958. doi: 10.1042/bj2620953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Rat heparins. A study of the relative sizes and antithrombin-binding characteristics of heparin proteoglycans, chains and depolymerization products from rat adipose tissue, heart, lungs, peritoneal cavity and skin. Biochem J. 1986 Nov 15;240(1):171–179. doi: 10.1042/bj2400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A., Young E. Asymmetric distribution of sites with high affinity for antithrombin III in rat skin heparin proteoglycans. J Biol Chem. 1982 Aug 10;257(15):8749–8754. [PubMed] [Google Scholar]
- Hovingh P., Piepkorn M., Linker A. Biological implications of the structural, antithrombin affinity and anticoagulant activity relationships among vertebrate heparins and heparan sulphates. Biochem J. 1986 Jul 15;237(2):573–581. doi: 10.1042/bj2370573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. R., Jennings C. A., Barrowcliffe T. W. Anticoagulant properties in vitro of heparan sulphates. Thromb Res. 1984 Sep 1;35(5):567–576. doi: 10.1016/0049-3848(84)90288-3. [DOI] [PubMed] [Google Scholar]
- Jacobsson K. G., Riesenfeld J., Lindahl U. Biosynthesis of heparin. Effects of n-butyrate on cultured mast cells. J Biol Chem. 1985 Oct 5;260(22):12154–12159. [PubMed] [Google Scholar]
- Lane D. A., Denton J., Flynn A. M., Thunberg L., Lindahl U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem J. 1984 Mar 15;218(3):725–732. doi: 10.1042/bj2180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. A., Pejler G., Flynn A. M., Thompson E. A., Lindahl U. Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor 4. J Biol Chem. 1986 Mar 25;261(9):3980–3986. [PubMed] [Google Scholar]
- Marcum J. A., Atha D. H., Fritze L. M., Nawroth P., Stern D., Rosenberg R. D. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986 Jun 5;261(16):7507–7517. [PubMed] [Google Scholar]
- Marcum J. A., McKenney J. B., Rosenberg R. D. Acceleration of thrombin-antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984 Aug;74(2):341–350. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparan sulfate proteoglycan and the vascular endothelium. Semin Thromb Hemost. 1987 Oct;13(4):464–474. doi: 10.1055/s-2007-1003523. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Pejler G., Bäckström G., Lindahl U., Paulsson M., Dziadek M., Fujiwara S., Timpl R. Structure and affinity for antithrombin of heparan sulfate chains derived from basement membrane proteoglycans. J Biol Chem. 1987 Apr 15;262(11):5036–5043. [PubMed] [Google Scholar]
- Reubsaet F. A., Langeveld J. P., Veerkamp J. H. Glycosaminoglycan content of glomerular and tubular basement membranes of various mammalian species. Biochim Biophys Acta. 1985 Jan 28;838(1):144–150. doi: 10.1016/0304-4165(85)90260-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Teien A. N., Abildgaard U., Hök M., Lindahl U. Anticoagulant activity of heparin: assay of bovine, human and porcine preparations by amidolytic and clotting methods. Thromb Res. 1977 Aug;11(2):107–117. doi: 10.1016/0049-3848(77)90029-9. [DOI] [PubMed] [Google Scholar]
- Teien A. N., Abildgaard U., Hök M. The anticoagulant effect of heparan sulfate and dermatan sulfate. Thromb Res. 1976 Jun;8(6):859–867. doi: 10.1016/0049-3848(76)90014-1. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Young E., Horner A. A. The assay and partial characterization of macromolecular heparin depolymerase activity in rat small intestine. Biochem J. 1979 Jun 15;180(3):587–596. doi: 10.1042/bj1800587. [DOI] [PMC free article] [PubMed] [Google Scholar]