Abstract
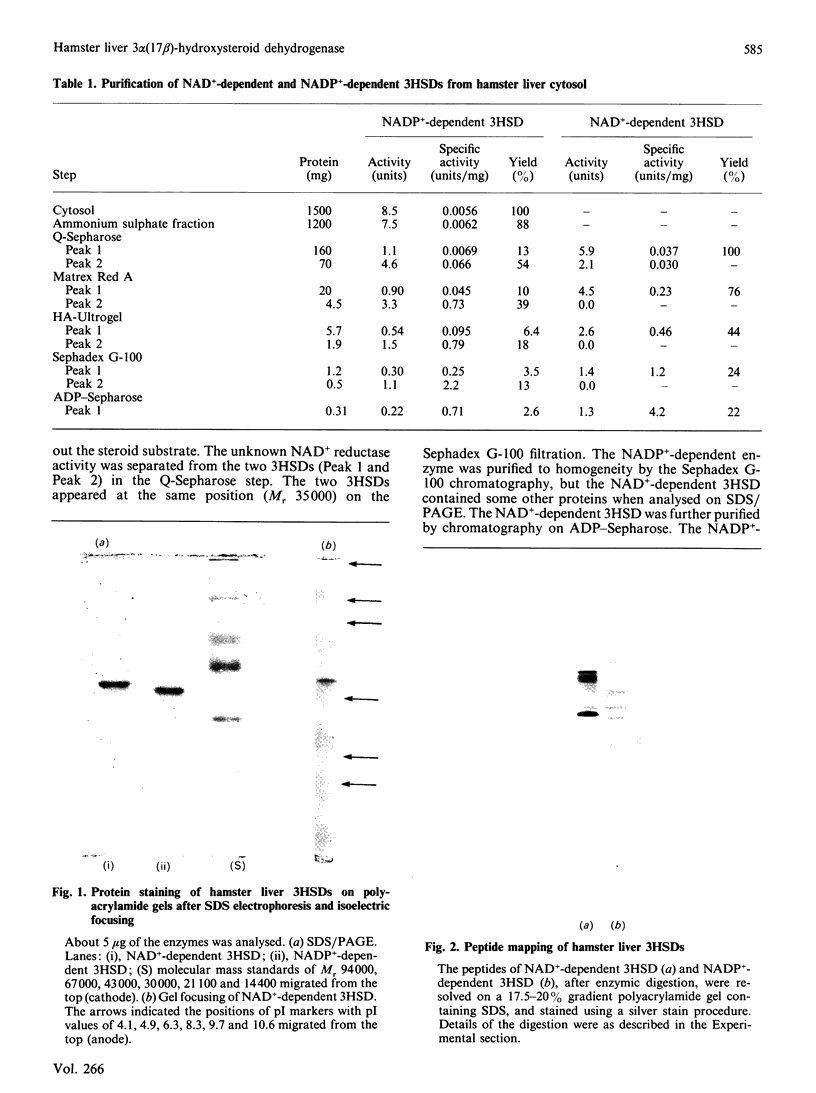
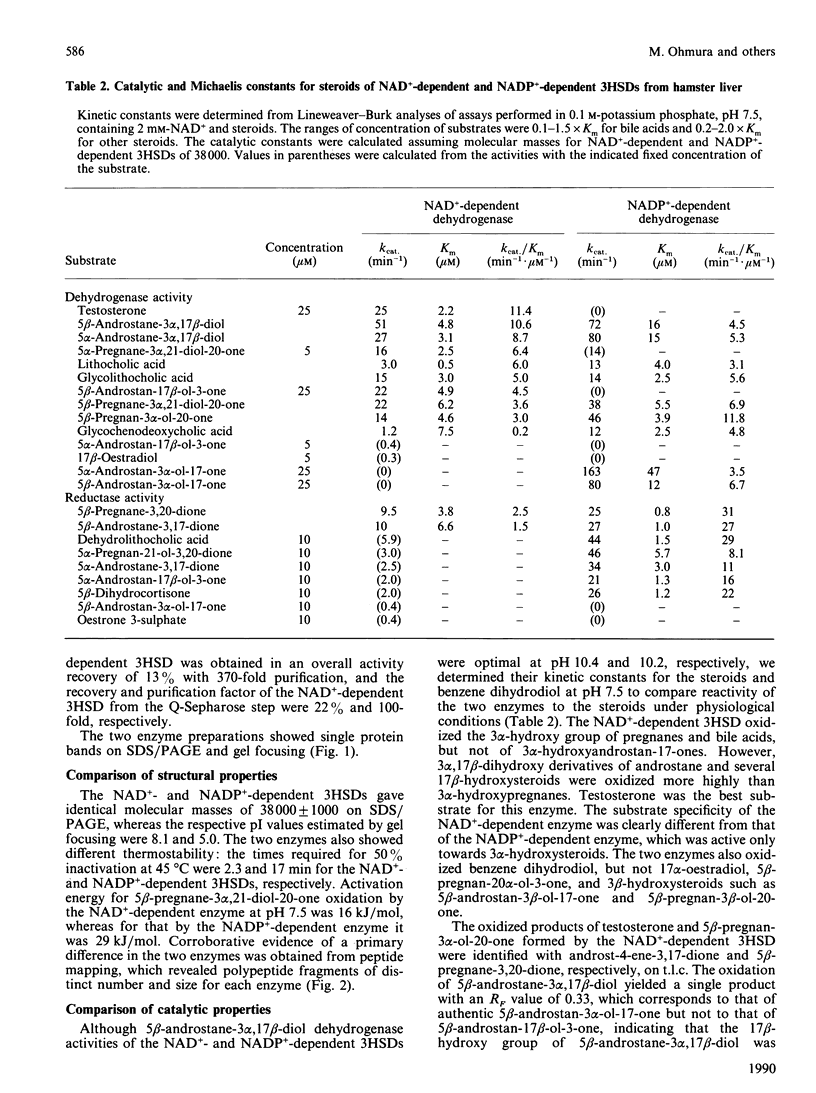
NAD(+)-linked and NADP(+)-linked 3 alpha-hydroxysteroid dehydrogenases were purified to homogeneity from hamster liver cytosol. The two monomeric enzymes, although having similar molecular masses of 38,000, differed from each other in pI values, activation energy and heat stability. The two proteins also gave different fragmentation patterns by gel electrophoresis after digestion with protease. The NADP(+)-linked enzyme catalysed the oxidoreduction of various 3 alpha-hydroxysteroids, whereas the NAD(+)-linked enzyme oxidized the 3 alpha-hydroxy group of pregnanes and some bile acids, and the 17 beta-hydroxy group of testosterone and androstanes. The thermal stabilities of the 3 alpha- and 17 beta-hydroxysteroid dehydrogenase activities of the NAD(+)-linked enzyme were identical, and the two enzyme activities were inhibited by mixing 17 beta- and 3 alpha-hydroxysteroid substrates, respectively. Medroxyprogesterone acetate, hexoestrol and 3 beta-hydroxysteroids competitively inhibited 3 alpha- and 17 beta-hydroxysteroid dehydrogenase activities of the enzyme. These results show that hamster liver contains a 3 alpha(17 beta)-hydroxysteroid dehydrogenase structurally and functionally distinct from 3 alpha-hydroxysteroid dehydrogenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AOSHIMA Y., KOCHAKIAN C. D. Activity, intracellular distribution and some properties of 17beta-hydroxy-C-19-steroid dehydrogenases in liver and kidney. Endocrinology. 1963 Jan;72:106–114. doi: 10.1210/endo-72-1-106. [DOI] [PubMed] [Google Scholar]
- Antoun G. R., Brglez I., Williamson D. G. A 17 beta-hydroxysteroid dehydrogenase of female rabbit liver cytosol. Purification and characterization of multiple forms of the enzyme. Biochem J. 1985 Jan 15;225(2):383–390. doi: 10.1042/bj2250383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hara A., Deyashiki Y., Nakagawa M., Nakayama T., Sawada H. Isolation of proteins with carbonyl reductase activity and prostaglandin-9-ketoreductase activity from chicken kidney. J Biochem. 1982 Dec;92(6):1753–1762. doi: 10.1093/oxfordjournals.jbchem.a134105. [DOI] [PubMed] [Google Scholar]
- Hara A., Hasebe K., Hayashibara M., Matsuura K., Nakayama T., Sawada H. Dihydrodiol dehydrogenases in guinea pig liver. Biochem Pharmacol. 1986 Nov 15;35(22):4005–4012. doi: 10.1016/0006-2952(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Hara A., Inoue Y., Nakagawa M., Naganeo F., Sawada H. Purification and characterization of NADP+-dependent 3 alpha-hydroxysteroid dehydrogenase from mouse liver cytosol. J Biochem. 1988 Jun;103(6):1027–1034. doi: 10.1093/oxfordjournals.jbchem.a122374. [DOI] [PubMed] [Google Scholar]
- Hara A., Kariya K., Nakamura M., Nakayama T., Sawada H. Isolation of multiple forms of indanol dehydrogenase associated with 17 beta-hydroxysteroid dehydrogenase activity from male rabbit liver. Arch Biochem Biophys. 1986 Aug 15;249(1):225–236. doi: 10.1016/0003-9861(86)90578-3. [DOI] [PubMed] [Google Scholar]
- Hara A., Kariya K., Nakamura M., Nakayama T., Sawada H. Isolation of multiple forms of indanol dehydrogenase associated with 17 beta-hydroxysteroid dehydrogenase activity from male rabbit liver. Arch Biochem Biophys. 1986 Aug 15;249(1):225–236. doi: 10.1016/0003-9861(86)90578-3. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Hattori H., Ikeda N., Hayakawa S., Ohmori S. Purification and characterization of the multiple forms of 3 alpha-hydroxysteroid dehydrogenase in rat liver cytosol. Hoppe Seylers Z Physiol Chem. 1984 Mar;365(3):377–391. doi: 10.1515/bchm2.1984.365.1.377. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kochakian C. D. 17beta-Hydroxy-C19-steroid dehydrogenases from male guinea pig liver. Purification and characterization. J Biol Chem. 1978 May 25;253(10):3635–3642. [PubMed] [Google Scholar]
- Krause J. E., Karavolas H. J. Properties of the hypothalamic 5 alpha-dihydroprogesterone NADH- and NADPH-linked 3 alpha-hydroxysteroid oxidoreductase activities. J Steroid Biochem. 1981 Jan;14(1):63–69. doi: 10.1016/0022-4731(81)90193-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Penning T. M., Mukharji I., Barrows S., Talalay P. Purification and properties of a 3 alpha-hydroxysteroid dehydrogenase of rat liver cytosol and its inhibition by anti-inflammatory drugs. Biochem J. 1984 Sep 15;222(3):601–611. doi: 10.1042/bj2220601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning T. M., Sharp R. B., Krieger N. R. Purification and properties of 3 alpha-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem. 1985 Dec 5;260(28):15266–15272. [PubMed] [Google Scholar]
- Sawada H., Hara A., Hayashibara M., Nakayama T. Guinea pig liver aromatic aldehyde-ketone reductases identical with 17 beta-hydroxysteroid dehydrogenase isozymes. J Biochem. 1979 Oct;86(4):883–892. doi: 10.1093/oxfordjournals.jbchem.a132620. [DOI] [PubMed] [Google Scholar]
- Sawada H., Hara A., Nakagawa M., Tsukada F., Ohmura M., Matsuura K. Separation and properties of multiple forms of dihydrodiol dehydrogenase from hamster liver. Int J Biochem. 1989;21(4):367–375. doi: 10.1016/0020-711x(89)90360-1. [DOI] [PubMed] [Google Scholar]
- Sawada H., Hara A., Nakayama T., Kato F. Reductases for aromatic aldehydes and ketones from rabbit liver. Purification and characterization. J Biochem. 1980 Apr;87(4):1153–1165. [PubMed] [Google Scholar]
- Taurog J. D., Moore R. J., Wilson J. D. Partial characterization of the cytosol 3 alpha-hydroxysteroid: NAD(P)+oxidoreductase of rat ventral prostate. Biochemistry. 1975 Feb 25;14(4):810–817. doi: 10.1021/bi00675a026. [DOI] [PubMed] [Google Scholar]