Abstract
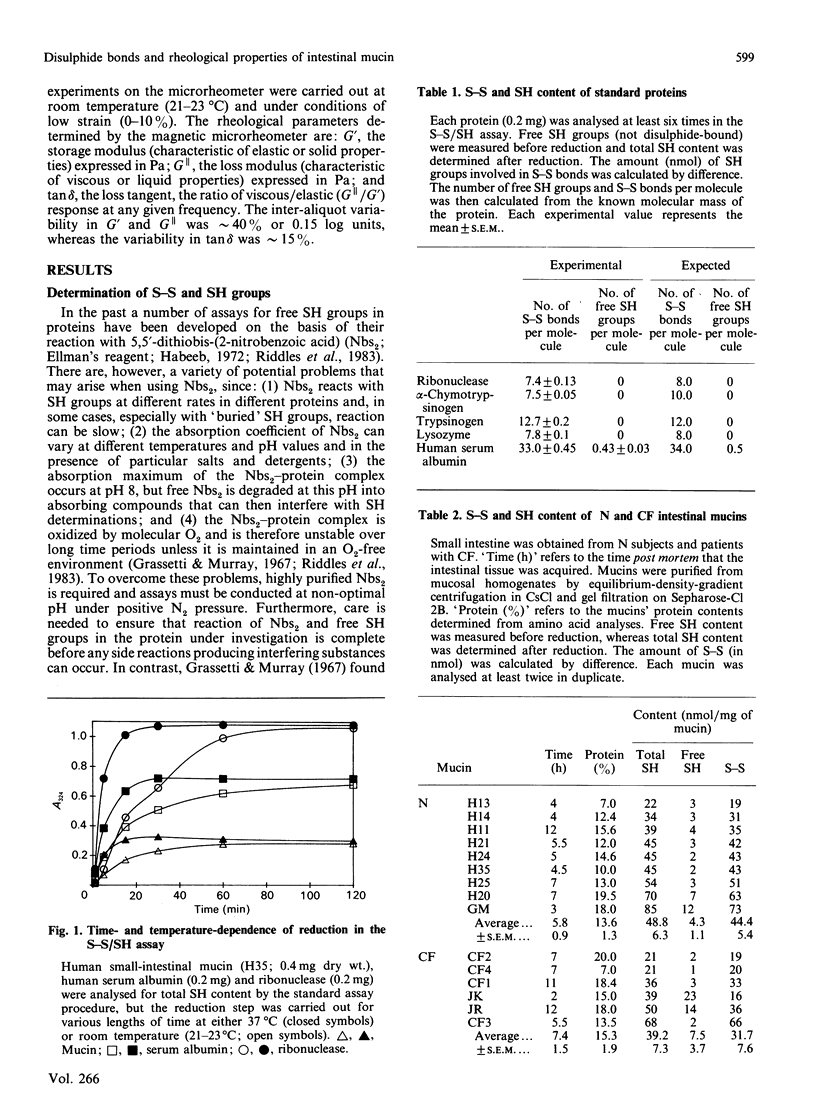
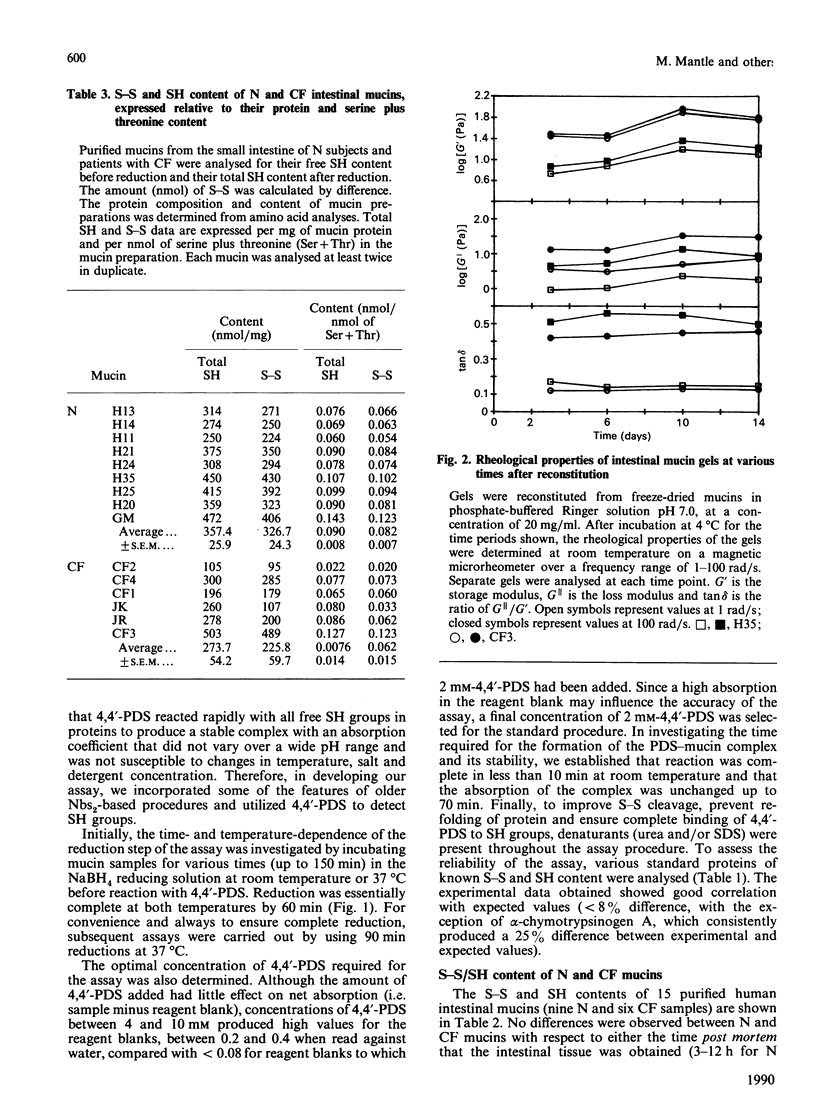
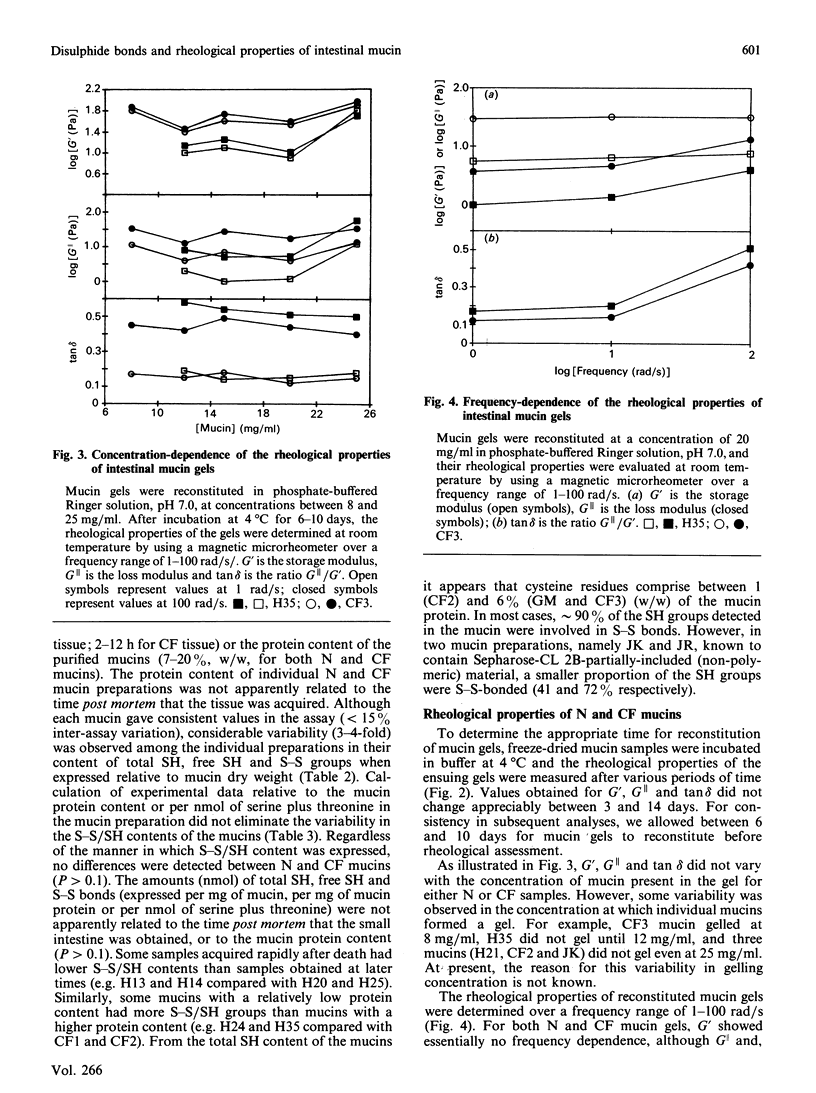
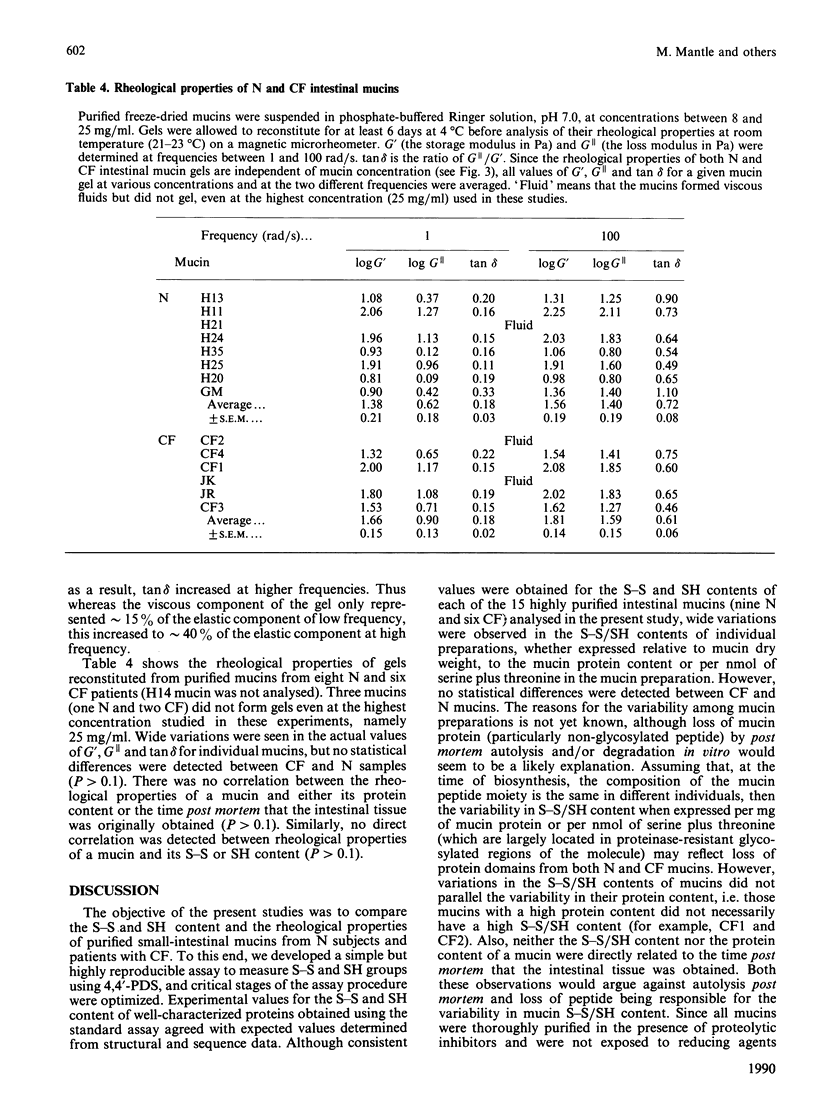
The disulphide/thiol (S-S/SH) content and rheological properties of highly purified small-intestinal mucins from normal (N) subjects and patients with cystic fibrosis (CF) were investigated. (1) An assay was developed to measure free SH groups (before reduction) and total SH content (after reduction) using 4,4'-dipyridyl disulphide. S-S bonds were calculated by difference. Experimental values for the S-S and SH contents of well-characterized proteins obtained with the assay showed good agreement with expected values. (2) The S-S and free SH contents of nine N and six CF mucins were variable: 44.4 +/- 5.4 nmol of S-S and 4.3 +/- 1.1 nmol of free SH per mg of N mucin and 31.7 +/- 7.6 nmol of S-S and 7.5 +/- 3.7 nmol of SH per mg of CF mucin. N and CF mucins were not statistically different. In most mucins, approximately 90% of the SH groups were involved in S-S bonds. (3) Gels were reconstituted from the same N and CF mucins at concentrations between 8 and 25 mg/ml and their rheological properties were assessed by using a magnetic microrheometer. (4) Once formed, mucin gels were stable and maintained the same mechanical properties over a long period of time (3-14 days). (5) The rheological profiles of both N and CF samples did not vary with the concentration of mucin present and were characteristic of weak, visco-elastic gels. (6) Although variations were seen in the visco-elastic properties of individual mucins, no systematic differences were detected between N and CF preparations. (7) There was no apparent correlation between the rheological properties of a mucin and its S-S/SH content.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell A. E., Sellers L. A., Allen A., Cunliffe W. J., Morris E. R., Ross-Murphy S. B. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985 Jan;88(1 Pt 2):269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Charman J., Reid L. Sputum viscosity in chronic bronchitis, bronchiectasis, asthma and cystic fibrosis. Biorheology. 1972 Sep;9(3):185–199. doi: 10.3233/bir-1972-9310. [DOI] [PubMed] [Google Scholar]
- FREYE H. B., KURTZ S. M., SPOCK A., CAPP M. P. LIGHT AND ELECTRON MICROSCOPIC EXAMINATION OF THE SMALL BOWEL OF CHILDREN WITH CYSTIC FIBROSIS. J Pediatr. 1964 Apr;64:575–579. doi: 10.1016/s0022-3476(64)80351-6. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feather E. A., Russell G. Sputum viscosity in cystic fibrosis of the pancreas and other pulmonary diseases. Br J Dis Chest. 1970 Oct;64(4):192–200. doi: 10.1016/s0007-0971(70)80015-8. [DOI] [PubMed] [Google Scholar]
- Forstner J. F., Forstner G. G. Effects of calcium on intestinal mucin: implications for cystic fibrosis. Pediatr Res. 1976 Jun;10(6):609–613. doi: 10.1203/00006450-197606000-00009. [DOI] [PubMed] [Google Scholar]
- Forstner J. F., Jabbal I., Findlay B. P., Forstner G. G. Interaction of mucins with calcium, H+ ion and albumin. Mod Probl Paediatr. 1976 Oct 24;19:54–65. [PubMed] [Google Scholar]
- Forstner J., Maxwell B., Roomi N. Intestinal secretion of mucin in chronically reserpine-treated rats. Am J Physiol. 1981 Nov;241(5):G443–G450. doi: 10.1152/ajpgi.1981.241.5.G443. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Jeanneret-Grosjean A., King M., Michoud M. C., Liote H., Amyot R. Sampling technique and rheology of human tracheobronchial mucus. Am Rev Respir Dis. 1988 Mar;137(3):707–710. doi: 10.1164/ajrccm/137.3.707. [DOI] [PubMed] [Google Scholar]
- Jeffrey I., Durrans D., Wells M., Fox H. The pathology of meconium ileus equivalent. J Clin Pathol. 1983 Nov;36(11):1292–1297. doi: 10.1136/jcp.36.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P. G., Kay R. Histochemistry of rectal mucus in cystic fibrosis of the pancreas. J Pathol. 1969 Dec;99(4):299–306. doi: 10.1002/path.1710990405. [DOI] [PubMed] [Google Scholar]
- Khan M. A., Wolf D. P., Litt M. Effect of mucolytic agents on the rheological properties of tracheal mucus. Biochim Biophys Acta. 1976 Sep 24;444(2):369–373. doi: 10.1016/0304-4165(76)90380-9. [DOI] [PubMed] [Google Scholar]
- King M. Is cystic fibrosis mucus abnormal? Pediatr Res. 1981 Feb;15(2):120–122. doi: 10.1203/00006450-198102000-00007. [DOI] [PubMed] [Google Scholar]
- King M. The role of mucus viscoelasticity in cough clearance. Biorheology. 1987;24(6):589–597. doi: 10.3233/bir-1987-24611. [DOI] [PubMed] [Google Scholar]
- King M. Viscoelastic properties of airway mucus. Fed Proc. 1980 Nov;39(13):3080–3085. [PubMed] [Google Scholar]
- List S. J., Findlay B. P., Forstner G. G., Forstner J. F. Enhancement of the viscosity of mucin by serum albumin. Biochem J. 1978 Nov 1;175(2):565–571. doi: 10.1042/bj1750565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Khan M. A., Wolf D. P. Mucus rheology: relation to structure and function. Biorheology. 1976 Feb;13(1):37–48. doi: 10.3233/bir-1976-13106. [DOI] [PubMed] [Google Scholar]
- Lopez-Vidriero M. T., Reid L. Chemical markers of mucous and serum glycoproteins and their relation to viscosity in mucoid and purulent sputum from various hypersecretory diseases. Am Rev Respir Dis. 1978 Mar;117(3):465–477. doi: 10.1164/arrd.1978.117.3.465. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner J. F. The effects of delipidation on the major antigenic determinant of purified human intestinal mucin. Biochem Cell Biol. 1986 Mar;64(3):223–228. doi: 10.1139/o86-032. [DOI] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Stewart G. Intestinal mucins from normal subjects and patients with cystic fibrosis. Variable contents of the disulphide-bound 118 kDa glycoprotein and different reactivities with an anti-(118 kDa glycoprotein) antibody. Biochem J. 1989 Apr 1;259(1):243–253. doi: 10.1042/bj2590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Morrissey S. M., Tymvios M. C. Acid mucins in human intestinal goblet cells. J Pathol. 1978 Dec;126(4):197–208. doi: 10.1002/path.1711260403. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Grand R. J., Trier J. S. Glycoprotein synthesis, transport, and secretion by epithelial cells of human rectal mucosa: normal and cystic fibrosis. Lab Invest. 1977 May;36(5):535–546. [PubMed] [Google Scholar]
- Neutra M. R., Trier J. S. Rectal mucosa in cystic fibrosis. Morphological features before and after short term organ culture. Gastroenterology. 1978 Oct;75(4):701–710. [PubMed] [Google Scholar]
- Park C. M., Reid P. E., Owen D. A., Sanker J. M., Applegarth D. A. Morphological and histochemical changes in intestinal mucosa in the reserpine-treated rat model of cystic fibrosis. Exp Mol Pathol. 1987 Aug;47(1):1–12. doi: 10.1016/0014-4800(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot R., Das I., Reid L. Pus, deoxyribonucleic acid, and sputum viscosity. Thorax. 1978 Apr;33(2):235–242. doi: 10.1136/thx.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mechanical characterization and properties of gastrointestinal mucus gel. Biorheology. 1987;24(6):615–623. doi: 10.3233/bir-1987-24614. [DOI] [PubMed] [Google Scholar]
- Wesley A. W., Forstner J. F., Forstner G. G. Structure of intestinal-mucus glycoprotein from human post-mortem or surgical tissue: inferences from correlation analyses of sugar and sulfate composition of individual mucins. Carbohydr Res. 1983 Apr 16;115:151–163. doi: 10.1016/0008-6215(83)88143-9. [DOI] [PubMed] [Google Scholar]
- Wesley A., Forstner J., Qureshi R., Mantle M., Forstner G. Human intestinal mucin in cystic fibrosis. Pediatr Res. 1983 Jan;17(1):65–69. doi: 10.1203/00006450-198301000-00013. [DOI] [PubMed] [Google Scholar]


