Abstract
The structures of the complexes formed between 9-amino-[N-(2-dimethyl-amino)butyl]acridine-4-carboxamide and d(CG5BrUACG)2 and d(CGTACG)2 have been solved by X-ray crystallography using MAD phasing methodology and refined to a resolution of 1.6 Å. The complexes crystallised in space group C222. An asymmetric unit in the brominated complex comprises two strands of DNA, one disordered drug molecule, two cobalt (II) ions and 19 water molecules (31 in the native complex). Asymmetric units in the native complex also contain a sodium ion. The structures exhibit novel features not previously observed in crystals of DNA/drug complexes. The DNA helices stack in continuous columns with their central 4 bp adopting a B-like motif. However, despite being a palindromic sequence, the terminal GC base pairs engage in quite different interactions. At one end of the duplex there is a CpG dinucleotide overlap modified by ligand intercalation and terminal cytosine exchange between symmetry-related duplexes. A novel intercalation complex is formed involving four DNA duplexes, four ligand molecules and two pairs of base tetrads. The other end of the DNA is frayed with the terminal guanine lying in the minor groove of the next duplex in the column. The structure is stabilised by guanine N7/cobalt (II) coordination. We discuss our findings with respect to the effects of packing forces on DNA crystal structure, and the potential effects of intercalating agents on biochemical processes involving DNA quadruplexes and strand exchanges. NDB accession numbers: DD0032 (brominated) and DD0033 (native).
INTRODUCTION
DNA intercalating agents that poison the enzyme topoisomerase II are important in the treatment of cancer (1), prominent examples being the anthracycline antibiotics adriamycin and daunomycin (1). 9-Aminoacridine-4-carboxamides are also potent DNA-binding poisons of topoisomerase II with antitumour activity against experimental leukaemias (2–4). The related des-9-amino compound, DACA (NSC 601316), is active in mouse solid tumour models and is currently in clinical trial (5–7). There are tight correlations between ligand structure, cytotoxicity and antitumour efficacy for the 9-aminoacridine-4-carboxamides (2,3,8–10). In order to probe the molecular determinants of these structure–activity relationships, so that we may begin to understand what structural features distinguish the DNA complexes of active versus inactive 9-aminoacridine-4-carboxamides, we have initiated X-ray crystallographic studies of their complexes with oligonucleotides.
The choice of DNA substrate in such studies is critical to success, having regard to obtaining crystals that are well-ordered and that diffract to high resolution, and for intercalating agents the hexanucleotides d(CGTACG)2 and d(CGATCG)2 have come to prominence, particularly for studies involving the anthracyclines which bind in between the terminal GC base pairs (for examples, see 11,12). Since the 9-aminoacridine-4-carboxamides bind selectively to GC-rich sequences (8,13), these oligonucleotides are especially suitable for our purpose. To date, we have solved the structures of 9-amino-DACA and three of its bromine and fluorine derivatives bound to d(CGTACG)2 and/or d(CG5BrUACG)2 (14–16). The complexes have similar structures in which the ligand intercalates at each CpG step with its carboxamide sidechain lying in the major groove, and its terminal protonated dimethylamino group making hydrogen bonding interactions with the O6/N7 atoms of guanine G2 (14–16). The complexes also share the feature that the carboxamide group lies in the plane of the acridine ring with its amide oxygen hydrogen bonded to the protonated N10 atom, and its NH group bound to the G2 phosphate by a bridging water molecule (15,16). These findings enabled us to formulate a molecular rationale for how the 9-aminoacridine-4-carboxamides might poison topoisomerase II (15,16). In the current work we have crystallised an inactive homologue of 9-amino-DACA, termed 9-aminobutyl-DACA, in which the sidechain is lengthened to four methylene groups (see Figure S1, Supplementary Material), with both d(CGTACG)2 and d(CG5BrUACG)2, in anticipation that the structure would provide further insight into the mechanism of action of this class of anticancer drug.
However, the 9-aminobutyl-DACA/hexamer complexes crystallised in a different space group and, to our surprise, the structures are remarkably different from those obtained previously. They contain several unusual features which appear to be novel, the most important of which is the formation of a pseudo-intercalation cavity involving four DNA duplexes that results in drug binding between two sets of base tetrads. In addition, despite the fact that the oligonucleotide is self-complementary, its ends adopt quite structurally distinct conformations in the crystal. Some of the unusual elements of the structure presented here, for example cytosine exchange, GC base pair overlap and frayed terminal GC base pairs, have been observed before. Cytosine exchange between two DNA molecules has been reported in the crystal structure of the Z-DNA fragment d(CCGCGG)2 (17), and in the structure of a porphyrin bound to the hexanucleotide d(CGATCG)2 (18). The overlap of two terminal GC base pairs with reciprocal hydrogen bonding interactions between their guanines in the minor groove, but without an intercalation cavity, is a ubiquitous feature of dodecamers related to the Dickerson–Drew sequence (19,20). Frayed GC base pairs at the ends of DNAs, and the interaction of the unravelled guanine with a GC base pair in the minor groove of the adjacent duplex, has been described in crystal structures of the decamers d(CGCAATTGCG)2 and d(CGTATATACG)2 (21,22), in the complex of the former with the minor groove binding drug netropsin (23), and in crystals of the Ca2+ forms of the Dickerson–Drew, and related, dodecamers (20,24). Our structure is unique in having an unusual combination of these features, and is the first crystallographic example of an intercalation site embedded within a DNA quadruplex. As far as we know, it is also the first example in which a palindromic DNA molecule crystallises in a form with fundamentally different structures at either end. We discuss the relevance of our findings in light of the effects of packing forces on DNA crystal structures and the conformational flexibility of DNA. In addition, we consider the potential biological implications of our structure, having regard to the effects of intercalating agents on unusual DNA quadruplexes or strand exchange processes in vivo.
MATERIALS AND METHODS
Crystallisation
The native and brominated DNAs used in this study were purchased from Oswel DNA Service, Southampton, UK. They were dissolved in water to give a 5 mM stock solution and stored frozen at 253 K. 9-Aminobutyl-DACA was synthesised as the dihydrochloride salt as described previously (2), and similarly dissolved in water to give a 10 mM stock solution and stored frozen at 253 K. Crystals were grown by the vapour diffusion method in sitting drops at 285 K. Crystals of complexes of 9-aminobutyl-DACA with d(CG5BrUACG)2 and d(CGTACG)2 grew in 10 µl drops which initially contained 20 mM sodium cacodylate buffer pH 6.5, 0.5 mM DNA (double-stranded concentration), 3 mM magnesium acetate, 0.5 mM Co(II) chloride, 4.5 mM spermine, 1 mM 9-aminobutyl-DACA and 7% MPD. The drops were equilibrated against reservoirs of 1 ml of 35% MPD. Yellow crystals, with approximate dimensions 0.2 × 0.2 × 0.2 mm, appeared after several months and were placed in Riedel de Haen perfluoropolyether RS 3000 oil, mounted in a cryoloop, and frozen at 110 K in a N2 cryostream.
Data collection
Diffraction data for crystals of both complexes were initially recorded on a Rigaku R-axis II image plate system mounted on a Rigaku RU-200 rotating-anode generator with focusing mirror optics (CuKα, 1.5418 Å). The data sets were each processed to a resolution of 1.6 Å with the DENZO and SCALEPACK programmes (25) which indicated that both crystals were in the space group C222 with cell dimensions of a = 28.96 Å, b = 52.60 Å and c = 40.46 Å for the d(CGTACG)2 complex and a = 29.01 Å, b = 52.70 Å and c = 40.53 Å for the d(CG5BrUACG)2 complex.
As we were unable to solve either of our structures by molecular replacement or SIR, a full four wavelength MAD data set was collected at the Advanced Photon Source, Chicago, IL, using the d(CG5BrUACG)2 complex crystal. The bromine fluorescence edge (13.49137 KeV) was measured on a sample consisting of several crystals of the complex. X-ray diffraction data were collected at four different energies around the fluorescence edge using a single complex crystal, the data sets being designated L1 (bromine fluorescence edge 13.4914 KeV), L2 (13.5019 KeV white line maximum energy), L3 (low energy off-set 13.4413 KeV) and L4 (high energy off-set 13.9000 KeV). At each energy, except L3, a data set of longer exposure (10 or 15 s) and shorter exposure (2 s with copper and iron filters) was collected to ensure recording of the maximum possible number of reflections, including the strong base-stacking reflections. The oscillation range for each exposure was 1°, and 110 images were collected in each data set. The Quantum 1 image plate was positioned 105 mm from the crystal for all the data sets except for the long exposure (15 s) at the highest energy where it was moved to a distance of 75 mm. Two data sets were collected at each energy and exposure time, starting with their φ values separated by 180° to ensure the Bijvoet pairs were observed. The data were processed to 2.0, 2.0, 2.15 and 1.6 Å for L1 to L4 with DENZO and SCALEPACK programmes (25) using the scale anomalous command, the averaged cell dimensions were a = 28.89 Å, b = 53.20 Å and c = 40.44 Å. Further details of the MAD data collection and processing may be found in Table S1 in the Supplementary Material.
Solution of the d(CG5BrUACG)2/9-aminobutyl-DACA structure
The MAD phasing solution was found using the program CNS, version 0.9a (26). The two Br positions were located by the heavy atom search procedure of CNS using the sum of the anomalous Patterson functions at L1 and L2 and the dispersive Patterson L1–L4. The sites were then refined using the MAD phase procedure to a resolution of 2.2 Å. The overall figure of merit for the 2992 reflections was 0.86. Electron density maps were calculated with the MAD phases and the observed amplitudes for L4. The correct ‘hand’ for the heavy atom was chosen by the better contrast features clearly recognisable as DNA in one and not the other. Solvent flattening and histogram matching procedures resulted in the electron density map shown in Figure 1. The structure of the d(CG5BrUACG)2/9-aminobutyl-DACA complex was immediately apparent from the experimental MAD-phased map, and the positions of the acridine ring, and all the phosphates, sugar and base positions, except for cytosine C1, could be clearly identified. The two strands of DNA (excluding cytosine C1) and the 9-aminoacridine portion of the drug were built into the density with the programme O (27) using the coordinates of our previous structure (15), with the thymine modified to a 5-bromouracil, as a starting model.
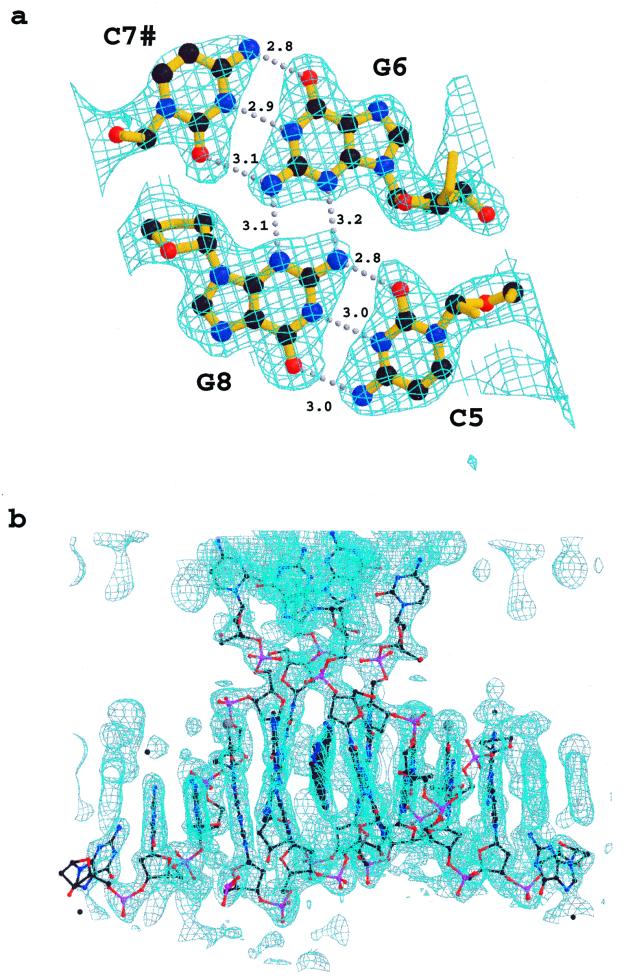
Figure 1.
Sections of the original MAD electron density map. (a) Contoured at 1.2 σ, superimposed on the 4 bp quadruplex refined structure, positions of hydrogen bonds are indicated by dotted lines, lengths of the hydrogen bonds are given. (b) Contoured at 1.0 σ, along the ab diagonal with the final refined structure superimposed.
Refinement of the d(CG5BrUACG)2/9-aminobutyl-DACA structure
The structure was refined with SHELX 97-2 (28) using the highest energy (L4) data set. Eight percent of the reflections were randomly selected for separation into a reference set to monitor Rfree. After 10 cycles of refinement the R factor was 0.34 and the Rfree 0.37 for the resolution shell 40–1.6 Å. An iterative refinement procedure was conducted interspersed with inspection of electron density maps and manual model rebuilding with the programme O (27). Bond lengths and bond angles within the DNA bases were restrained to target values as specified in the Shelx 2000 DNA dictionary. All other chemically equivalent DNA bond lengths and bond angles were restrained by similarity distance restraints but without specification of an actual target value. The DNA bases and the acridine ring were restrained to be planar whereas all other torsion angles remained unrestrained. The largest peak in the initial (Fo–Fc) difference maps was identified as a Co(II) ion and included in the refinement because of the observed electron densities, proximity to the N7 of G12 (2.3 Å) and its octahedral water coordination. Where the density was unequivocal, water molecules were also included, five of which were observed on 2-fold axes. One water was given 0.25 occupancy because it lies on a 2-fold axis and is disordered. Regions of diffuse solvent were modelled using Babinet’s principle as implemented in the SWAT command in SHELXL 97-2 (28). An additional Co(II) ion, coordinated to the N7 of guanine G2, was included in this structure after refinement of the native complex, where a Co(II) ion was clearly observed bound to the G2 N7 nitrogen and octahedrally coordinated to five water molecules. In the brominated structure this second Co(II) atom appears to be disordered and was given 50% occupancy. We were unable to identify electron density corresponding to cytosine C1 in either the brominated or native structure. The position of the 9-aminobutyl-DACA is clearly disordered. We identified the position of the major component (60%) of the ligand from the original MAD density map but after including this in the refinement there remained significant difference density in the area around the chromophore. This remaining density was modelled as an additional chromophore location with total occupancy of 40%, divided as two components of 20% occupancy lying across the 2-fold axis. We were unable to position any portion of the ligand 4-carboxamide sidechain. The final R-factor for the structure is 0.23 and Rfree 0.25 (see Table S2, Supplementary Material). The coordinates and structure factors have been deposited with the Nucleic Acid Data Base (NDB), accession number DD0032 and the Protein Data Base (PDB), accession number 1FN1.
Solution and refinement of the d(CGTACG)/9-aminobutyl-DACA structure
The structure of the non-brominated complex was solved using SHELX 97-2, as described above, with a starting model that included the two strands of DNA and the one drug molecule as defined in the d(CG5BrUACG)2/9-aminobutyl-DACA structure. After 30 cycles of refinement during which the resolution range was increased in three steps, the R factor was 0.37 and the Rfree 0.41 for the resolution range 40–1.6 Å. Initially, the density for the acridine was not as clear in OMIT maps as it was in similar maps for the brominated structure, but after metal ions and water were added, the acridine position became well defined at 50% occupancy. The density for the Co(II) ion coordinated to the N7 of guanine G2 and five water molecules was the largest peak in the initial difference density map. The second Co(II) ion, coordinated with four water molecules and the N7 of guanine G12 of a symmetry-related molecule, fitted well into the density adjacent to the N7 atom of guanine G12. The sodium ion, which appeared in the difference density maps as a peak of similar magnitude to water molecules was distinguished from the latter by its octahedral coordination to four phosphate oxygen atoms and two water molecules. Thirty-one water molecules were observed in this structure compared with 19 in the brominated complex. The final R-factor was 0.25 and the Rfree 0.30. The coordinates and structure factors have been deposited with the NDB (DD0033) and the PDB (1FN2).
Thermal parameters and bond statistics
The average thermal parameters for the d(CG5BrUACG)2/9-aminobutyl-DACA structure are: 40 Å2 for phosphates, 29 Å2 for furanose atoms, 26 Å2 for bases and 26 Å2 for the acridine ring (average of the disordered positions). For the d(CGTACG)2/9-aminobutyl-DACA structure the corresponding values are: 38 Å2 for phosphates, 30 Å2 for furanose atoms, 24 Å2 for bases and 25 Å2 for the acridine ring at 50% occupancy. Root mean square (r.m.s.) deviations from ideality for bond lengths are 0.018 and 0.009 Å for the brominated and non-brominated structures respectively. For those distances defining bond angles, the r.m.s. deviations from ideality are 0.031 and 0.017 Å. The r.m.s. deviations from planarity of the nucleotide bases and acridine rings are 0.002 and 0.011 Å. Structural parameters for the DNA were analysed using the Curves 5.2 program (29).
RESULTS
Structure of the d(CG5BrUACG)2/9-aminobutyl-DACA complex
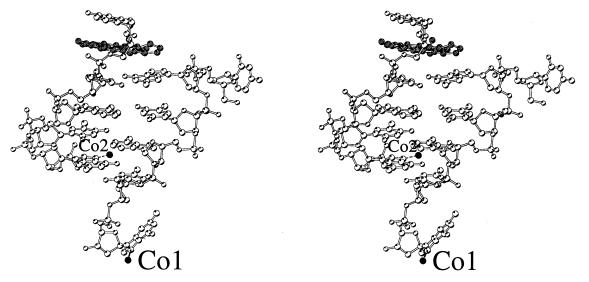
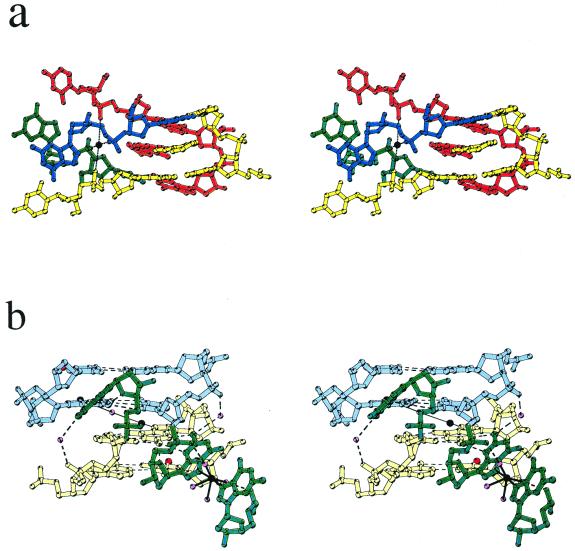
Global structure and crystal packing. An asymmetric unit for the 9-aminobutyl-DACA/d(CG5BrUACG)2 complex contains two strands of DNA, one disordered drug molecule, 19 water molecules and two Co(II) ions given 50% occupancy because one lies on a 2-fold axis and the other is disordered (Fig. 2). No spermine was observed in the electron density maps. The nucleotides are labelled in the 5′ to 3′ direction from C1 to G6 for strand A and C7 to G12 for strand B (Fig. 3). The DNA strands form duplexes, with Watson–Crick base pairing, which stack in continuous columns along the ab diagonals of the unit cell (see Figure S2, Supplementary Material). Figure 3 illustrates the main features of the global structure of the complex. The central 4 bp constitute a right-handed B-DNA-like structure. At one end of the duplex the C5/G8 base pair forms a pseudo-intercalation cavity with a base pair comprising G6 of the same duplex hydrogen-bonded to cytosine C7 from a symmetry-related molecule in the column of duplexes lying at an angle of 60° (Fig. 4a). The displaced cytosine from the first duplex invades the second helix in a similar manner, so that there is a reciprocal exchange of cytosines between these two DNAs. At the pseudo-intercalation site, contiguous helices within the same column of duplexes are not stacked end-to-end, but overlap, so that their terminal two GC base pairs interact with each other in their minor grooves. The angle between the average planes of the overlapping base pairs is ∼30°, which results in the formation of an almost planar GC:GC hydrogen-bonded base quadruplet where the inter-duplex hydrogen bonding contacts are between the 2-amino groups and N3 atoms of the guanines (G6 N2# to G8 N3, 3.1 Å; G6 N3# to G8 N2, 3.2 Å; see Fig. 1a). This arrangement extends the intercalation cavity, which is between the two planes of quadruplets, across the two adjacent DNA helices in the column. A disordered drug molecule intercalates from each major groove side, spearing the cavity so that its major axis aligns obliquely with respect to the long axes of the base pairs (Figs 4a and 5).
Figure 2.
Stereo view of the double-stranded d(CG5BrUACG)2 with bound 9-aminobutyl-DACA in grey, the Co(II) ions (labelled) are in black.
Figure 3.
Schematic representation of the overall structure. The asymmetric unit is enclosed in a box, the 2-fold axes are indicated by filled ovals, 9ABD is 9-aminobutyl-DACA, # indicates that cytosine C7 is from another duplex, within the nominated asymmetric unit the symmetry operation for C7# is –x, y, –z.
Figure 4.
(a) Stereo view of the double intercalation site of the d(CGTACG) complex. DNA and corresponding 9-aminobutyl-DACA molecules from different duplexes are represented in different colours. Nucleotides C7 and G8 from four different duplexes are shown, nucleotides C5 and G6 for two duplexes only (yellow and red) are drawn. The sodium ion is drawn in black. Black dashed lines indicate sodium coordination bonds to the guanine G8 phosphate oxygens. (b) Stereo view of the unpaired end of the d(CG5BrUACG) showing the Co(II) ion coordinated to the N7 atoms of two guanine G12s. The G12 nucleotides are coloured green, Co(II) ions black, bromine atoms red and water oxygen atoms pink. Nucleotide pairs A10/U3 and C11/G2 from the two duplexes are shown in different colours (blue and yellow) to distinguish the different duplexes. Dashed lines indicate hydrogen bonds and solid black lines indicate Co(II) coordination bonds.
Figure 5.
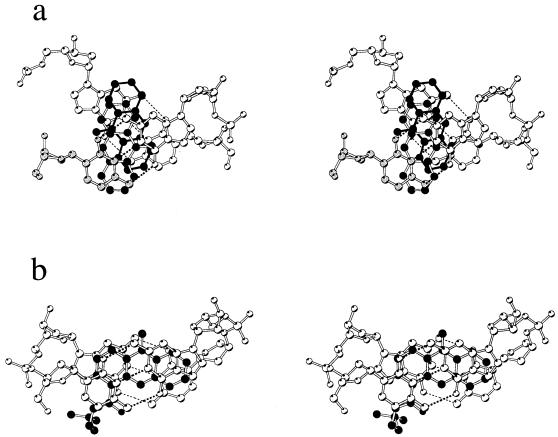
Stereo view of a projection down the helix axis of the (pseudo) intercalation site: (a) this work, with the symmetry related cytosine C7 atoms grey, and, for comparison, (b) previous structure of 9-amino-DACA intercalated into d(CGTACG)2 (15). The ligand is depicted with black atoms for (b) and the major component of (a), and with black atoms and black bonds for the minor component of (a).
At the other end of the duplex the terminal guanine, G12, is unpaired from cytosine C1 and the G2/C11 base pair stacks on the G2/C11 base pair of the abutting duplex in the column. These two end-stacked base pairs are separated by a distance of 3.4 Å and there is a large pseudo-helix twist angle of 65° between them. The N7 nitrogen atom of guanine G2 is co-ordinated to a partially occupied Co(II) ion. The unpaired G12 swings into its own major groove as a consequence of a flip in its sugar pucker, and comes to rest in the minor groove of the next symmetry-related duplex where it makes specific hydrogen bonding contacts (Fig. 4b).
Within the crystal lattice, the DNA molecules align in columns, forming sheets in the ab plane (see Figure S2, Supplementary Material). The centres of consecutive sheets are separated along the c axis by 20.2 Å with the DNA columns lying at an angle of 60° to each other. These sheets are held together by intermolecular interactions between the crossed columns of duplexes, a different type of interaction occurring between alternate layers. On one face of the sheet the reciprocal C7 cytosine exchanges occur at 2-fold axes along the ab diagonal twice within each unit cell. On the other face of the sheet, at the intervening 2-fold axes, intermolecular interactions are mediated by a Co(II) ion which is coordinated to the N7 atoms of two guanine G12s. By these means, the structure extends fully in three dimensions throughout the lattice, which gives added stability to this crystal form.
Conformation of the central base pairs. The backbone torsion angles and sugar puckers (Table 1) for the central 4 bp of the duplex fall within the normal range for B-DNA except for those of guanine G8 where the sugar pucker is C3′-endo and the P-O5′ bond torsional angle is 69°. These changes, together with concomitant modifications to the mainchain torsion angles of cytosine C7, cause the G8 phosphate group to swing away from the duplex, and the C7 base to flip out of the helical stack, thus enabling it to form a hydrogen-bonded base pair with guanine G6 of a symmetry-related DNA molecule (Fig. 4a). In contrast, some of the helicoidal properties of the central base pairs and base-pair steps are quite perturbed compared to B-DNA (Table 2) as a consequence of intercalation and intermolecular base pair stacking. For example, the twist value for the A4pC5 step is reduced by 9°, its roll angle is widened to 5° and its shift and slide values differ from those of B-DNA by ∼1 Å, changes which serve to slide the underwound C5/G8 base pair out of the helical stack in the direction of the guanine and position it more towards the major groove.
Table 1. Sugar–phosphate torsion angles, glycosidic angles (°) and furanose conformations for the d(CGTACG)2/9-aminobutyl-DACA complex, data for the complex with d(CG5BrUACG)2 are in bolda.
| α P-O5′ | β O5′-C5′ | γ C5′-C4′ | δ C4′-C3′ | ɛ C3′-O3′ | ζ O3′-P | χ C1′-N | P | τm | pucker | |
|---|---|---|---|---|---|---|---|---|---|---|
| G2 | 67 27 | 139 153 | –172 –167 | –97 –97 | –118 –117 | 160 180 | 42 40 | C2′-endo C2′-endo | ||
| 5BrU3/T3 | –50 –48 | 168 164 | 43 34 | 109 111 | 169 168 | –83 –87 | –122 –120 | 112 111 | 42 43 | C1′-exo C1′-exo |
| A4 | –69 –53 | –171 –172 | 46 28 | 132 138 | –177 –172 | –92 –88 | –113 –117 | 140 146 | 40 38 | C2′-endo C2′-endo |
| C5 | –61 –67 | 170 165 | 42 52 | 108 109 | –165 –158 | –94 –87 | –126 –128 | 99 110 | 50 42 | O1′-endo C1′-exo |
| G6 | –79 –64 | –150 –150 | 60 36 | 93 81 | –88 –88 | 19 20 | 38 43 | C3′-endo C3′-endo | ||
| C7 | 169 180 | 151 133 | –131 –134 | 68 75 | –119 –117 | 156 149 | 47 37 | C2′-endo C2′-endo | ||
| G8 | 69 47 | 147 156 | 38 60 | 83 84 | –169 –174 | –70 –55 | –162 –161 | 14 21 | 42 32 | C3′-endo C3′-endo |
| 5BrU3/ T9 | –70 –66 | –171 –179 | 68 60 | 144 136 | 179 178 | –102 –82 | –127 –128 | 158 150 | 38 35 | C2′-endo C2′-endo |
| A10 | –64 –71 | –168 –165 | 48 41 | 141 144 | –171 –162 | –88 –90 | –117 –119 | 162 164 | 41 38 | C2′-endo C2′-endo |
| C11 | –63 –54 | 168 171 | 45 33 | 128 131 | –160 –160 | –84 –76 | –110 –106 | 134 132 | 52 42 | C1′-exo C1′-exo |
| G12 | –60 –57 | –166 –176 | 64 61 | 98 99 | –60 –65 | 347 335 | 39 42 | C2′-exo C2′-exo | ||
| B DNAb | –62 | BI 176 | 48 | BI 128 | BI –176 | BI –95 | BIR-102 | 160 | 35 | C2′-endo |
| BII 146 | BII 144 | BII –114 | BII 174 | BIY-119 | ||||||
| BII –89 | ||||||||||
| A DNAb | –175 | 174 | 56 | 81 | –157 | –71 | –161 | 15 | 35 | C3′-endo |
| –67 | 150 |
P, pseudorotation phase angle; τm, pseudorotation amplitude.
aAll parameters were calculated using the Curves 5.2 programme (29).
bAverage values for DNA taken from Schneider et al. (45).
Table 2. Geometrical properties of base-pair steps and base pairs for the d(CGTACG)2/9-aminobutyl-DACA complex, data for the d(CG5BrUACG)2 complex are in bolda.
| Base pair or base | Step | Shift (Å) | Slide (Å) | Rise (Å) | Tilt (°) | Roll (°) | Twist (°) | Propeller twist (°) | Buckle (°) |
|---|---|---|---|---|---|---|---|---|---|
| G2–C11 | 3.2 2.7 | –9.33 –11.8 | |||||||
| 2 | –0.1 –0.2 | –0.4 –0.5 | 3.2 3.1 | –2.0 –1.7 | 2.7 3.3 | 34 31 | |||
| 5BrU3/T3–A10 | –3.9 –2.4 | 1.6 3.0 | |||||||
| 3 | –0.1 0.0 | –0.9 –0.8 | 3.2 3.4 | 3.9 1.3 | 1.8 6.4 | 36 35 | |||
| A4–T9/5BrU9 | 3.5 3.6 | 2.4 –5.7 | |||||||
| 4 | 1.1 0.8 | –0.9 –0.6 | 3.1 2.9 | –2.0 –3.0 | 5.0 2.2 | 27 31 | |||
| C5–G8 | –3.6 –2.5 | 15.2 15.9 | |||||||
| 5c | 3.0 2.5 | 2.5 2.5 | 6.8 6.8 | 1.4 –0.3 | –1.7 –1.8 | 55 51 | |||
| G6– | |||||||||
| B DNA b | 0 | 0 | 3.4 | 2 | 1 | 36 | –11 | ||
| A DNAb | –4.4 | –1.5 | 2.9 | 12 | 6 | 31 | –8 |
aAll parameters were calculated using the Curves 5.2 programme (29).
bAverage values for DNA taken from Neidle (46).
cSingle-strand values used for last step.
Structure of the intercalation site. The C3′-endo pucker of guanine G8, and the movement of its base away from the helix axis, is accompanied by a shift of the cytosine C5 sugar to an O1′-endo conformation. This flattens the sugar ring and pushes the pyrimidine towards the helix axis, so maintaining normal Watson–Crick hydrogen bonding distances. Thus, the structural parameters for the base pairs at step 4 (A4pC5), taken together, suggest that the DNA conformation is somewhat dislocated at this point, as the structure adapts to accommodate an underwound C2′-endo–C3′-endo sugar pucker junction. The propeller twist of C5/G8 is low, being almost flat, but its buckle angle of 15° is large and positive, and the base pair presents a concave ‘face’ to the pseudo-intercalation cavity (Fig. 4a). The rise between base pair planes at the binding site of 6.8 Å is as expected for intercalation of an acridine (Table 2). However, the helical twist angle of 55° (Table 2; Fig. 5a), as defined by the Curves programme for the rotation between guanine G6 and cytosine C5, is 19° larger than the canonical B-DNA value. Thus, in the pseudo-intercalation cavity itself the major axes of the sandwiching G/C base pairs make an angle of ∼60° to each other in projection along the helix axis (Fig. 5a). This overwinding contrasts with our recent findings (15), where the base pairs at the (true) intercalation site are generally underwound by ∼8° for this DNA with these types of ligands (see Fig. 5 for comparison). Here, on the continuous DNA strand, the cavity is generated by a 34° rotation in the mainchain torsion angle β for guanine G6 and by a move to a C3′-endo sugar pucker (Table 1), changes that again contrast with our previous work where the binding site was largely produced by modifications to the α and γ torsion angles (15). The slide and shift parameters show that guanine G6 has also moved substantially out of the helical stack towards its own sugar–phosphate backbone and into the major groove. In order to bring its base into alignment with the helical stack for hydrogen bonding with cytosine C7, its glycosidic angle rotates 73° from the canonical C3′-endo A-DNA position. In contrast, the sugar pucker and glycosidic angle for cytosine C7 adopt normal B-motif values. The G6/C7 base pair is practically flat and buckles slightly in the opposite direction to the C5/G8 base pair (Fig. 4a).
The phosphate group of guanine G8 is swung out of its normal helical position by rotations about the O3′-P and P-O5′ bonds (Table 1). The drug intercalates from the major groove and comes to lie with its long axis at an angle of ∼60° to the major axes of the base pairs. It is disordered, so that the electrons of the 4-carboxamide sidechain are not detected, and we have modelled two conformations for the aromatic ring in the binding site related by a 180° rotation about the long axis of the chromophore. In the major conformation, the acridine ring stacks, rather poorly, on cytosine C7 and guanine G8 with its 9-amino group sandwiched between cytosine C5 and guanine G6 (Figs 4a and 5). This stacking pattern is strikingly different from that seen with 9-amino-DACA for this DNA (15) where the long axis of the drug is oriented parallel to the major axes of the flanking base pairs, which are themselves arranged in close alignment (Fig. 5).
Structure of the unpaired end. The mainchain torsion angles between cytosine C11 and the unpaired guanine G12 are typical of B-DNA, whereas the sugar pucker of the guanine has the unusual C2′-exo conformation and its glycosidic angle is rotated 50–60° from the B-DNA position. This manoeuvre flips both the sugar and the base out of the helical stack into their own major groove where they interact with the minor groove of the G2/C11 base pair of the adjacent duplex in the column (Fig. 4b). The G12 base lies at an angle of ∼45° to the plane of the G2/C11 base pair and makes several hydrogen-bonding contacts with it. The 2-amino groups and N3 nitrogen atoms of guanines G12 and G2 are reciprocally hydrogen bonded (both 3.0 Å), and the furanose oxygen of G12 hydrogen bonds to the 2-amino group of G2 (2.9 Å). In addition, the N1 nitrogen of guanine G12 is bound by a hydrogen-bonded bridging water molecule to the O1P phosphate oxygen of cytosine C11 in its own duplex (Fig. 4b). The G2/C11 base pair has little propeller twist but has a large negative buckle which appears to be associated with unfavourable steric interactions between the furanose oxygen of guanine G12 and the carbonyl oxygen of the symmetry-related cytosine C11 (3.2 Å), and between the coordinated Co(II) on the N7 of guanine G2 and the 5-Br atom of 5BrU3 (Fig. 4b). This Co(II) ion lies at a distance of 2.2 Å from the N7 of G2 and is weakly bound to a trans water molecule at 3.4 Å. No other water molecules were found associated with it. The bound water molecule, being on a 2-fold axis, is also coordinated to the Co(II) ion on the symmetry-related guanine G2 of the abutting base pair in the stack (Fig. 4b). In contrast, the Co(II) ion coordinated to N7 of guanine G12 (2.2 Å) is directly bound to the N7 atom of a symmetry-related G12 (Fig. 4b), and this ion has a full hydration shell. Two of the bound water molecules are hydrogen bonded to the O1P oxygen atoms of the two symmetry-related G12 guanines (2.7 Å), which may account for the unusually large H2O-Co(II)-H2O angle of 137° (Fig. 4b). These cobalt-mediated linkages between symmetry-related guanine G2s and G12s are important elements in stabilising the three-dimensional crystal lattice. To date, Co(II) ions have rarely been found in DNA crystals, there being a single report describing the direct coordination of Co(II) to the N7 atoms of guanines in Z-DNA and an anthracycline/DNA complex (30).
Structure of the d(CGTACG)2/9-aminobutyl-DACA complex
An asymmetric unit in the native complex again contains two strands of DNA, one disordered drug molecule and two Co(II) ions. But in this structure, we located more (31) water molecules and found that one of the Co(II) ions is at 50% occupancy (because it lies on the 2-fold axis) and one at full occupancy. We also identified an octahedrally coordinated Na+ ion at the junction of 2-fold axes 2.5 Å from four G8 phosphate oxygens (Fig. 4a) and 2.2 Å from two disordered water molecules. In contrast to the brominated complex, the Co(II) ion coordinated to guanine G2 (2.2 Å) has 100% occupancy and full octahedral water coordination of regular geometry. Otherwise, we can detect only minor differences between the structures of the native and brominated complexes.
DISCUSSION
The most significant finding of our study is the discovery of a novel intercalation complex involving four DNA duplexes, four ligand molecules, and two sets of paired (un)stacked base tetrads. Many DNA–ligand complexes have been studied crystallographically (31), but this is the first example where an intercalation cavity involves two pairs of intermolecularly hydrogen-bonded Watson–Crick base pairs. The cavity itself is formed by a number of elements, principal amongst which are the overlap of the terminal two GC base pairs of two duplexes to create the pair of tetrads, and the reciprocal exchange of the terminal cytosines with a symmetry-related pair of tetrads in the next plane. At this exchange point, four DNA duplexes form a cross-like structure in projection, with an angle of 60° between the arms, that has some similarities to crossover points in recombination events (32,33). Quadruplex structures containing tetrads are commonly observed in guanine-rich single-stranded DNAs related to telomeric and other biologically relevant sequences (34–36). For these tetrads, the hydrogen-bonding scheme involves interactions between guanine O6/N7 acceptor atoms and N1H/2-NH2 donor groups (34–36). There is also an example of two GC base pairs forming a tetrad in which bonding comprises Watson–Crick pairing and additional interactions between guanine 2-amino groups and cytosine O2 oxygen atoms (37). The intermolecular interaction and hydrogen bonding scheme we see here, Watson–Crick plus guanine–guanine 2-NH2/N3 interactions, although ubiquitously found in the structures of dodecamers related to the Dickerson–Drew sequence (38 and references therein), has not generally been perceived as being a ‘base tetrad’. Perhaps this is because the angle between the planes of the Watson–Crick base pairs in the Dickerson–Drew-type structures is ∼45° (38). In the present structure this angle is flattened to 30°, and the four-way interaction between the GC bases is more identifiable as quadruplex-like (Fig. 1a).
The extraordinary differences in the form of the crystal structures of the complexes of d(CGTACG)2 with 9-amino-DACA (15) and 9-aminobutyl-DACA are surprising, given that the free energy of binding to DNA of these compounds is experimentally indistinguishable (2,8). Indeed, we have observed that 9-amino-DACA forms crystals in both space groups in the same drop indicating that there is no large free energy difference between different molecular conformations in different crystal forms. Evidently, the selection of either form depends on crystal growth kinetics. The crystal structure of the 9-aminobutyl-DACA complex is most unlikely to be representative of the solution-phase interactions of ligand and hexanucleotide given that the series of interactions observed form an infinite three-dimensional polymer in the crystal. It seems, therefore, that the form of the complex revealed here is strongly influenced by the competing forces involved in crystal formation. There is much debate about the effect of such forces on the conformation and packing arrangements of DNA in crystal structures (39, see also 38 and references therein). A general finding for B-type decamers and dodecamers is that in order to fit in the observed lattices, oligonucleotides with CpG ends modify their effective helical repeat by overlapping their termini in the manner of the Dickerson–Drew interaction (19,38), or by fraying the end and placing the terminal guanine in the minor groove of the adjacent duplex (20–24). Both manoeuvres effectively result in a shorter helix to pack end-to-end into the crystal lattice. In the 9-amino-DACA/d(CGTACG)2 complex, duplexes are stacked end-to-end in columns and the structure repeats every ninth position along the stack. This is achieved by packing together two intercalated drug molecules, six base pairs and a third drug molecule, the latter lodged between duplexes on a 2-fold axis (15). In order to secure a similar 9-fold repeat, the DNA in the 9-aminobutyl-DACA complex adopts both strategies for helix shortening, i.e. CpG base pair overlap at one end and frayed guanine–minor groove interaction at the other. Given the palindromic nature of the DNA, this necessarily results in the formation of an asymmetric complex.
Cytosine exchange enables the geometry of the binding cavity in the 9-aminobutyl-DACA complex to deviate substantially from that normally seen in intercalation complexes (for example, see 11,12,14,15), so that the base pairs are overwound, rather than underwound, and the helical rotation between them is much larger than could be accommodated in a regular duplex (Fig. 5). The structure is stabilised at the point of exchange by maintaining the glycosidic angle of the cytosine in its preferred anti conformation and by the interaction of a water molecule/Na+ ion with the phosphate groups of the four symmetry-related G8 guanines (Fig. 4a). Significantly, the cytosine exchange process itself contributes to stabilising the observed crystal form since it provides bonding interactions between successive planes in the structure on the opposite face to the strong G12-Co(II)-G12 interactions. The potential general importance of interactions of the latter type in stabilising unusual DNA crystal structures is shown by the recent observation of Ni2+–N7 guanine interactions between flipped-up terminal GC base pairs in the structure of d(CGTATATACG)2 (21). The pseudo-intercalation cavity itself is directly stabilised by binding of the dicationic 9-aminobutyl-DACA, which intercalates obliquely so as to maximise chromophore–base pair stacking interactions (Fig. 5), the unusual binding orientation being the consequence of the large overwinding of the CG base pairs.
In summary, it appears that the geometrical requirements to pack the hexanucleotide into a crystal lattice in the presence of Co(II) and the ligand are the major determinants of the drug–DNA complex we see, with the flexibility of the geometry of the pseudo-intercalation cavity conferred by strand exchange, being the point at which adjustments can be made in the fine structure. Given this conclusion, and the evident disorder in the location of the acridine chromophore, it is probable that the nature of the intercalating ligand is not a major consideration, and that other intercalators could be accommodated in this crystal form. Finally, we note that the unique intercalation geometry we observe here, involving four DNA duplexes, GC tetrads and cytosine strand exchanges, may provide new insights into understanding the biological properties of intercalating agents. Strand invasion and/or the formation of multiple-stranded helices are components of recombination (40), integration (41), and DNA repair events (42), all of which are potentially sensitive to modification by intercalators. From a cancer drug development perspective, our findings might have relevance to the search for inhibitors of the enzyme telomerase that are designed to work by stabilising telomere quadruplexes (43,44), and they raise the interesting issue as to whether intercalating agents, as employed in the clinical setting, have any effects on the repair of DNA double-strand breaks, the cytotoxic lesion induced by radiation treatment.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We wish to thank Barry Fields for help with collection of the MAD data set, the Association for International Cancer Research (grant to C.A.C. and L.P.G.W.) and the National Health and Medical Research Council of Australia (grant to J.M.G. and W.A.D.) for financial support. Access to BioCARS Sector 14 at the Advanced Photon Source at Argonne, IL, was provided by the Australian Synchrotron Research Programme, which is funded by the Commonwealth of Australia as a Major National Research Facility. BioCARS Sector 14 is supported by a grant RR07707 from the US National Institutes of Health, National Centre for Research Resources. The Advanced Photon Source is supported by the US Department of Energy, Basic Energy Sciences, Office of Research, under Contract No. W-31-109-Eng-38.
PDB accession nos 1FN1, 1FN2
REFERENCES
- 1.Malonne H. and Atassi,G. (1997) AntiCancer Drugs, 8, 811–822. [DOI] [PubMed] [Google Scholar]
- 2.Atwell G.J., Cain,B.F., Baguley,B.C., Finlay,G.J. and Denny,W.A. (1984) J. Med. Chem., 27, 1481–1485. [DOI] [PubMed] [Google Scholar]
- 3.Denny W.A., Roos,I.A.G. and Wakelin,L.P.G. (1986) Anticancer Drug Des., 1, 141–147. [PubMed] [Google Scholar]
- 4.Finlay G.J., Riou,J.F. and Baguley,B.C. (1996) Eur. J. Cancer, Part A, 32A, 708–714. [Google Scholar]
- 5.Atwell G.J., Rewcastle,G.W., Baguley,B.C. and Denny,W.A. (1987) J. Med. Chem., 30, 664–669. [DOI] [PubMed] [Google Scholar]
- 6.Baguley B.C., Zhuang,L. and Marshall,E. (1995) Cancer Chemother. Pharmacol., 36, 244–248. [DOI] [PubMed] [Google Scholar]
- 7.McCrystal M.R., Evans,B.D., Harvey,V.J., Thompson,P.I., Porter,D.J. and Baguley,B.C. (1999) Cancer Chemother. Pharmacol., 44, 39–44. [DOI] [PubMed] [Google Scholar]
- 8.Rewcastle G.W., Atwell,G.J., Chambers,D., Baguley,B.C. and Denny,W.A. (1986) J. Med. Chem., 29, 472–477. [DOI] [PubMed] [Google Scholar]
- 9.Denny W.A., Atwell,G.J., Rewcastle,G.W. and Baguley,B.C. (1987) J. Med. Chem., 30, 658–663. [DOI] [PubMed] [Google Scholar]
- 10.Wakelin L.P.G., Atwell,G.J., Rewcastle,G.W. and Denny,W.A. (1987) J. Med. Chem., 30, 855–861. [DOI] [PubMed] [Google Scholar]
- 11.Wang A.H.J., Ughetto,G., Quigley,G.J. and Rich,A. (1987) Biochemistry, 26, 1152–1163. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y. and Wang,A.H.J. (1995) J. Biomol. Struct. Dyn., 13, 103–118. [DOI] [PubMed] [Google Scholar]
- 13.Bailly C., Denny,W.A., Mellor,L.E., Wakelin,L.P.G. and Waring,M.J. (1992) Biochemistry, 31, 3514–3524. [DOI] [PubMed] [Google Scholar]
- 14.Todd A.K., Adams,A., Thorpe,J.H., Denny,W.A., Wakelin,L.P.G. and Cardin,C.J. (1999) J. Med. Chem., 42, 536–540. [DOI] [PubMed] [Google Scholar]
- 15.Adams A., Guss,J.M., Collyer,C.A., Denny,W.A. and Wakelin,L.P.G. (1999) Biochemistry, 38, 9221–9233. [DOI] [PubMed] [Google Scholar]
- 16.Adams A., Guss,J.M., Collyer,C.A., Denny,W.A., Prakash,A.S. and Wakelin,L.P.G. (2000) Mol. Pharmacol., 58, 649–658. [DOI] [PubMed] [Google Scholar]
- 17.Malinina L., Urpi,L., Salas,X., Huynhdinh,T. and Subirana,J.A. (1994) J. Mol. Biol., 243, 484–493. [DOI] [PubMed] [Google Scholar]
- 18.Lipscomb L.A., Zhou,F.X., Presnell,S.R., Woo,R.J., Peek,M.E., Plaskon,R.R. and Williams,L.D. (1996) Biochemistry, 35, 2818–2823. [DOI] [PubMed] [Google Scholar]
- 19.Wing R., Drew,H., Takano,T., Broka,C., Tanaka,S., Itakura,K. and Dickerson,R.E. (1980) Nature, 287, 755–758. [DOI] [PubMed] [Google Scholar]
- 20.Minasov G., Tereshko,V. and Egli,M. (1999) J. Mol. Biol., 291, 83–99. [DOI] [PubMed] [Google Scholar]
- 21.Abrescia N.G.A., Malinina,L., Fernandez,L.G., Huynh-Dinh,T., Neidle,S. and Subirana,J.A. (1999) Nucleic Acids Res., 27, 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spink N., Nunn,C.M., Vojtechovsky,J., Berman,H.M. and Neidle,S. (1995) Proc. Natl Acad. Sci. USA, 92, 10767–10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunn C.M., Garman,E. and Neidle,S. (1997) Biochemistry, 36, 4792–4799. [DOI] [PubMed] [Google Scholar]
- 24.Liu J. and Subirana,J.A. (1999) J. Biol. Chem., 274, 24749–24752. [DOI] [PubMed] [Google Scholar]
- 25.Otwinowski Z. (1993) In Sawyer,L., Isaacs,N. and Bailey,S.S. (eds), Proceedings of the CCP4 Study Weekend: Data Collection and Processing. SERC Daresbury Laboratory, Warrington, UK, pp. 56–62.
- 26.Brunger A.T., Adams,P.D., Clore,G.M., DeLano,W.L., Gros,P., Grosse-Kunstleve,R.W., Jiang,J.-S., Kuszewski,J., Nilges,M., Pannu,N.S., Read,R.J., Rice,L.M., Simonson,T. and Warren,G.L. (1998) Acta Crystallogr. Sec. D Biol. Crystallogr., D54, 905–921. [DOI] [PubMed] [Google Scholar]
- 27.Jones T.A., Zou,J.-Y., Cowan,S.W. and Kjeldgaard,M. (1991) Acta Crystallogr., A47, 110–119. [Google Scholar]
- 28.Sheldrick G.M. and Schneider,T.R. (1997) Methods Enzymol., 277, 319–343. [PubMed] [Google Scholar]
- 29.Lavery R. and Sklenar,H. (1989) J. Biomol. Struct. Dynam., 6, 655–667. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y.G., Sriram,M. and Wang,A.H.J. (1993) Nucleic Acids Res., 21, 4093–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman H.M., Olson,W.K., Beveridge,D.L., Westbrook,J., Gelbin,A., Demeny,T., Hsieh,S.H., Srinivasan,A.R. and Schneider,B. (1992) Biophys. J., 63, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz-Lombardia M., Gonzalez,A., Eritja,R., Aymami,J., Azorin,F. and Coll,M. (1999) Nature Struct. Biol., 6, 913–917. [DOI] [PubMed] [Google Scholar]
- 33.Lilley D.M.J. and Norman,D.G. (1999) Nature Struct. Biol., 6, 897–899. [DOI] [PubMed] [Google Scholar]
- 34.Kang C.H., Zhang,X., Ratliff,R., Moyzis,R. and Rich,A. (1992) Nature, 356, 126–131. [DOI] [PubMed] [Google Scholar]
- 35.Phillips K., Dauter,Z., Murchie,A.I.H., Lilley,D.M.J. and Luisi,B. (1997) J. Mol. Biol., 273, 171–182. [DOI] [PubMed] [Google Scholar]
- 36.Laughlan G., Murchie,A.I.H., Norman,D.G., Moore,M.H., Moody,P.C.E., Lilley,D.M. and Luisi,B. (1994) Science, 265, 520–524. [DOI] [PubMed] [Google Scholar]
- 37.Leonard G.A., Zhang,S., Peterson,M.R., Harrop,S.J., Helliwell,J.R., Cruse,W.B.T., Langlois d’Estaintot,B., Kennard,O., Brown,T. et al. (1995) Structure, 3, 335–340. [DOI] [PubMed] [Google Scholar]
- 38.Tereshko V. and Subirana,J.A. (1999) Acta Crystallogr. Sec. D Biol. Crystallogr., D55, 810–819. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson R.E., Goodsell,D.S. and Neidle,S. (1994) Proc. Natl Acad. Sci. USA, 91, 3579–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gopaul D.N. and Van Duyne,G.D. (1999) Curr. Opin. Struct. Biol., 9, 14–20. [DOI] [PubMed] [Google Scholar]
- 41.Curcio M.J. and Morse,R.H. (1996) Trends Genet., 12, 436–438. [DOI] [PubMed] [Google Scholar]
- 42.Lindahl T. and Wood,R.D. (1999) Science, 286, 1897–1905. [DOI] [PubMed] [Google Scholar]
- 43.Perry P.J., Reszka,A.P., Wood,A.A., Read,M.A., Gowan,S.M., Dosanjh,H.S., Trent,J.O., Jenkins,T.C., Kelland,L.R. and Neidle,S. (1998) J. Med. Chem., 41, 4873–4884. [DOI] [PubMed] [Google Scholar]
- 44.Izbicka E., Wheelhouse,R.T., Raymond,E., Davidson,K.K., Lawrence,R.A., Sun,D., Windle,B.E., Hurley,L.H. and Hoff,D.D.V. (1999) Cancer Res., 59, 639–644. [PubMed] [Google Scholar]
- 45.Schneider B., Neidle,S. and Berman,H.M. (1997) Biopolymers, 42, 113–124. [DOI] [PubMed] [Google Scholar]
- 46.Neidle S. (1994) DNA Structure and Recognition. In Rickwood,D.M.D. (ed.), Focus. Oxford University Press, Oxford, UK. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.