Abstract
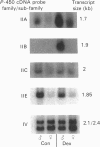
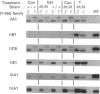
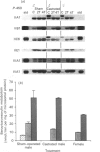
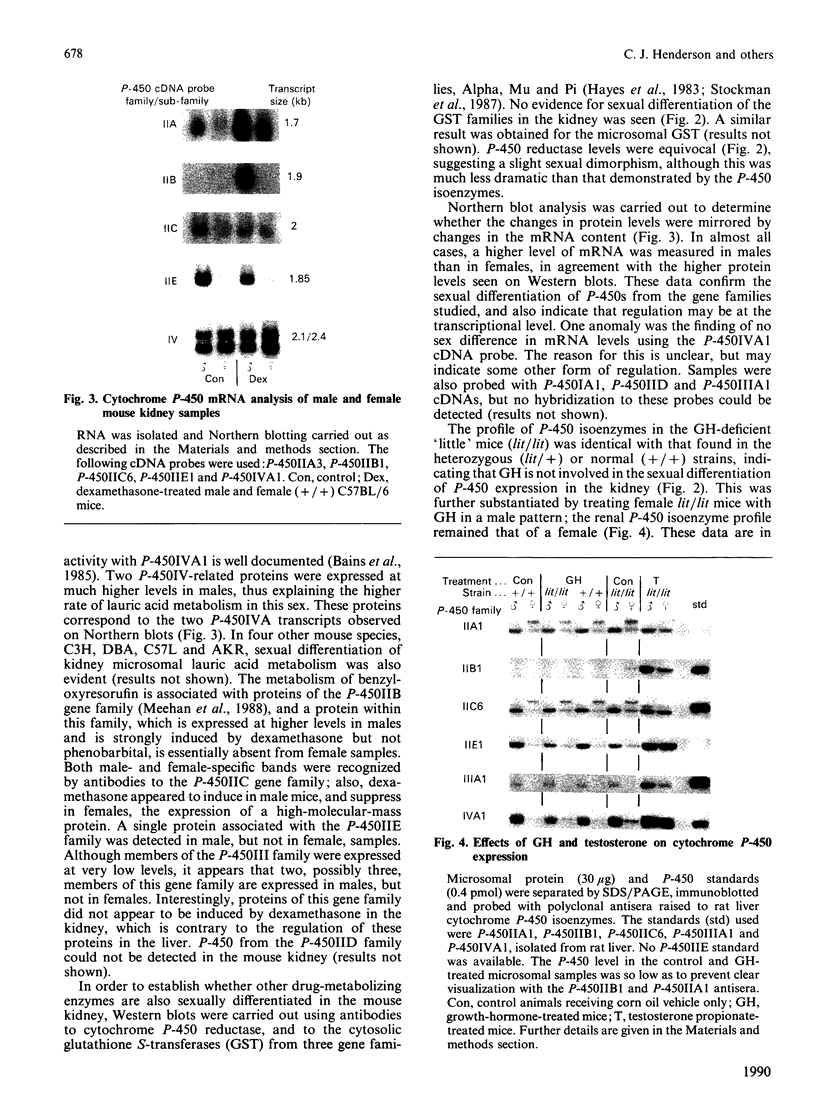
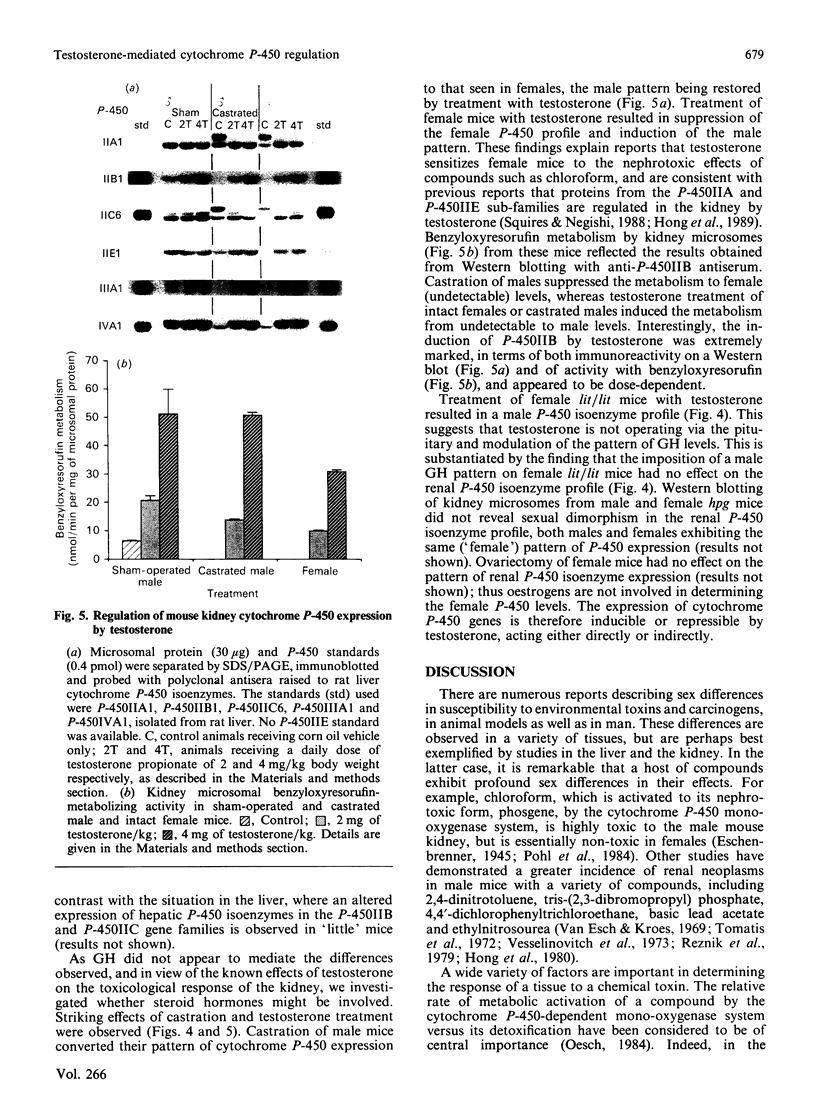
We have studied the extent to which mouse renal cytochrome P-450 isoenzymes are sexually differentiated, and the factor(s) regulating this dimorphism. Intriguingly, sex differences were not seen in the expression of a single cytochrome P-450 enzyme, but were observed in the expression of all P-450 isoenzymes detectable, encoded by six gene families or sub-families. This effect was mediated by testosterone, which had the capacity to both induce and repress P-450 gene expression, and which was independent of growth hormone. The changes in protein content were mirrored in all but one case by changes in the levels of mRNA, indicating that these genes contain hormone-responsive elements. These findings are consistent with numerous reports of sex differences in the susceptibility of the mouse kidney to the toxic and carcinogenic effects of drugs and environmental chemicals, many of which are metabolized to cytotoxic products by the cytochrome P-450-dependent mono-oxygenases. These data imply that circulating androgen levels will be an important factor in susceptibility of the kidney to toxic or carcinogenic compounds which require metabolic activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bains S. K., Gardiner S. M., Mannweiler K., Gillett D., Gibson G. G. Immunochemical study on the contribution of hypolipidaemic-induced cytochrome P-452 to the metabolism of lauric acid and arachidonic acid. Biochem Pharmacol. 1985 Sep 15;34(18):3221–3229. doi: 10.1016/0006-2952(85)90338-7. [DOI] [PubMed] [Google Scholar]
- Beamer W. H., Eicher E. M. Stimulation of growth in the little mouse. J Endocrinol. 1976 Oct;71(1):37–45. doi: 10.1677/joe.0.0710037. [DOI] [PubMed] [Google Scholar]
- Bendele A. M., Carlton W. W., Krogh P., Lillehoj E. B. Ochratoxin A carcinogenesis in the (C57BL/6J X C3H)F1 mouse. J Natl Cancer Inst. 1985 Oct;75(4):733–742. [PubMed] [Google Scholar]
- Blanck A., Aström A., Hansson T., De Pierre J. W., Gustafsson J. A. Pituitary regulation of cytochrome P-450-mediated metabolism of steroids and xenobiotics in rat liver microsomes. Carcinogenesis. 1986 Apr;7(4):575–582. doi: 10.1093/carcin/7.4.575. [DOI] [PubMed] [Google Scholar]
- Bloom H. J. Proceedings: Hormone-induced and spontaneous regression of metastatic renal cancer. Cancer. 1973 Nov;32(5):1066–1071. doi: 10.1002/1097-0142(197311)32:5<1066::aid-cncr2820320507>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Burke M. D., Thompson S., Elcombe C. R., Halpert J., Haaparanta T., Mayer R. T. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985 Sep 15;34(18):3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Iddon C. A., Charlton H. M., Chiappa S. A., Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977 Sep 22;269(5626):338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- Cheng T. C., Beamer W. G., Phillips J. A., 3rd, Bartke A., Mallonee R. L., Dowling C. Etiology of growth hormone deficiency in little, Ames, and Snell dwarf mice. Endocrinology. 1983 Nov;113(5):1669–1678. doi: 10.1210/endo-113-5-1669. [DOI] [PubMed] [Google Scholar]
- Clark R. G., Robinson I. C. Effects of a fragment of human growth hormone-releasing factor in normal and 'Little' mice. J Endocrinol. 1985 Jul;106(1):1–5. doi: 10.1677/joe.0.1060001. [DOI] [PubMed] [Google Scholar]
- Earnshaw D., Dale J. W., Goldfarb P. S., Gibson G. G. Differential splicing in the 3' non-coding region of rat cytochrome P-452 (P450 IVA1) mRNA. FEBS Lett. 1988 Aug 29;236(2):357–361. doi: 10.1016/0014-5793(88)80055-3. [DOI] [PubMed] [Google Scholar]
- Edén S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979 Aug;105(2):555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Beamer W. G. Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J Hered. 1976 Mar-Apr;67(2):87–91. doi: 10.1093/oxfordjournals.jhered.a108682. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felder M. R., Watson G., Huff M. O., Ceci J. D. Mechanism of induction of mouse kidney alcohol dehydrogenase by androgen. Androgen-induced stimulation of transcription of the Adh-1 gene. J Biol Chem. 1988 Oct 5;263(28):14531–14537. [PubMed] [Google Scholar]
- Hawke R. L., Welch R. M. Major differences in the specificity and regulation of mouse renal cytochrome P-450-dependent monooxygenases. A comparison of xenobiotic and endogenous substrates. Mol Pharmacol. 1985 Sep;28(3):283–289. [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Hong J. Y., Pan J. M., Ning S. M., Yang C. S. Molecular basis for the sex-related difference in renal N-nitrosodimethylamine demethylase in C3H/HeJ mice. Cancer Res. 1989 Jun 1;49(11):2973–2979. [PubMed] [Google Scholar]
- Jansson J. O., Downs T. R., Beamer W. G., Frohman L. A. Receptor-associated resistance to growth hormone-releasing factor in dwarf "little" mice. Science. 1986 Apr 25;232(4749):511–512. doi: 10.1126/science.3008329. [DOI] [PubMed] [Google Scholar]
- Kandala J. C., Kistler M. K., Kistler W. S. Androgen regulated genes from prostate and seminal vesicle share upstream sequence homologies. Biochem Biophys Res Commun. 1985 Jan 31;126(2):948–952. doi: 10.1016/0006-291x(85)90277-3. [DOI] [PubMed] [Google Scholar]
- Koenig H., Goldstone A., Blume G., Lu C. Y. Testosterone-mediated sexual dimorphism of mitochondria and lysosomes in mouse kidney proximal tubules. Science. 1980 Aug 29;209(4460):1023–1026. doi: 10.1126/science.7403864. [DOI] [PubMed] [Google Scholar]
- Koths K. E., Godchaux W., 3rd, Doeg L. H., Doeg K. The effect of testosterone on the synthesis of mouse kidney mitochondrial and microsomal proteins in vivo and in vitro. Endocrinology. 1972 Jul;91(1):125–134. doi: 10.1210/endo-91-1-125. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod J. N., Shapiro B. H. Growth hormone regulation of hepatic drug-metabolizing enzymes in the mouse. Biochem Pharmacol. 1989 May 15;38(10):1673–1677. doi: 10.1016/0006-2952(89)90316-x. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Barlow D. P., Hill R. E., Hogan B. L., Hastie N. D. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984 Aug;3(8):1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Forrester L. M., Stevenson K., Hastie N. D., Buchmann A., Kunz H. W., Wolf C. R. Regulation of phenobarbital-inducible cytochrome P-450s in rat and mouse liver following dexamethasone administration and hypophysectomy. Biochem J. 1988 Sep 15;254(3):789–797. doi: 10.1042/bj2540789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J. S., Bickmore W., Brook J. D., McLaren A. W., Meehan R., Wolf C. R. Close linkage of the human cytochrome P450IIA and P450IIB gene subfamilies: implications for the assignment of substrate specificity. Nucleic Acids Res. 1989 Apr 25;17(8):2907–2917. doi: 10.1093/nar/17.8.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mode A., Gustafsson J. A., Jansson J. O., Edén S., Isaksson O. Association between plasma level of growth hormone and sex differentiation of hepatic steroid metabolism in the rat. Endocrinology. 1982 Nov;111(5):1692–1697. doi: 10.1210/endo-111-5-1692. [DOI] [PubMed] [Google Scholar]
- Mode A., Norstedt G., Eneroth P., Gustafsson J. A. Purification of liver feminizing factor from rat pituitaries and demonstration of its identity with growth hormone. Endocrinology. 1983 Oct;113(4):1250–1260. doi: 10.1210/endo-113-4-1250. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Norstedt G., Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell. 1984 Apr;36(4):805–812. doi: 10.1016/0092-8674(84)90030-8. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Orton T. C., Parker G. L. The effect of hypolipidemic agents on the hepatic microsomal drug-metabolizing enzyme system of the rat. Induction of cytochrome(s) P-450 with specificity toward terminal hydroxylation of lauric acid. Drug Metab Dispos. 1982 Mar-Apr;10(2):110–115. [PubMed] [Google Scholar]
- Pohl L. R., George J. W., Satoh H. Strain and sex differences in chloroform-induced nephrotoxicity. Different rates of metabolism of chloroform to phosgene by the mouse kidney. Drug Metab Dispos. 1984 May-Jun;12(3):304–308. [PubMed] [Google Scholar]
- Reznik G., Ward J. M., Hardisty J. F., Russfield A. Renal carcinogenic and nephrotoxic effects of the flame retardant tris(2,3-dibromopropyl) phosphate in F344 rats and (C57BL/6N X C3H/HeN)F1 mice. J Natl Cancer Inst. 1979 Jul;63(1):205–212. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rush G. F., Smith J. H., Newton J. F., Hook J. B. Chemically induced nephrotoxicity: role of metabolic activation. Crit Rev Toxicol. 1984;13(2):99–160. doi: 10.3109/10408448409034079. [DOI] [PubMed] [Google Scholar]
- Smith J. H., Maita K., Sleight S. D., Hook J. B. Effect of sex hormone status on chloroform nephrotoxicity and renal mixed function oxidases in mice. Toxicology. 1984 Apr 16;30(4):305–316. doi: 10.1016/0300-483x(84)90141-0. [DOI] [PubMed] [Google Scholar]
- Squires E. J., Negishi M. Reciprocal regulation of sex-dependent expression of testosterone 15 alpha-hydroxylase (P-450(15 alpha)) in liver and kidney of male mice by androgen. Evidence for a single gene. J Biol Chem. 1988 Mar 25;263(9):4166–4171. [PubMed] [Google Scholar]
- Stockman P. K., McLellan L. I., Hayes J. D. Characterization of the basic glutathione S-transferase B1 and B2 subunits from human liver. Biochem J. 1987 May 15;244(1):55–61. doi: 10.1042/bj2440055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini P. P., Masson H. A., Bains S. K., Makowski R. J., Morris B., Gibson G. G. Multiple forms of hepatic cytochrome P-450. Purification, characterisation and comparison of a novel clofibrate-induced isozyme with other major forms of cytochrome P-450. Eur J Biochem. 1984 Mar 1;139(2):235–246. doi: 10.1111/j.1432-1033.1984.tb07999.x. [DOI] [PubMed] [Google Scholar]
- Tomatis L., Turusov V., Day N., Charles R. T. The effect of long-term exposure to DDT on CF-1 MICE. Int J Cancer. 1972 Nov;10(3):489–506. doi: 10.1002/ijc.2910100308. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch G. J., Kroes R. The induction of renal tumours by feeding basic lead acetate to mice and hamsters. Br J Cancer. 1969 Dec;23(4):765–771. doi: 10.1038/bjc.1969.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesselinovitch S. D., Itze L., Mihailovich N., Rao K. V., Manojlovski B. Role of hormonal environment, partial hepatectomy, and dose of ethylnitrosourea in renal carcinogenesis. Cancer Res. 1973 Feb;33(2):339–341. [PubMed] [Google Scholar]
- Watson G., Paigen K. mRNA synthesis rates in vivo for androgen-inducible sequences in mouse kidney. Mol Cell Biol. 1988 May;8(5):2117–2124. doi: 10.1128/mcb.8.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L., McDonald C., Higgins S. Sequence organisation of rat seminal vesicle F gene: location of transcriptional start point and sequence comparison with six other androgen-regulated genes. Nucleic Acids Res. 1985 Feb 11;13(3):659–672. doi: 10.1093/nar/13.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C. R., Moll E., Friedberg T., Oesch F., Buchmann A., Kuhlmann W. D., Kunz H. W. Characterization, localization and regulation of a novel phenobarbital-inducible form of cytochrome P450, compared with three further P450-isoenzymes, NADPH P450-reductase, glutathione transferases and microsomal epoxide hydrolase. Carcinogenesis. 1984 Aug;5(8):993–1001. doi: 10.1093/carcin/5.8.993. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Seilman S., Oesch F., Mayer R. T., Burke M. D. Multiple forms of cytochrome P-450 related to forms induced marginally by phenobarbital. Differences in structure and in the metabolism of alkoxyresorufins. Biochem J. 1986 Nov 15;240(1):27–33. doi: 10.1042/bj2400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Mode A., Norstedt G., Gustafsson J. A. Regulation of sexual differentiation in drug and steroid metabolism. Trends Pharmacol Sci. 1989 Apr;10(4):149–153. doi: 10.1016/0165-6147(89)90167-3. [DOI] [PubMed] [Google Scholar]