Abstract
Purpose
Patients with spinal metastases undergoing surgical treatment face challenges related to preoperative anemia, intraoperative blood loss, and frailty, emphasizing the significance of perioperative blood management. This retrospective analysis aimed to assess the correlation between hemoglobin-related parameters and outcomes, identifying key markers to aid in blood management.
Methods
A retrospective review was performed to identify patients who underwent surgical treatment for spinal metastases. Hb-related parameters, including baseline Hb, postoperative nadir Hb, predischarge Hb, postoperative nadir Hb drift, and predischarge Hb drift (both in absolute values and percentages) were subjected to univariate and multivariate analyses. These analyses were conducted in conjunction with other established variables to identify independent markers predicting patient outcomes. The outcomes of interest were postoperative short-term (6-week) mortality, long-term (1-year) mortality, and postoperative 30-day morbidity.
Results
A total of 289 patients were included. Our study demonstrated that predischarge Hb (OR 0.62, 95% CI 0.44–0.88, P = 0.007) was an independent prognostic factor of short-term mortality, while baseline Hb (OR 0.76, 95% CI 0.66–0.88, P < 0.001) was identified as an independent prognostic factor of long-term mortality. Additionally, nadir Hb drift (OR 0.82, 95% CI 0.70–0.97, P = 0.023) was found to be an independent prognostic factor for postoperative 30-day morbidity.
Conclusions
This study demonstrated that predischarge Hb, baseline Hb, and nadir Hb drift are prognostic factors for outcomes. These findings provide a foundation for precise blood management strategies. It is crucial to consider Hb-related parameters appropriately, and prospective intervention studies addressing these markers should be conducted in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-07748-9.
Keywords: Spinal metastases, Surgery, Hemoglobin, Blood management, Outcome.
Background
In addition to the lungs and liver, the spine is also a frequently observed location of malignant metastasis. Approximately 60–70% of patients diagnosed with systemic cancer develop spinal metastases, with about 10% experiencing symptoms [1, 2]. Surgical intervention for patients with spinal metastases offers pain relief, protection, and improvement of neurological function, as well as the maintenance and restoration of spinal stability [3, 4].
In patients with metastatic disease, the presence of preoperative anemia, significant intraoperative blood loss, and frailty underscores the critical clinical importance of perioperative blood management. In the perioperative period, multiple hemoglobin (Hb) monitoring is often conducted. The results at different time points and their correlations are closely associated with postoperative complications and patient prognosis [5–8]. A comprehensive, impartial analysis of the correlation between Hb-related parameters and patient outcomes is crucial for identifying important parameters. It is necessary to implement specific interventions based on these markers to improve patient prognosis.
The baseline Hb level prior to treatment serves as an indicator of the patient’s long-term physical condition. The baseline Hb level is included as a prognostic variable in certain scoring systems for spinal metastasis [5, 6]. Nadir Hb refers to the lowest recorded level of Hb for a patient during medical evaluation or monitoring. Postoperative Hb drift is defined as the difference between the preoperative Hb level and the postoperative nadir Hb level [9]. Studies have found these variables to be strongly associated with postoperative complications and prognosis [10–12]. In a few studies, predischarge Hb has also been examined [13–15].
In this study, we analyzed various Hb-related parameters, including baseline Hb, postoperative nadir Hb, predischarge Hb, postoperative nadir Hb drift, and predischarge Hb drift (both in absolute values and percentages). Our objective was to investigate their impact on postoperative morbidity and short- and long-term mortality. By providing these insights, our aim was to provide valuable information to enhance perioperative blood management strategies for patients with spinal metastases. We hope that these markers will attract the attention of clinicians and offer preliminary clues for further study.
Methods
Study participants
We conducted a retrospective review of the medical records of all patients who underwent spinal metastases surgery at our institution between January 2017 and December 2020. This study was approved by the institutional review board of our hospital (no. 2022-437-002).
Therapeutic approaches were managed by a multidisciplinary team, and the decision to perform surgery was based on the patient’s medical fitness, clinical presentation (neurological deficits, spinal instability, and intractable pain), oncological status, and the feasibility of surgical treatment.
Two deep incision drains were inserted postoperatively. Anticoagulation therapy was initiated 48–72 hours after surgery. The decision to administer intraoperative transfusion was based on a combination of factors, including blood loss, hemodynamic status, and intraoperative Hb levels. For postoperative transfusion, a threshold of 8 g/dL is typically used as the trigger. No intraoperative cell saver was used. In addition, patient symptoms and underlying medical conditions were considered during the decision-making process.
Follow-ups were prospectively administered to our hospital staff. Following surgery, scheduled follow-up assessments were performed at 3, 6, and 12 months in the first year, followed by semiannual evaluations for the subsequent two years, and annually thereafter.
The inclusion criteria were as follows: (1) a pathologically confirmed diagnosis of spinal metastasis; (2) spinal surgery for excision, osteotomy, decompression, fusion, or fixation; and (3) age greater than 18 years at the time of surgery. The exclusion criteria were as follows: (1) loss to follow-up within one year after surgery, precluding the definitive determination of 1-year survival, (2) underwent unplanned reoperation during hospitalization, and (3) exclusive reliance on percutaneous vertebroplasty.
Hb-related parameters and other factors
Preoperative blood tests were performed as part of standard patient evaluation. Routine blood tests were performed on the first postoperative day and monitored every 2–4 days unless indications of severe anemia or increased drainage were evident. The baseline Hb level was determined based on the most recent Hb level prior to surgery. The nadir Hb level was defined as the lowest postoperative Hb level recorded during hospitalization. The predischarge Hb level was defined as the final Hb test result before patient discharge. Absolute nadir Hb drift was defined as the difference between the baseline and postoperative nadir Hb levels. The percentage nadir Hb drift was calculated as (baseline Hb - nadir Hb) / baseline Hb × 100%. Absolute predischarge Hb drift was determined as the difference between the baseline and predischarge Hb levels. The percentage of predischarge Hb drift was computed as (baseline Hb–predischarge Hb) / baseline Hb × 100%.
The following variables were analyzed in conjunction with Hb-related parameters: age, primary tumor site (classified as slow, moderate, or rapid growth according to the classification proposed by Katagiri [7]), visceral metastases, number of spine metastases, presence of other bone metastases, Eastern Cooperative Oncology Group (ECOG) performance status, American Spinal Injury Association (ASIA) Impairment Scale, and transfusion.
Outcome measures
The outcomes of interest were postoperative short-term (6-week) mortality, long-term (1-year) mortality, and postoperative 30-day morbidity. Although 30-day mortality was also used in many studies as short-term mortality, this study opted for a 6-week postoperative criterion due to the lower short-term mortality rates in Asian populations [16, 17] and to mitigate the occurrence of fewer outcome events. The postoperative morbidities included in this study were systemic infection, pneumonia, cardiac arrest/failure/arrhythmia, myocardial infarction, delirium, stroke, venous thromboembolism, gastrointestinal bleeding, urinary tract infection, wound infection, and/or dehiscence [18–20].
Statistical analysis
Continuous variables were summarized as mean and standard deviation, while categorical variables were presented as frequencies and percentages. Initial univariate logistic regression analyzed each factor, and those with P < 0.10 underwent multivariate logistic regression with backward stepwise elimination. For correlated Hb-related parameters (absolute and percentage drift), only the variable with the lowest P value underwent multivariate analysis. In the final model, P < 0.05 was considered significant. The optimal cutoff value for plotting the survival curve was determined through logistic regression, selecting the split with the highest significance using the “cutoff " package in R. We performed statistical analysis using R version 4.1.3 for Windows (R Project for Statistical Computing, http://www.r-project.org/).
Results
Patient demographics and clinical outcomes
A total of 289 patients (138 male, 151 female) with an average age of 55.7 ± 10.5 were included in this study, adhering to the inclusion and exclusion criteria. Among the primary tumor site pathologies, lung cancer (n = 79, 27.3%) was the most prevalent, followed by breast cancer (n = 56, 19.4%), liver cancer (n = 18, 6.2%), and kidney cancer (n = 13, 4.5%). With reference to Katagiri’s classification of primary tumor sites of malignancy [7], 103 (36%) patients are of slow growth, 75 (26%) patients are of moderate growth, and 111 (38%) patients are of rapid growth. There were 37 patients with an ECOG score of 37, 62 with a score of 2, 90 with a score of 3, and 100 with a score of 4.
The baseline Hb level was 12.3 ± 1.8 g/dL, the nadir Hb was 9.7 ± 1.8 g/dL, and the predischarge Hb was 10.8 ± 1.6 g/dL. According to the formula presented above, the absolute nadir Hb drift was − 2.6 ± 1.7 g/dL, and the percentage nadir Hb drift was − 21% ± 13%. The absolute predischarge Hb drift was − 1.6 ± 1.9 g/dL, and the percentage predischarge Hb drift was − 12% ± 15%. The demographic and clinical characteristics of the patients are presented in Table 1.
Table 1.
Baseline characteristics of the patients (n = 289)
| Factors | Value |
|---|---|
| Age (years) | 55.7 ± 10.5 |
| Male sex | 138 (48%) |
| Primary tumor site | |
| Slow growth | 103 (36%) |
| Moderate growth | 75 (26%) |
| Rapid growth | 111 (38%) |
| Location | |
| Cervical | 23 (8.0%) |
| Thoracic | 159 (55%) |
| Lumbar-Sacral | 77 (27%) |
| Combined | 30 (10%) |
| No. of spine metastases | |
| 1 level | 92 (32%) |
| 2 levels | 35 (12%) |
| ≥ 3 levels | 162 (56%) |
| ECOG performance status | |
| Score 0–2 | 99 (34%) |
| Score 3–4 | 190 (66%) |
| Other bone metastases | 187 (65%) |
| ASIA scale | |
| A to C | 56 (19%) |
| D or E | 233 (81%) |
| Visceral metastases | 127 (44%) |
| Surgery type | |
| Corpectomy with stabilization | 137 (47%) |
| Decompression and stabilization | 79 (27%) |
| Decompression alone | 73 (25%) |
| Prior local radiotherapys | 29 (10%) |
| Previous systemic therapys | 162 (56%) |
| Blood loss (ml) | 1128.3 ± 1053.5 |
| Transfusions | 189 (65%) |
| Baseline Hb (g/dL) | 12.3 ± 1.8 |
| Postoperative nadir Hb (g/dL) | 9.7 ± 1.8 |
| Predischarge Hb (g/dL) | 10.8 ± 1.6 |
| Nadir Hb drift | |
| Absolute (g/dL) | −2.6 ± 1.7 |
| Percentage | −21% ± 13% |
| Predischarge Hb drift | |
| Absolute (g/dL) | −1.6 ± 1.9 |
| Percentage | −12% ± 15% |
ECOG, Eastern Cooperative Oncology Group; ASIA, American Spinal Injury Association
The short-term (6-week) mortality, was 5.5%. The long-term (1-year) mortality was 45.3%. Postoperative morbidity within 30 days occurred in 20.4% of patients, including 16 cases of pneumonia,14 cases of systemic infection, 13 cases of wound infection and/or dehiscence, 5 cases of venous thromboembolism, 4 cases of myocardial infarction and delirium, and 3 cases of stroke.
The baseline characteristics and outcomes of patients with different surgery types are summarized in Table S1.
Correlation between Hb-related parameters and clinical outcomes
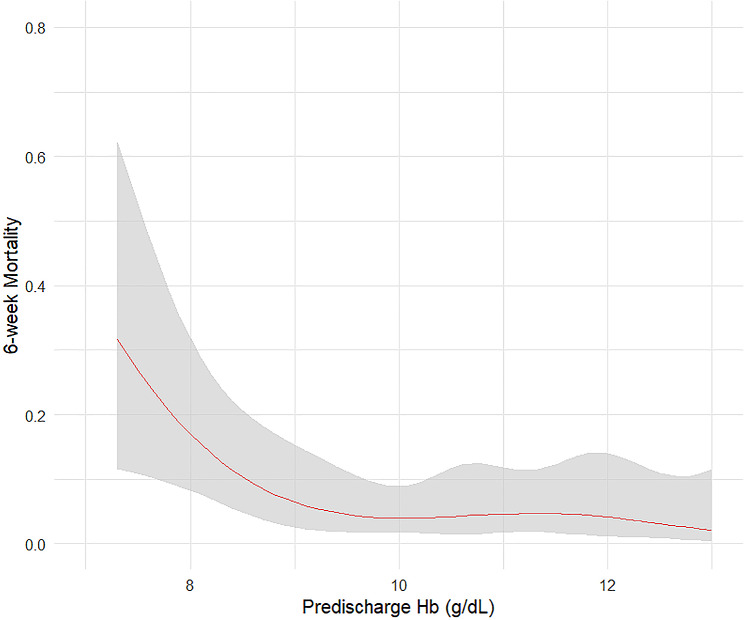
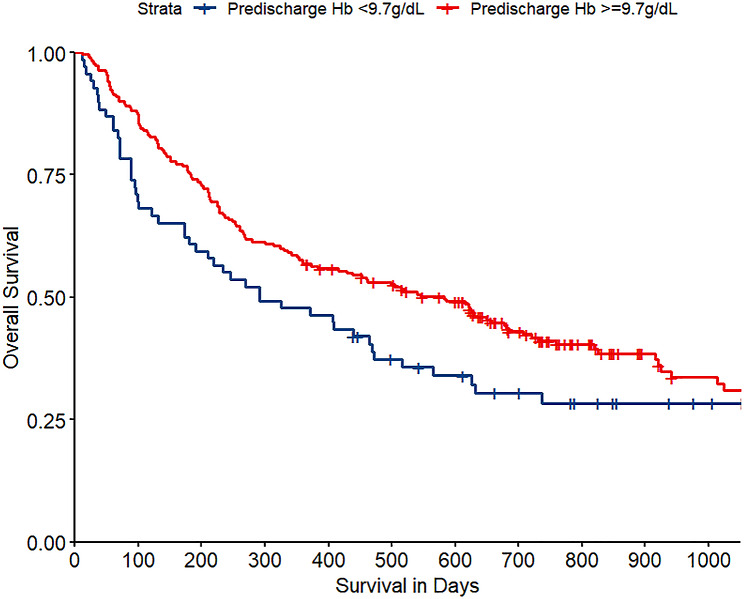
In the study, univariate analysis identified factors associated with short-term mortality, including nadir Hb, predischarge Hb, primary tumor site, visceral metastases, and ASIA. In the multivariate analysis, predischarge Hb remained independently associated with short-term mortality (OR 0.61, 95% CI 0.42–0.87, P = 0.006) (Table 2). Figure 1 showed a rapid risk reduction of postoperative short-term mortality with increasing Hb levels, plateauing around 9.5 g/dL. According to the method described above, the optimal cut-off value for predischarge Hb was 9.7 g/dL. The survival curves for patients with predischarge Hb levels greater and less than 9.7 g/dL are shown in Fig. 2.
Table 2.
Univariate and multivariate analysis of the factors related to postoperative short-term (6-week) mortality
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Baseline Hb | 0.89 (0.67–1.16) | 0.384 | ||
| Postoperative nadir Hb | 0.75 (0.57-1.00) | 0.048 | ||
| Predischarge Hb | 0.62 (0.44–0.88) | 0.007 | 0.61 (0.42–0.87) | 0.006 |
| Nadir Hb drift | ||||
| Absolute | 0.85 (0.64–1.12) | 0.239 | ||
| Percentage | 0.05 (0.00-2.38) | 0.127 | ||
| Predischarge Hb drift | ||||
| Absolute | 0.80 (0.61–1.06) | 0.116 | ||
| Percentage | 0.03 (0.00-1.66) | 0.087 | ||
| Age | 0.98 (0.94–1.03) | 0.522 | ||
| Primary tumor site | ||||
| Slow growth | Ref | Ref | ||
| Moderate growth | 7.46 (0.85–65.34) | 0.070 | 6.03 (0.65–55.86) | 0.114 |
| Rapid growth | 9.49 (1.18–76.38) | 0.034 | 8.57 (1.02–72.25) | 0.048 |
| Visceral metastases | 2.70 (0.90–8.14) | 0.077 | ||
| No. of spine metastases | ||||
| 1 level | Ref | |||
| 2 levels | 2.25 (0.57–8.90) | 0.250 | ||
| ≥ 3 levels | 0.79 (0.24–2.55) | 0.688 | ||
| Other bone metastases | 2.47 (0.69–8.86) | 0.167 | ||
| ECOG | ||||
| Score 0–2 | Ref | |||
| Score 3–4 | 2.08 (0.57–7.58) | 0.265 | ||
| ASIA | ||||
| A to C | Ref | Ref | ||
| D or E | 0.26 (0.09–0.75) | 0.013 | 0.16 (0.05–0.49) | 0.002 |
| Transfusion | 1.17 (0.40–3.48) | 0.772 | ||
| Blood loss > 1000 ml | 0.76 (0.26–2.26) | 0.624 | ||
| Surgery type | ||||
| Corpectomy with stabilization | Ref | |||
| Decompression and stabilization | 0.99 (0.28–3.49) | 0.988 | ||
| Decompression alone | 1.37 (0.42–4.46) | 0.606 | ||
| 30-day morbidity | 2.49 (0.87–7.16) | 0.090 |
ECOG, Eastern Cooperative Oncology Group; ASIA, American Spinal Injury Association
Fig. 1.
The restricted cubic spline figure between predischarge Hb and postoperative short-term (6-week) mortality
Fig. 2.
Kaplan-Meier curve of overall survival for patients with predischarge Hb < 9.7 g/dL and ≥ 9.7 g/dL
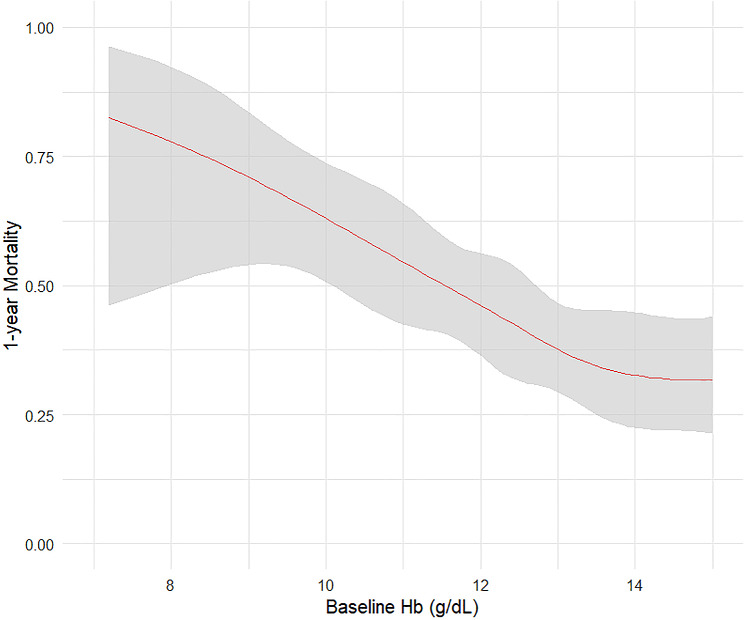
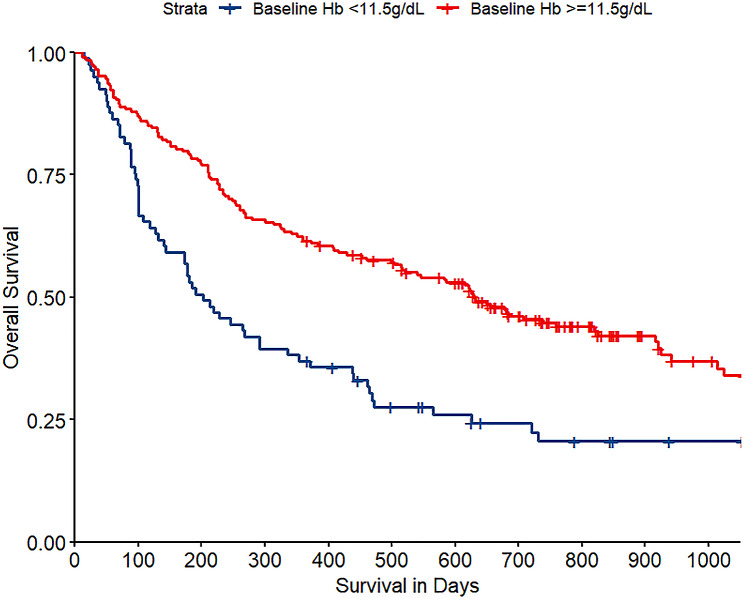
Long-term mortality associations included baseline Hb, nadir Hb, predischarge Hb, absolute predischarge Hb drift (only the absolute value of predischarge Hb drift was included in the multivariate model due to its lower P value compared to the predischarge Hb drift in percentage), the primary tumor site, number of spine metastases, and ECOG performance. In the multivariate analysis, baseline Hb remained independently associated with long-term mortality rate (OR 0.82, 95% CI 0.68–0.99, P = 0.039) (Table 3). Figure 3 indicated decreasing long-term mortality with increasing baseline Hb, plateauing near 13.0 g/dL. The optimal cut-off value for the baseline Hb level was 11.5 g/dL. The survival curves for patients with baseline Hb levels above and below 11.5 g/dL are shown in Fig. 4.
Table 3.
Univariate and multivariate analysis of the factors related to postoperative long-term (1-year) mortality
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Baseline Hb | 0.76 (0.66–0.88) | < 0.001 | 0.82 (0.68–0.99) | 0.039 |
| Postoperative nadir Hb | 0.84 (0.74–0.96) | 0.009 | ||
| Predischarge Hb | 0.88 (0.77–1.02) | 0.091 | ||
| Nadir Hb drift | ||||
| Absolute | 1.10 (0.96–1.27) | 0.152 | ||
| Percentage | 2.04 (0.34–12.05) | 0.432 | ||
| Predischarge Hb drift | ||||
| Absolute | 1.17 (1.03–1.33) | 0.016 | ||
| Percentage | 7.14 (1.42–35.83) | 0.017 | ||
| Age | 1.01 (0.99–1.03) | 0.354 | ||
| Primary tumor site | ||||
| Slow growth | Ref | Ref | ||
| Moderate growth | 2.59 (1.36–4.91) | 0.004 | 3.3063450 1.6138291 6.9572681 | 0.001 |
| Rapid growth | 5.76 (3.18–10.45) | < 0.001 | 7.9279341 3.9575718 16.6267541 | < 0.001 |
| Visceral metastases | 1.44 (0.90–2.30) | 0.126 | ||
| No. of spine metastases | ||||
| 1 level | Ref | Ref | ||
| 2 levels | 2.68 (1.21–5.96) | 0.016 | 2.7888459 1.0993837 7.2847946 | 0.033 |
| ≥ 3 levels | 1.62 (0.96–2.74) | 0.072 | 1.5532853 0.7849035 3.1158877 | 0.209 |
| Other bone metastases | 1.30 (0.80–2.11) | 0.295 | ||
| ECOG | ||||
| Score 0–2 | Ref | Ref | ||
| Score 3–4 | 3.22 (1.90–5.46) | < 0.001 | 2.4252384 1.2857389 4.6674838 | 0.007 |
| ASIA | ||||
| A to C | Ref | |||
| D or E | 0.35 (0.19–0.64) | < 0.001 | ||
| Transfusion | 0.96 (0.59–1.56) | 0.868 | ||
| Blood loss > 1000 ml | 0.60 (0.37–1.27) | 0.685 | ||
| Surgery type | ||||
| Corpectomy with stabilization | Ref | |||
| Decompression and stabilization | 0.91 (0.52–1.60) | 0.733 | ||
| Decompression alone | 2.01 (1.13–3.58) | 0.018 |
ECOG, Eastern Cooperative Oncology Group; ASIA, American Spinal Injury Association
Fig. 3.
The restricted cubic spline figure between baseline Hb and postoperative long-term (1-year) mortality
Fig. 4.
Kaplan-Meier curve of overall survival for patients with baseline Hb < 11.5 g/dL and ≥ 11.5 g/dL
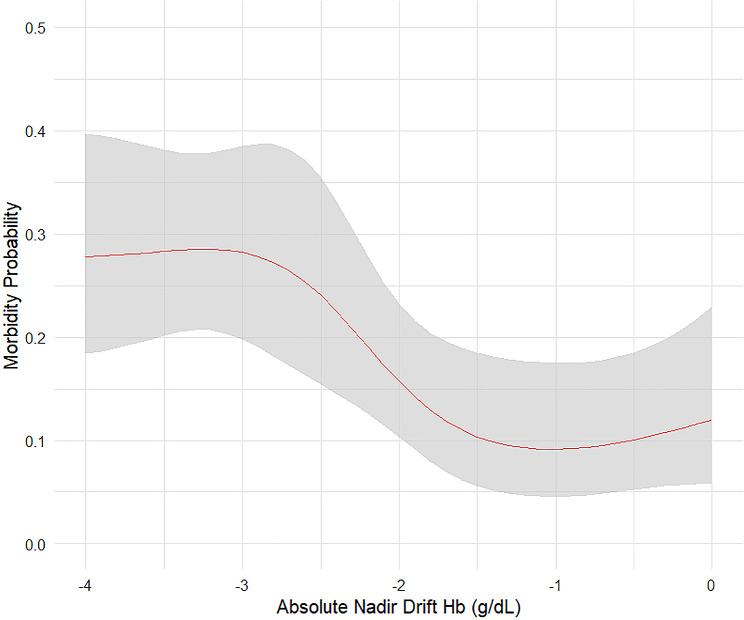
For 30-day morbidity, only the nadir Hb drift in absolute value and visceral metastases were subjected to the multivariate analysis. Absolute nadir Hb drift remained independently associated with the 30-day morbidity rate (OR 0.82, 95% CI 0.70–0.97, P = 0.023) (Table 4). Figure 5 displayed a high morbidity probability for absolute nadir Hb drift values less than − 3 g/dL, decreasing rapidly with increasing drift. Beyond − 1.5 g/dL, the morbidity probability plateaued or slightly elevated.
Table 4.
Univariate and multivariate analysis of the factors related to postoperative 30-day morbidity
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Baseline Hb | 1.05 (0.90–1.23) | 0.528 | ||
| Postoperative nadir Hb | 0.88 (0.75–1.03) | 0.113 | ||
| Predischarge Hb | 0.95 (0.80–1.13) | 0.568 | ||
| Nadir Hb drift | ||||
| Absolute | 0.82 (0.70–0.97) | 0.021 | 0.82 (0.70–0.97) | 0.023 |
| Percentage | 0.07 (0.01–0.67) | 0.022 | ||
| Predischarge Hb drift | ||||
| Absolute | 0.91 (0.78–1.07) | 0.259 | ||
| Percentage | 0.44 (0.06–3.21) | 0.418 | ||
| Age | 1.00 (0.97–1.02) | 0.791 | ||
| Primary tumor site | ||||
| Slow growth | Ref | |||
| Moderate growth | 0.85 (0.41–1.73) | 0.646 | ||
| Rapid growth | 0.60 (0.31–1.19) | 0.144 | ||
| Visceral metastases | 1.68 (0.95–2.99) | 0.076 | ||
| No. of spine metastases | ||||
| 1 level | Ref | |||
| 2 levels | 0.60 (0.21–1.75) | 0.349 | ||
| ≥ 3 levels | 0.96 (0.51–1.78) | 0.888 | ||
| Other bone metastases | 0.98 (0.54–1.79) | 0.957 | ||
| ECOG | ||||
| Score 0–2 | Ref | |||
| Score 3–4 | 1.69 (0.89–3.22) | 0.112 | ||
| ASIA | ||||
| A to C | Ref | |||
| D or E | ||||
| Transfusion | 1.39 (0.75–2.60) | 0.296 | ||
| Blood loss > 1000 ml | 1.11 (0.62-2.00) | 0.727 | ||
| Surgery type | ||||
| Corpectomy with stabilization | Ref | |||
| Decompression and stabilization | 1.05 (0.52–2.14) | 0.893 | ||
| Decompression alone | 1.58 (0.80–3.11) | 0.189 |
ECOG, Eastern Cooperative Oncology Group; ASIA, American Spinal Injury Association
Fig. 5.
The restricted cubic spline figure between nadir Hb drift and postoperative 30-day morbidity
Discussion
In this study, we systematically assessed the prognostic significance of Hb-related parameters in patients with spinal metastases undergoing surgery, filling a gap in existing research. Predischarge Hb levels independently predicted short-term mortality, while baseline Hb levels prognosticated long-term mortality. Absolute nadir Hb drift emerged as an independent prognostic factor for 30-day morbidity. Our findings underscore the crucial role of perioperative blood management and highlight specific markers for improved patient outcomes. This study, the first of its kind in the context of spinal metastases, emphasizes the importance of vigilant monitoring and timely interventions in enhancing patient prognosis.
Surgical interventions benefit symptomatic spinal metastases patients, but intraoperative bleeding is a significant challenge due to tumor hypervascularity and dilated epidural veins [21], resulting in an average blood loss of 2,180 mL [22]. The unique physical characteristics of oncology patients and bleeding risks highlight the critical need for perioperative blood management. Balancing surgical risks and blood resource consumption is essential. Investigating the clinical relevance of Hb-related parameters and their correlations with treatment outcomes is crucial for a rational approach to blood management in these cases.
Postoperative short-term mortality is common among patients with spinal metastases [23, 24], indicating that surgical decision-making does not primarily offer palliative results. Previous studies have predominately focused on preoperative prognostic factors and baseline variables. However, in this study, the predischarge Hb level was identified as an independent prognostic factor for short-term mortality, which has not been previously studied in the field of spinal metastases. This can serve as an early warning indicator for identifying high-risk patients prior to discharge. Our findings suggest that maintaining Hb levels > 10.0 g/dL before discharge can significantly improve patient outcomes. For patients with lower predischarge Hb levels, close monitoring is suggested.
In this study, we identified lower baseline Hb level as an independent prognostic factor for postoperative 1-year mortality in patients with spinal metastases. One possible explanation for this finding is that anemia serves as an indicator of the underlying comorbid disease burden and physiological reserve, which are pivotal factors in achieving long-term survival. The evaluation of systemic components in patients with spinal metastases is critical for surgical decision-making, especially considering the increased fragility of these patients due to disease progression, treatments, and cachexia [25]. Anemia is a common manifestation of frailty, particularly prevalent among oncology patients due to direct and indirect causes [26]. The impaired oxygen-carrying capacity caused by the presence of anemia may contribute to multiple clinical manifestations of cancer, including fatigue, dyspnea, dizziness, and exhaustion. Notably, cancer-related anemia significantly affects patients’ quality of life and is associated with an elevated risk of mortality and treatment-related adverse events [27]. Prediction scoring systems for survival in metastatic spine disease should consider incorporating baseline Hb as an important predictor [5, 6]. Consequently, we confirm that steady-state Hb levels in patients can serve as a marker for long-term survival. Aggressive interventions focusing on the etiology of anemia and nutritional supplementation have the potential to benefit patients.
Nadir Hb drift was initially researched by Karkouti et al., who found that nadir Hb drift, rather than nadir Hb, was independently associated with an increased risk of adverse outcomes in cardiac surgery [28]. Similar associations have been reported in various surgical contexts, including gastrointestinal surgery [11], head and neck surgery [12], brain tumor surgery [10], and non-oncological spinal surgery [8]. These studies recommended considering postoperative Hb drift in addition to Hb levels when assessing the need for transfusion. Our research indicates that postoperative morbidity is associated with nadir Hb drift rather than nadir Hb alone. As previously mentioned, a patient’s baseline Hb level is influenced by the underlying comorbid disease and the physiological reserve state to which the patient has adapted to a certain degree. Consequently, the body may exhibit greater sensitivity to a relative change in Hb level than to a specific low value. Research has shown that patients with abnormally high Hb levels may not tolerate sudden Hb reductions, even if their Hb levels remain within the normal range, because they require above-normal Hb levels to ensure adequate oxygen delivery to tissues [29]. To minimize Hb fluctuations, bleeding control techniques such as minimally invasive surgery and preoperative embolization should be considered. Additionally, maintaining hemodynamic stability through rational blood transfusion is crucial. Notably, minor reductions did not increase the probability of persistent complications. This is demonstrated in Fig. 5, where the risk of complications reaches a plateau or may even slightly increase above − 1.5 g/dL, possibly owing to the adverse effects of allogeneic blood transfusion. Therefore, there is no need for excessive transfusions, and consideration should be given to the scarcity of blood resources and the adverse effects of transfusions.
This study has several limitations that should be acknowledged when interpreting the findings. Firstly, it was an observational study, and the efficacy of a focused intervention on these significant parameters in improving patient outcomes remains to be established. Secondly, owing to disparities in healthcare policies, patients across various countries receive varying levels of medical support after discharge. However, the effects of predischarge Hb levels may differ. Thirdly, further studies are required to investigate the importance of Hb-related parameters in various patient subgroups.
Conclusions
Our study illustrates that various perioperative Hb-related parameters correlate with the prognosis of patients with spinal metastases. Predischarge Hb, baseline Hb, and nadir Hb drift were independent prognostic factors for postoperative short- and long-term mortality and surgical morbidity, respectively. These correlations remained statistically significant even after conducting multifactorial analyses, considering other established variables. Our findings provide a foundation for precise blood management. It is crucial to appropriately assess Hb-related parameters, and prospective intervention studies addressing these markers should be conducted in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
ZL and PZ designed the study and made substantial contributions to its conception and development. ZL drafted the paper. WY, XW, SL and JW critically revised the manuscript and helped prepare the final draft. All authors have read and approved the final manuscript.
Funding
The study was funded by Henan Medical Science and Technology Research and Development Plan Key Projects Jointly Constructed by the Provincial and Ministerial Departments in 2023 (SBGJ202302021).
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethical approval and consent to participate
This study was approved by the institutional review board of Affiliated Cancer Hospital of Zhengzhou University (no. 2022-437-002). Clinical information were obtained from medical management systems of hospital. All methods were carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus. 2001;11:e10. 10.3171/foc.2001.11.6.11. 10.3171/foc.2001.11.6.11 [DOI] [PubMed] [Google Scholar]
- 2.Sundaresan N, Digiacinto GV, Hughes JE, Cafferty M, Vallejo A. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645–50. 10.1097/00006123-199111000-00001. 10.1097/00006123-199111000-00001 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm. 2007;64. 10.2146/ajhp060601. S5-13; quiz S28-30. [DOI] [PubMed]
- 4.Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–51. 10.1634/theoncologist.2012-0293. 10.1634/theoncologist.2012-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulino Pereira NR, Janssen SJ, van Dijk E, Harris MB, Hornicek FJ, Ferrone ML, et al. Development of a prognostic survival algorithm for patients with metastatic spine disease. J Bone Joint Surg Am. 2016;98:1767–76. 10.2106/JBJS.15.00975. 10.2106/JBJS.15.00975 [DOI] [PubMed] [Google Scholar]
- 6.Karhade AV, Thio Q, Ogink PT, Bono CM, Ferrone ML, Oh KS, et al. Predicting 90-Day and 1-Year mortality in spinal metastatic disease: Development and Internal Validation. Neurosurgery. 2019;85:E671. 10.1093/neuros/nyz070. -671E681. 10.1093/neuros/nyz070 [DOI] [PubMed] [Google Scholar]
- 7.Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359–67. 10.1002/cam4.292. 10.1002/cam4.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purvis TE, Goodwin CR, Molina CA, Frank SM, Sciubba DM. Percentage change in hemoglobin level and morbidity in spine surgery patients. J Neurosurg Spine. 2018;28:345–51. 10.3171/2017.7.SPINE17301. 10.3171/2017.7.SPINE17301 [DOI] [PubMed] [Google Scholar]
- 9.George TJ, Beaty CA, Kilic A, Haggerty KA, Frank SM, Savage WJ, et al. Hemoglobin drift after cardiac surgery. Ann Thorac Surg. 2012;94:703–9. 10.1016/j.athoracsur.2012.03.038. 10.1016/j.athoracsur.2012.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Jia L, Tian Y, He J, He M, Chen L, et al. Association of Postoperative Drift in Hemoglobin with Mortality after Brain Tumor Craniotomy. Neurosurgery. 2023;93:168–75. 10.1227/neu.0000000000002396. 10.1227/neu.0000000000002396 [DOI] [PubMed] [Google Scholar]
- 11.Spolverato G, Kim Y, Ejaz A, Frank SM, Pawlik TM. Effect of relative decrease in blood hemoglobin concentrations on postoperative morbidity in patients who undergo major gastrointestinal surgery. JAMA Surg. 2015;150:949–56. 10.1001/jamasurg.2015.1704. 10.1001/jamasurg.2015.1704 [DOI] [PubMed] [Google Scholar]
- 12.Pehlivan B, Zouhair A, Luthi F, Bron L, Pasche P, Dragusanu D, et al. Decrease in hemoglobin levels following surgery influences the outcome in head and neck cancer patients treated with accelerated postoperative radiotherapy. Ann Surg Oncol. 2009;16:1331–6. 10.1245/s10434-009-0355-2. 10.1245/s10434-009-0355-2 [DOI] [PubMed] [Google Scholar]
- 13.Sim YE, Sim SD, Seng C, Howe TS, Koh SB, Abdullah HR. Preoperative Anemia, functional outcomes, and Quality of Life after hip fracture surgery. J Am Geriatr Soc. 2018;66:1524–31. 10.1111/jgs.15428. 10.1111/jgs.15428 [DOI] [PubMed] [Google Scholar]
- 14.Pizano A, Ray HM, Cambiaghi T, Saqib NU, Afifi R, Khan S, et al. Initial experience and early outcomes of the management of acute pulmonary embolism using the FlowTriever mechanical thrombectomy device. J Cardiovasc Surg (Torino). 2022;63:222–8. 10.23736/S0021-9509.21.12081-6. 10.23736/S0021-9509.21.12081-6 [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura Y, Wakabayashi H, Shiraishi A, Nagano F, Bise T, Shimazu S. Hemoglobin Improvement Is Positively Associated with Functional outcomes in Stroke patients with Anemia. J Stroke Cerebrovasc Dis. 2021;30:105453. 10.1016/j.jstrokecerebrovasdis.2020.105453. 10.1016/j.jstrokecerebrovasdis.2020.105453 [DOI] [PubMed] [Google Scholar]
- 16.Yang JJ, Chen CW, Fourman MS, Bongers M, Karhade AV, Groot OQ, et al. International external validation of the SORG machine learning algorithms for predicting 90-day and one-year survival of patients with spine metastases using a Taiwanese cohort. Spine J. 2021;21:1670–8. 10.1016/j.spinee.2021.01.027. 10.1016/j.spinee.2021.01.027 [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Guo L, Guo B, Zhang P, Wang J, Wang X, et al. Evaluation of different scoring systems for spinal metastases based on a Chinese cohort. Cancer Med. 2023;12:4125–36. 10.1002/cam4.5272. 10.1002/cam4.5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groot OQ, van Steijn NJ, Ogink PT, Pierik RJ, Bongers M, Zijlstra H, et al. Preoperative embolization in surgical treatment of spinal metastases originating from non-hypervascular primary tumors: a propensity score matched study using 495 patients. Spine J. 2022;22:1334–44. 10.1016/j.spinee.2022.03.001. 10.1016/j.spinee.2022.03.001 [DOI] [PubMed] [Google Scholar]
- 19.Rampersaud YR, Anderson PA, Dimar JR 2nd, Fisher CG. Spinal adverse events Severity System, version 2 (SAVES-V2): inter- and intraobserver reliability assessment. J Neurosurg Spine. 2016;25:256–63. 10.3171/2016.1.SPINE14808. 10.3171/2016.1.SPINE14808 [DOI] [PubMed] [Google Scholar]
- 20.Paulino Pereira NR, Ogink PT, Groot OQ, Ferrone ML, Hornicek FJ, van Dijk CN, et al. Complications and reoperations after surgery for 647 patients with spine metastatic disease. Spine J. 2019;19:144–56. 10.1016/j.spinee.2018.05.037. 10.1016/j.spinee.2018.05.037 [DOI] [PubMed] [Google Scholar]
- 21.Bilsky MH, Fraser JF. Complication avoidance in vertebral column spine tumors. Neurosurg Clin N Am. 2006;17:317 – 29, vii. 10.1016/j.nec.2006.04.007 [DOI] [PubMed]
- 22.Chen Y, Tai BC, Nayak D, Kumar N, Chua KH, Lim JW, et al. Blood loss in spinal tumour surgery and surgery for metastatic spinal disease: a meta-analysis. Bone Joint J. 2013;95–B:683–8. 10.1302/0301-620X.95B5.31270. 10.1302/0301-620X.95B5.31270 [DOI] [PubMed] [Google Scholar]
- 23.Karhade AV, Thio Q, Ogink PT, Shah AA, Bono CM, Oh KS, et al. Development of machine learning algorithms for prediction of 30-Day mortality after surgery for spinal metastasis. Neurosurgery. 2019;85:E83–8391. 10.1093/neuros/nyy469. 10.1093/neuros/nyy469 [DOI] [PubMed] [Google Scholar]
- 24.Schoenfeld AJ, Leonard DA, Saadat E, Bono CM, Harris MB, Ferrone ML. Predictors of 30- and 90-Day survival following Surgical intervention for spinal metastases: a prognostic study conducted at four academic centers. Spine (Phila Pa 1976). 2016;41:E503–9. 10.1097/BRS.0000000000001273. 10.1097/BRS.0000000000001273 [DOI] [PubMed] [Google Scholar]
- 25.Massaad E, Bridge CP, Kiapour A, Fourman MS, Duvall JB, Connolly ID, et al. Evaluating frailty, mortality, and complications associated with metastatic spine tumor surgery using machine learning-derived body composition analysis. J Neurosurg Spine. 2022;1–11. 10.3171/2022.1.SPINE211284. [DOI] [PubMed]
- 26.De la Garza Ramos R, Goodwin CR, Jain A, Abu-Bonsrah N, Fisher CG, Bettegowda C, et al. Development of a metastatic spinal tumor Frailty Index (MSTFI) using a Nationwide Database and its Association with Inpatient Morbidity, Mortality, and length of stay after spine surgery. World Neurosurg. 2016;95:548–55. .e4. 10.1016/j.wneu.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol. 2020;145:102837. 10.1016/j.critrevonc.2019.102837. 10.1016/j.critrevonc.2019.102837 [DOI] [PubMed] [Google Scholar]
- 28.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, van Rensburg A, Beattie WS. The influence of baseline hemoglobin concentration on tolerance of anemia in cardiac surgery. Transfusion. 2008;48:666–72. 10.1111/j.1537-2995.2007.01590.x. 10.1111/j.1537-2995.2007.01590.x [DOI] [PubMed] [Google Scholar]
- 29.Vongpatanasin W, Brickner ME, Hillis LD, Lange RA. The Eisenmenger syndrome in adults. Ann Intern Med. 1998;128:745–55. 10.7326/0003-4819-128-9-199805010-00008. 10.7326/0003-4819-128-9-199805010-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.