Abstract
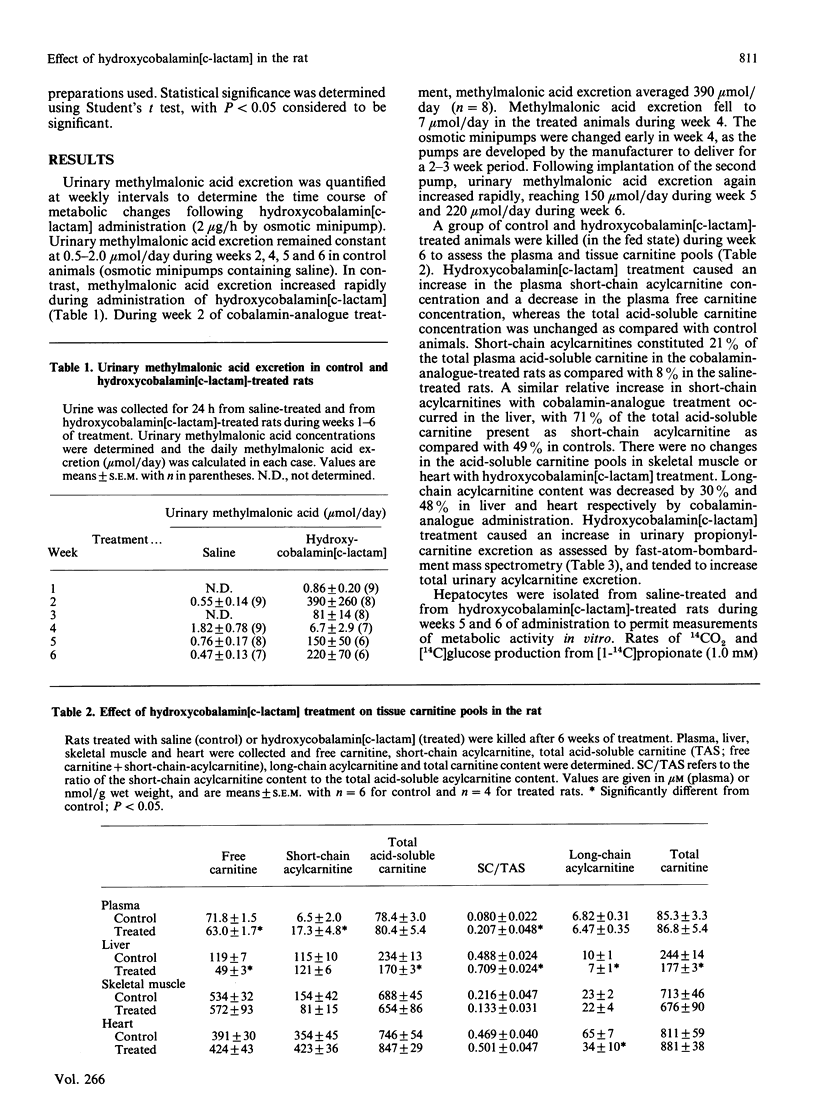
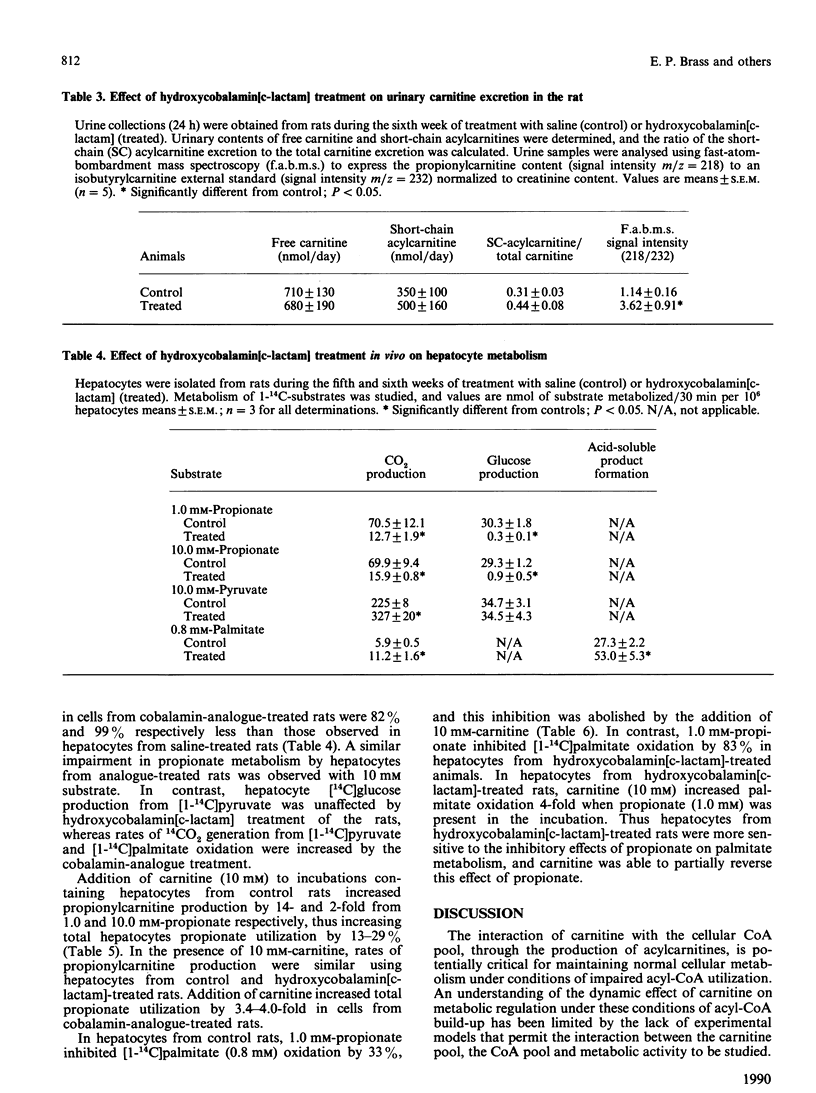
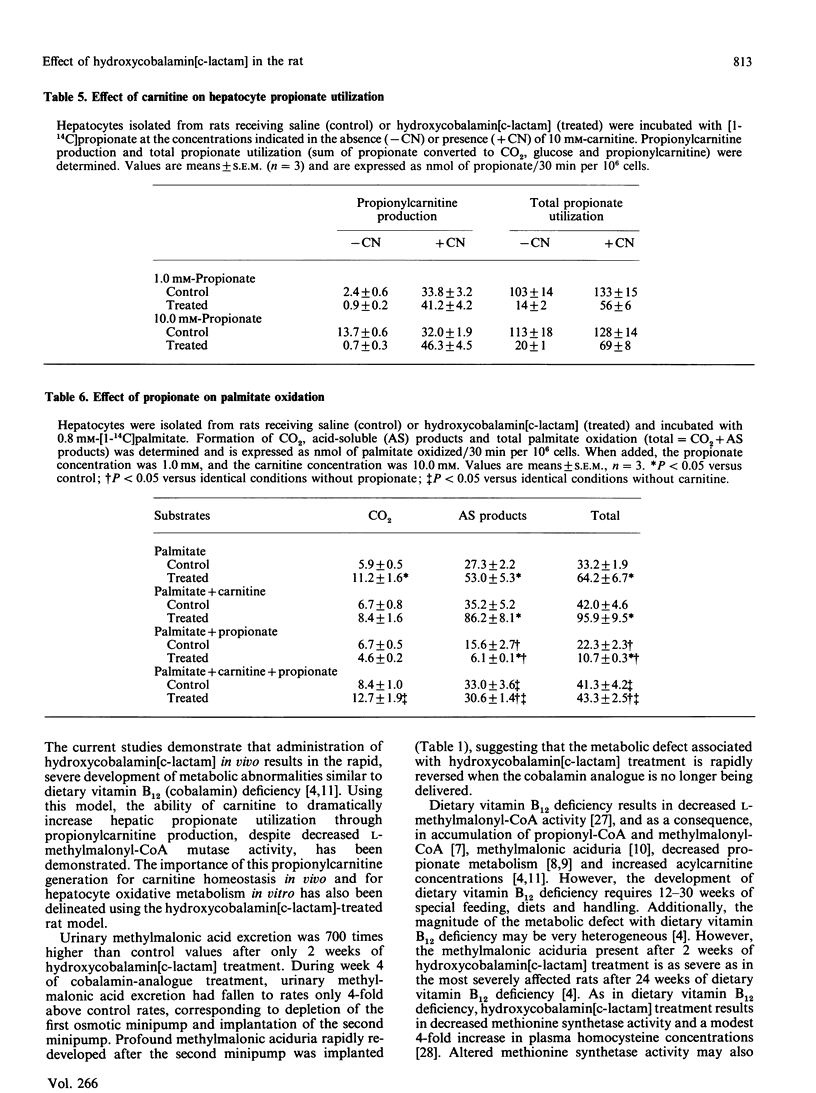
The administration in vivo of the cobalamin analogue hydroxycobalamin[c-lactam] inhibits hepatic L-methylmalonyl-CoA mutase activity. The current studies characterize in vivo and in vitro the hydroxycobalamin[c-lactam]-treated rat as a model of disordered propionate and methylmalonic acid metabolism. Treatment of rats with hydroxycobalamin[c-lactam] (2 micrograms/h by osmotic minipump) increased urinary methylmalonic acid excretion from 0.55 mumol/day to 390 mumol/day after 2 weeks. Hydroxycobalamin[c-lactam] treatment was associated with increased urinary propionylcarnitine excretion and increased short-chain acylcarnitine concentrations in plasma and liver. Hepatocytes isolated from cobalamin-analogue-treated rats metabolized propionate (1.0 mM) to CO2 and glucose at rates which were only 18% and 1% respectively of those observed in hepatocytes from control (saline-treated) rats. In contrast, rates of pyruvate and palmitate oxidation were higher than control in hepatocytes from the hydroxycobalamin[c-lactam]-treated rats. In hepatocytes from hydroxycobalamin[c-lactam]-treated rats, propionylcarnitine was the dominant product generated from propionate when carnitine (10 mM) was present. The addition of carnitine thus resulted in a 4-fold increase in total propionate utilization under these conditions. Hepatocytes from hydroxycobalamin[c-lactam]-treated rats were more sensitive than control hepatocytes to inhibition of palmitate oxidation by propionate. This inhibition of palmitate oxidation was partially reversed by addition of carnitine. Thus hydroxycobalamin[c-lactam] treatment in vivo rapidly causes a severe defect in propionate metabolism. The consequences of this metabolic defect in vivo and in vitro are those predicted on the basis of propionyl-CoA and methylmalonyl-CoA accumulation. The cobalamin-analogue-treated rat provides a useful model for studying metabolism under conditions of a metabolic defect causing acyl-CoA accretion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber L. L., Emaus R., Valkner K., Farrell S. Possible functions of short-chain and medium-chain carnitine acyltransferases. Fed Proc. 1982 Oct;41(12):2858–2862. [PubMed] [Google Scholar]
- Binder M., Kolhouse J. F., Van Horne K. C., Allen R. H. High-pressure liquid chromatography of cobalamins and cobalamin analogs. Anal Biochem. 1982 Sep 15;125(2):253–258. doi: 10.1016/0003-2697(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Beyerinck R. A. Effects of propionate and carnitine on the hepatic oxidation of short- and medium-chain-length fatty acids. Biochem J. 1988 Mar 15;250(3):819–825. doi: 10.1042/bj2500819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass E. P., Beyerinck R. A. Interactions of propionate and carnitine metabolism in isolated rat hepatocytes. Metabolism. 1987 Aug;36(8):781–787. doi: 10.1016/0026-0495(87)90117-x. [DOI] [PubMed] [Google Scholar]
- Brass E. P. Effect of alpha-ketobutyrate on palmitic acid and pyruvate metabolism in isolated rat hepatocytes. Biochim Biophys Acta. 1986 Aug 29;888(1):18–24. doi: 10.1016/0167-4889(86)90065-0. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Fennessey P. V., Miller L. V. Inhibition of oxidative metabolism by propionic acid and its reversal by carnitine in isolated rat hepatocytes. Biochem J. 1986 May 15;236(1):131–136. doi: 10.1042/bj2360131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass E. P., Garrity M. J., Robertson R. P. Inhibition of glucagon-stimulated hepatic glycogenolysis by E-series prostaglandins. FEBS Lett. 1984 Apr 24;169(2):293–296. doi: 10.1016/0014-5793(84)80336-1. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Carnitine metabolism in the fasting rat. J Biol Chem. 1978 Apr 25;253(8):2688–2693. [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Relationship between acid-soluble carnitine and coenzyme A pools in vivo. Biochem J. 1980 Sep 15;190(3):495–504. doi: 10.1042/bj1900495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass E. P., Ruff L. J. Effect of carnitine on propionate metabolism in the vitamin B-12--deficient rat. J Nutr. 1989 Aug;119(8):1196–1202. doi: 10.1093/jn/119.8.1196. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Stabler S. P. Carnitine metabolism in the vitamin B-12-deficient rat. Biochem J. 1988 Oct 1;255(1):153–159. doi: 10.1042/bj2550153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton W. C., Frenkel E. P. Effect of vitamin B-12 deficiency on the hepatic tissue concentration of acyl carnitines. Biochim Biophys Acta. 1975 Aug 25;398(2):217–223. doi: 10.1016/0005-2760(75)90137-x. [DOI] [PubMed] [Google Scholar]
- Cardinale G. J., Dreyfus P. M., Auld P., Abeles R. H. Experimental vitamin B12 deficiency: its effect on tissue vitamin B12-coenzyme levels and on the metabolism of methylmalonyl-CoA. Arch Biochem Biophys. 1969 Apr;131(1):92–99. doi: 10.1016/0003-9861(69)90108-8. [DOI] [PubMed] [Google Scholar]
- Cederblad G., Lindstedt S. A method for the determination of carnitine in the picomole range. Clin Chim Acta. 1972 Mar;37:235–243. doi: 10.1016/0009-8981(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Ciman M., Rossi C. R., Siliprandi N. On the mechanism of the antiketogenic action of propionate and succinate in isolated rat liver mitochondria. FEBS Lett. 1972 Apr 15;22(1):8–10. doi: 10.1016/0014-5793(72)80205-9. [DOI] [PubMed] [Google Scholar]
- Cooper B. A., Rosenblatt D. S. Inherited defects of vitamin B12 metabolism. Annu Rev Nutr. 1987;7:291–320. doi: 10.1146/annurev.nu.07.070187.001451. [DOI] [PubMed] [Google Scholar]
- Frenkel E. P., Kitchens R. L., Hersh L. B., Frenkel R. Effect of vitamin B12 deprivation on the in vivo levels of coenzyme A intermediates associated with propionate metabolism. J Biol Chem. 1974 Nov 10;249(21):6984–6991. [PubMed] [Google Scholar]
- Frenkel E. P., White J. D. Characterization of an animal model of vitamin B12 deprivation. Lab Invest. 1973 Dec;29(6):614–619. [PubMed] [Google Scholar]
- Glasgow A. M., Chase H. P. Effect of propionic acid on fatty acid oxidation and ureagenesis. Pediatr Res. 1976 Jul;10(7):683–686. doi: 10.1203/00006450-197607000-00010. [DOI] [PubMed] [Google Scholar]
- Lysiak W., Lilly K., DiLisa F., Toth P. P., Bieber L. L. Quantitation of the effect of L-carnitine on the levels of acid-soluble short-chain acyl-CoA and CoASH in rat heart and liver mitochondria. J Biol Chem. 1988 Jan 25;263(3):1151–1156. [PubMed] [Google Scholar]
- Lysiak W., Toth P. P., Suelter C. H., Bieber L. L. Quantitation of the efflux of acylcarnitines from rat heart, brain, and liver mitochondria. J Biol Chem. 1986 Oct 15;261(29):13698–13703. [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. ENZYMOLOGICAL DETERMINATION OF FREE CARNITINE CONCENTRATIONS IN RAT TISSUES. J Lipid Res. 1964 Apr;5:184–187. [PubMed] [Google Scholar]
- Marcell P. D., Stabler S. P., Podell E. R., Allen R. H. Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal Biochem. 1985 Oct;150(1):58–66. doi: 10.1016/0003-2697(85)90440-3. [DOI] [PubMed] [Google Scholar]
- Martin-Requero A., Corkey B. E., Cerdan S., Walajtys-Rode E., Parrilla R. L., Williamson J. R. Interactions between alpha-ketoisovalerate metabolism and the pathways of gluconeogenesis and urea synthesis in isolated hepatocytes. J Biol Chem. 1983 Mar 25;258(6):3673–3681. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler S. P., Marcell P. D., Podell E. R., Allen R. H., Lindenbaum J. Assay of methylmalonic acid in the serum of patients with cobalamin deficiency using capillary gas chromatography-mass spectrometry. J Clin Invest. 1986 May;77(5):1606–1612. doi: 10.1172/JCI112476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wałajtys-Rode E., Coll K. E., Williamson J. R. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. II. Interactions with gluconeogenesis and urea synthesis. J Biol Chem. 1979 Nov 25;254(22):11521–11529. [PubMed] [Google Scholar]
- Weidemann M. J., Hems R., Williams D. L., Spray G. H., Krebs H. A. Gluconeogenesis from propionate in kidney and liver of the vitamin B12-deficient rat. Biochem J. 1970 Mar;117(1):177–181. doi: 10.1042/bj1170177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Spray G. H., Newman G. E., O'Brien J. R. Dietary depletion of vitamin B12 and the excretion of methylmalonic acid in the rat. Br J Nutr. 1969 Jun;23(2):343–352. doi: 10.1079/bjn19690041. [DOI] [PubMed] [Google Scholar]


