Abstract
Background
The Republic of Guinea, where malaria represents the leading cause of morbidity and mortality among children, the seasonal malaria chemoprevention (SMC) is deployed only in areas with very seasonal modes of transmission. It should target children at the highest risk of serious illness. The objective of the study was to prevent uncomplicated and serious cases of malaria in the target population. This study aimed to analyse the monthly trends in malaria-related morbidity among children under the age of 5 in Guinea.
Methods
This was a quasi-experimental study with routine data from the National Health Information System (SNIS). The two districts Mamou (the SMC intervention site) and Kindia (the control site) were selected to compare the monthly trends in malaria cases among children under the age of 5, from July to October, covering the years from 2015 to 2020. The statistical analysis used interrupted time series to estimate the effects of the SMC.
Results
The SMC programme contributed to a significant average reduction in the number of malaria cases of 225 cases per month in the intervention district (95% CI − 362 to − 88; p = 0.002), compared to the control district. However, the study also revealed that the effect of SMC varied between cycles, presenting different monthly malaria cases.
Conclusion
The SMC contributed to a significant reduction in malaria cases among children under the age of 5 in the health district of Mamou from 2018 to 2020. However, this reduction varied by monthly SMC cycle. This study suggests extending the SMC in other areas with high perennial seasonal transmission respecting the World Health Organization SMC eligibility criteria, as a strategy in the dynamic of reducing malaria cases in children under the age of 5 in Guinea.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-024-05060-4.
Keywords: Seasonal Malaria Chemoprevention, Malaria, Effect, Monthly trends, Children under 5, Guinea
Background
The number of malaria cases was estimated at 249 million in 2022 in 85 malaria-endemic countries and territories. In 2022, the World Health Organization (WHO) African region represented ~ 94% of estimated cases in the world [1]. In the African region, the number of deaths due to malaria has declined from 808,000 in 2000 to 548,000 in 2017, before increasing to 604,000 in 2020 (due to a disruption caused by the COVID pandemic) [1, 2].
To reduce the mortality rate in areas with high endemicity, since 2012 the WHO has recommended Seasonal Malaria Chemoprevention (SMC) as a tool for preventing malaria in children in areas where malaria transmission is highly seasonal and where the majority (> 60%) of clinical malaria cases occur within 4 consecutive months. [3]. The SMC strategy consists of administering three or four cycles of sulfadoxine-pyrimethamine (SP) and amodiaquine (AQ) at monthly intervals to children aged from 3 to 59 months in areas of high seasonal malaria transmission (at least 60% of cases occurring over a 4-month period). [4, 5]. The effectiveness of the SMC was proven in research conducted in Ghana on the evaluation of the implementation of the seasonal malaria chemoprevention on the morbidity among children, that the proportion of children with malaria increased significantly, from 9 to 21% in the district without intervention. However, in the area where the SMC was implemented, the proportion of children carrying malaria decreased significantly, from 31 to 12% [2]. A ratio of intervention to non-intervention district of 0.52 giving protective effectiveness of 48% (p = 0.62). A study carried out in Mali reported a 61% reduction in malaria cases in localities benefiting from the SMC, while in the control district an increase of 23% was recorded. In sites considered suitable for the SMC, the median fraction of the incidence occurring in the 4 consecutive months of peak transmission was 77% and the average of 75.7%, so 75% of the annual burden occurred in the SMC period [6]. Case–control studies carried out in 2015 in Mali and The Gambia, as well as in 2016 in Burkina Faso, Chad, Mali, Nigeria and The Gambia, revealed a protective effectiveness of SMC of 88% against malaria [7]. Several studies carried out in different countries confirm the remarkable effectiveness of the SMC in the fight against malaria and recommend its extension to other regions having recorded a high incidence of malaria [6, 8].
A study carried out in Guinea used a malaria indicator analysis approach based on the annual trends (January–November), including the SMC and the non-SMC months. This study demonstrated that the incidence of simple and severe malaria was significantly lower in the SMC district than in the control district [9]. In Guinea, malaria remains the main cause of morbidity and mortality among children under the age of 5, with 2,422,445 confirmed cases and 1.029 deaths reported in 2021 [9]. The SMC was introduced in the country in 2015, then it was extended to 17 districts in 2021. Nevertheless, the effect of this intervention remains under-documented in the Guinean context. The only published study on the effect of the SMC did not examine the monthly trends before and during its implementation, nor did it consider the comparison of these monthly trends before and during the intervention. To fill this gap in knowledge, this study will answer the following question in order to better evaluate the effect of the SMC.
This study aimed to overcome this knowledge gap by carrying out a comparative analysis between a health district covered by the SMC and another district which is not covered by the SMC. The objective of this study was to analyse the monthly trends in malaria-related morbidity among children under the age of 5 in Guinea. Specifically, the aim was to describe the monthly trends in malaria cases in children under the age of 5 in a SMC-covered area compared with a SMC-not-covered area during the SMC cycles (July–October), in order to isolate the net effect of each cycle.
Methods
Priority target regions for the implementation of the SMC:
The malaria transmission is highly seasonal and the majority (> 60%) of clinical malaria cases occur within 4 consecutive months. When malaria data from the health management information system are unreliable, rainfall data can be used as an indicator of seasonality of incidence (at least 60% of annual rainfall in 4 consecutive months)
The clinical attack rate of malaria (without the SMC) is at least 0.1 episodes per child during the transmission season in the target group.
Type of study
This was a quasi-experimental comparative study before and after non-equivalent using data from the National Health Information System.
Study framework
General framework
The Republic of Guinea, in West Africa, extends over a total area of 245,857 km2, with an estimated population of 13.5 million inhabitants in 2022. The rainy season is associated with maximum malaria transmission, with the peak of cases generally observed between July and November. Approximately 95% of malaria cases in Guinea are attributable to Plasmodium falciparum [10–12].
Specific framework
This study was carried out in the health districts of Mamou and Kindia. The intervention zone was the district of Mamou, located at 268 km east of Conakry (the capital city of the Republic of Guinea). The SMC was implemented in Mamou in 2017. The duration of the peak rainy season in each district is 6 months from June to November. Since its start in the country in six (6) health districts, the extension to other districts has been done gradually depending on the availability of funding.
The district of Kindia was chosen as a control area, being a neighbouring district with high seasonal rainfall and a malaria transmission pattern similar to Mamou. Kindia is located at 138 km east of Conakry. The peak season rainfall was about 1 954 mm both in Mamou and in the district of Kindia [13]. The level of malaria incidence in children under the age of 5 is strongly correlated with the season. More than 60% of malaria cases occur during the 4 months (July, August, September and October) in the intervention zone and in the control zone (Bulletin Annual Malaria Epidemiology).
Source of data
Data were extracted into the district health information software (DHIS-2), which collects data from the National Health Information System (SNIS). These data come from consultation registers, are entered, aggregated monthly, and then validated. Data were collected from malaria in the two districts for the period from 2015 to. The malaria indicators included the number of suspected cases, the number of cases tested, the number of malaria cases and the number of malaria-related deaths.
Data were collected from malaria in the two districts for the period from 2015 to 2020. Malaria indicators included the number of suspected cases, the number of cases tested, the number of malaria cases and the number of malaria-related deaths.
Study population and data analysis
Study variables
Dependent variable
The study is a controlled interrupted time-series design [14]. The dependent variable was the number of parasitologically and rapid diagnostic test (RDT) confirmed malaria cases per month. A separate variable was created to represent the application of the SMC in Mamou. The monthly mean of malaria cases was compared before the intervention with the one observed during the intervention. In the contexts where populations are relatively stable, the raw cases of malaria may be sufficient to detect temporal trends. For example, an increase or decrease in the number of cases over time may indicate changes in disease transmission. The malaria cases are a more reliable and relevant measure for assessing the progress of national programmes, especially in the contexts of high transmission where data are more limited. This facilitates the monitoring of the disease trends and the evaluation of the effectiveness of malaria control interventions. The malaria cases provide a direct and easily understood measure of the disease burden. The policy-makers, the general public and even some healthcare professionals may find it more intuitive to understand and respond to a total number of the cases rather than the incidence, which is a rate and requires an understanding of epidemiological concepts to be correctly interpreted [15, 16].
Independent variables
The usual variables necessary for time trend analysis were included [17]. For each district, a group variable was established to identify malaria cases. The time variable was coded sequentially to indicate the months since the start of the observation periods in each district. Any temporal change in this variable should logically influence the malaria cases.
T: Time from start of study to end (2015–2020).
X: Is the SMC intervention, coded 0 if absent and 1 if present.
XT: Is the interaction between T and X.
They are explanatory variables which are applied using the ITSA (Interrupted time-series analysis) method in Stata software.
The study population consisted of children under the age of 5 diagnosed with malaria in health posts and centers in the health districts of Mamou and Kindia. The monthly malaria case data from each district were entered centrally by the study team into Excel software and then imported into Stata version16 for the analysis. First, a descriptive analysis was carried out to determine the mean of the malaria cases by district. Next, linear regression analyses with mixed effects were used to estimate the effects of the intervention on the malaria cases. Segmented ordinary least squares regression applying Newey-West standard errors was used to account for serial autocorrelation. The change in malaria case levels and the trends would be identical between the intervention district and the control district if the former had not been exposed to the intervention. The introduction of the intervention would result in a variation in the intercept, as well as a monthly variation in trends. Also, suppose that pre-intervention (2015–2017) and post-intervention (2018–2020) trends did not follow a linear trajectory. Additionally, considering that the intersections and trends varied across districts. These assumptions led to the equation below:
In this model, Yt represents the monthly number of the malaria cases at time t. β0 is the number of malaria cases at the start of the pre-intervention period. β1 estimates the average monthly change in the number of the malaria cases during the period preceding the SMC, Tt is the time elapsed since the start of the study, β2 represents the change in the level of the number of the malaria cases occurring during the period immediately after the administration of the first dose of the SMC (the SMC period designated by the indicator variable Xt) β3 represents the difference between the trend of the malaria cases during the SMC compared to the period before the SMC, β4 represents the change in the number of the malaria cases during the period immediately after the SMC, β5 represents the difference between the trend in the malaria cases during the period following the start of each SMC cycle and the period during the SMC cycles, β6 represents the difference in the average monthly variation of the malaria cases after the SMC in the SMC district versus the control district, β7 represents the difference in the average monthly variation of the malaria cases before the SMC and after the SMC in the district of the SMC versus the control district and εt the random error term. The autocorrelation up to four lags was taken into account in the model. The effects of the intervention over time were estimated by assuming that the malaria cases among children under the age of 5 would have increased in the absence of the intervention. All analyses were carried out with Stata software version 16 and the significance level of statistical tests was set at 0.05.
Ethical considerations
The study obtained the authorization from the National Malaria Control Programme in Guinea with the approval number 025/PNLP/2024 of March 12, 2024. The authorization to use these data was obtained after clearly explaining the objectives of the study.
Results
Table 1 presents the malaria case data in the SMC district (Mamou) and the control district (Kindia). During the period from 2015 to 2017, the Health District of Mamou recorded 25 696 cases of malaria, while the Health District of Kindia recorded 18,294 cases of malaria. However, during the period from 2018 to 2020, cumulative cases of malaria were 34,542 cases in Mamou and 32,616 cases in Kindia.
Table 1.
Description of the sample in the two districts before and after the introduction of the chemoprevention of seasonal malaria 2015–2020
| Health district SMC (Mamou) |
Health district No SMC (Kindia) |
|
|---|---|---|
| Number of malaria cases during the pre-intervention period (2015–2017) | 25,696 | 18,294 |
| Number of malaria cases during the post-intervention period (2018–2020) | 34,542 | 32,616 |
| Malaria incidence during the pre-intervention period (2015–2017) | 378 for 1000 | 195 for 1000 |
| Malaria incidence during the post-intervention period (2018–2020) | 465 for 1000 | 319 for 1000 |
| Number of the average monthly malaria cases during the pre-intervention period (SD) (2015–2017) | 2141 (948) | 1525 (374) |
| Number of the average monthly malaria cases during the post-intervention period (SD) (2018–2020) | 2878 (557) | 2718 (730) |
SD standard deviation
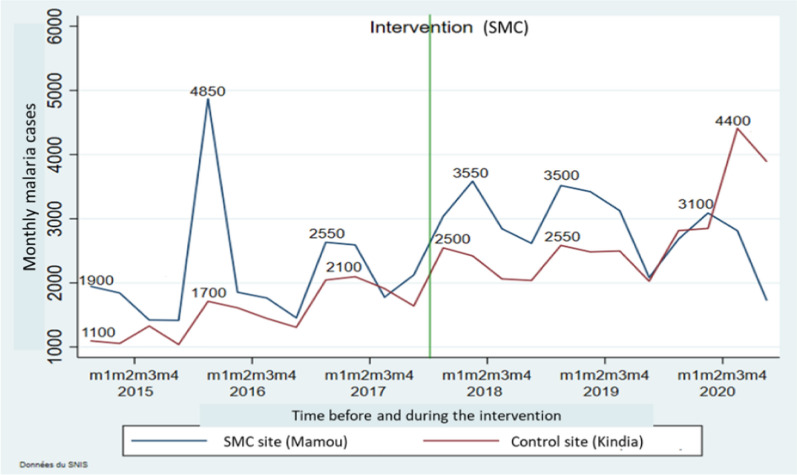
Figure 1 illustrates the estimated monthly trends in the malaria cases before and during the SMC. The curves show that there were non-similar fluctuations per SMC cycle between the intervention district and the control district.
Fig. 1.
Estimated monthly trends in the malaria cases before and during the SMC in the districts
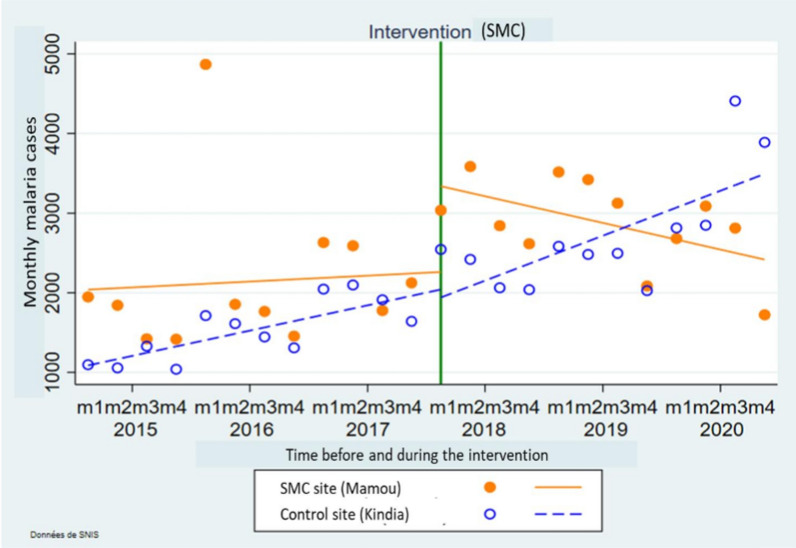
Figure 2 presents comparisons of the evolution of the number of the malaria cases before and during the SMC in the district of Mamou and Kindia. Before the implementation of the seasonal malaria chemoprevention, an increasing trend was observed in the SMC district (Mamou) compared to the control district (Kindia). During the administration of the SMC, a downward trend was observed in the district, which benefited from the intervention. The effects of the intervention on the number of the malaria cases decreased over time in Mamou. During the same period of time the number of the malaria cases increased in Kindia as illustrated in the Fig. 2.
Fig. 2.
Comparison of the evolution of the number of the malaria cases in the SMC districts
Table 2 presents the evolution of the monthly trend of malaria cases in the intervention district (Mamou) and in the control district (Kindia). Before the implementation of the SMC, the average number of the malaria cases in the control district was 1008 cases, with an average monthly variation of approximately 80 cases per month, which was statistically significant (95% CI 42–117; p < 0.001). Immediately after the implementation of the SMC, a significant decrease in the average number of malaria cases of ~ 1013 cases per month was observed (95% CI 22–2005; p = 0.045). However, after the intervention, the monthly mean difference between post-intervention and pre-intervention malaria cases was 60 cases per month, but this variation was not statistically significant (95% CI − 157 to 36; p = 0.210). The difference in average monthly change in the malaria cases before the SMC between the intervention district and the control district was less than 61 cases, without statistical significance (95% CI − 54 to 178; p = 0.288). In contrast, the difference in average monthly change in the malaria cases after the SMC between the intervention district and the control district was 1,177 cases, with statistical significance (95% CI 180–2175; p = 0.022). The gap in the difference in the average monthly variation of post-intervention malaria cases compared to the pre-intervention variation was less than 164 cases. This difference was statistically significant (95% CI − 328 to 0.4; p = 0.049). Overall, the intervention resulted in an average significant reduction in the number of malaria cases of 225 cases per month in the intervention district (95% CI − 362 to − 88; p = 0.002).
Table 2.
Comparisons of the monthly trends in the malaria cases among children under the age of 5 in the intervention district (Mamou) and in the control district (Kindia)
| Variables | Monthly cases of malaria | ||
|---|---|---|---|
| Coefficients (β) | 95% CI | P. value | |
| Number of malaria cases at the start of the pre-intervention period in the control group (β0) | 1008*** | 826 to 1190 | < 0.001 |
| The average monthly variation of malaria cases during the pre-intervention period in the control group (β1) | 80*** | 42 to 117 | < 0.001 |
| The average monthly change in malaria cases during the intervention in the control group (β2) | 1013* | 22 to 2005 | 0.045 |
| Difference in the malaria case trends during the intervention and the period before the intervention in the control group (β3) | − 61 | − 157 to 36 | 0.210 |
| Average monthly difference in the malaria cases between the intervention and control groups before the intervention (β4) | − 100 | − 863 to 662 | 0.792 |
| Average monthly difference in the malaria case trends between intervention and control groups before intervention (β5) | 61 | − 54 to 178 | 0.288 |
| Average monthly difference in the malaria cases between intervention and control groups immediately after introduction of the intervention (β6) | 1177* | 180 to 2175 | 0.022 |
| Average monthly difference in the malaria case trends between the intervention group and the control group after intervention (β7) | − 164* | − 328 to 0.4 | 0.049 |
| Net effect of the CPS (β3 + β7) | − 225** | − 362 to − 89 | 0.002 |
*p < 0.05, **p < 0.01, ***p < 0.001
Discussion
The results of this study show that the SMC contributed to a significant drop the in malaria cases in the health district of Mamou compared to that of Kindia where the SMC was not implemented. This drop was estimated at an average of 225 cases per month. However, the trend of reduction in the malaria cases during the SMC was not linear over the months. These results have important implications for the fight against malaria in Guinea. A study carried out in Guinea on the malaria trends in the districts which were targeted and not-targeted for seasonal malaria chemoprevention reported that malaria incidence was significantly lower in the SMC (median = 1.8%) compared with the non-SMC (median = 11.5%) districts. The implementation of the SMC has made it possible to reduce the cases of malaria in the Guinean context. Several previous studies have also proven a reduction in the malaria cases in areas where the SMC has been implemented [18–23]. Similarly, an evaluation of the ACCESS-SMC program using DHIS2 data observed a 45.0% and 55.2% reduction in the number of the malaria cases in Burkina Faso and The Gambia [24]. Controlled clinical trials and quasi-experimental study have demonstrated the effectiveness of SMC [25–28]. The results found in the study could be explained by the fact that the implementation strategy used for the SMC in Guinea is effective. This indicates that the SMC is a key strategy in the fight against malaria in Guinea.
The results are a new information which adds to the existing literature. To our knowledge, this is the first study to use non-linear monthly trends in the malaria cases during the SMC in a SMC area. This study also reveals that the decrease was not linear over the months of the implementation of the SMC. This demonstrates that the effects of the SMC vary from one cycle to another. The difference between the SMC cycles in terms of effects on the malaria prevalence would be linked to different organizational reasons. Firstly, certain cycles could be marked by low coverage of the target children compared to other cycles. The distributing agents did not go to all households, either due to the lack of logistics, availability of distributing agents, parents' refusal due to fear of the side effects or distrust of the public health services. These reasons could lead to an increase in the malaria cases and deaths despite the SMC campaigns. This underlines the importance of supportive supervision of the motivation and logistical support of the field agents for effective coverage of all children eligible for the SMC. In addition, it would also be necessary to improve awareness among parents of children by emphasizing what to do in the event of the side effects and the effectiveness of the SMC. Information and reminders from parents of children on the dates of the next SMC cycles by the distributing agents and other information channels. Another reason for the non-coverage of households would be linked to the fact that some parents are not aware of the arrival of the distributing agents and probably leave the households.
Limitations and strengths
There are a number of limitations to this study. Firstly, the data used being secondary and aggregated, the risk of missing data could not be controlled at the level of the primary sources of health posts and centers. Some factors such as the increasing annual trends in the malaria cases in the control district, testing and reporting practices, significant population growth or a change in health service utilization have not been studied. Secondly, the eligible age to benefit from the SMC is from 3 to 59 months in Guinea. In this study, the data used were routine data of children under the age of 5. Children aged less than 3 months, although not considered a vulnerable group, may present a low proportion of malaria cases during periods of the high transmission. This could have led to bias in the estimates of the SMC effects. The third limitation concerns the potential contribution of other ongoing interventions that could have an effect on the malaria cases, such as the use of long-acting insecticide-treated nets and the adequate management of the malaria cases. Therefore, these results cannot be attributed solely to the SMC, as it is a complementary intervention to other measures. However, despite its limitations, this study has strengths. Especially, the evaluation was carried out by a research team external to the implementation of the SMC. The study used a population of children under the age of 5 of the SMC district and the control district, from similar geographic areas, allowing for potential confounding factors to be taken into account. The use of a control district before and during the implementation of the SMC allowed a clear estimation of the effect of this intervention.
Implications for the research and practice
The results reveal that chemoprevention of seasonal malaria leads to a reduction in the malaria cases among children under the age of 5 in Guinea. However, the effects were not linear between the SMC cycles. This observation is an argument in favour of the policy of extending the SMC in areas with high perennial seasonal transmission, as a strategy in the dynamic of reducing malaria cases in the country.
Conclusion
This study demonstrates that in Guinea, the SMC contributed to a significant reduction in malaria cases among children under the age of 5 in the health district of Mamou from 2018 to 2020. However, this reduction varied by monthly SMC cycle. This study suggests the extension of chemoprevention of seasonal malaria in other areas with high perennial seasonal transmission with respect to the WHO SMC eligibility criteria, as a strategy in the dynamic of reducing the malaria cases in children under the age of 5 in Guinea. In addition, it would also be necessary to continue the efforts (the involvement of the Prefectural Health Directorate and partners, the involvement of the administrative and the community authorities, the training of distributing agents before deployment), aiming at improving the effects of all cycles.
Supplementary Information
Acknowledgements
We would like to thank the National Malaria Control Program for their authorization to use data from the National Health Information System of Guinea.
Abbreviations
- DHIS2
District Health Information System
- ITSA
Interrupted time-series analysis
- RDT
Rapid diagnostic test
- SMC
Chemoprevention of Seasonal Malaria
- SNIS
National Health Information System
- WHO
World Health Organization
Author contributions
KSK, BSC, KK and AC participated in the design of the study. KSK, SC, FB, TS, RD and MT participated in the analysis and interpretation of the results. KSK, BSC, KK and MDB contributed to the writing of the manuscript. BSC and MDB contributed to revising the manuscript. All authors read and approved the final manuscript.
Funding
This section is not applicable.
Availability of data and materials
The data used in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study used only already existing routine (secondary) data collected for the purposes of planning and implementing public health programs. The study obtained authorization from the National Malaria Control Programme to use these data with the approval number 025/PNLP/2024 on March 12, 2024.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World malaria report 2023: 23 years of global progress and challenges. Geneva, World Health Organization, 2023, Available: https://www.who.int/publications/i/item/9789240086173.
- 2.Ansah PO, Ansah NA, Malm K, Awuni D, Peprah N, Dassah S, et al. Evaluation of pilot implementation of seasonal malaria chemoprevention on morbidity in young children in Northern Sahelian Ghana. Malar J. 2021;20:440. 10.1186/s12936-021-03974-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide. Geneva: World Health Organization; 2023. [Google Scholar]
- 4.WHO. Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide. Geneva: World Health Organization; 2013. [Google Scholar]
- 5.Meremikwu MM, Donegan S, Sinclair D, Esu E, Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev. 2012;2012:CD003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns M, Roca-Feltrer A, Garske T, Wilson AL, Diallo D, Milligan PJ, et al. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat Commun. 2012;3:881. 10.1038/ncomms1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traore A, Donovan L, Sawadogo B, Ward C, Smith H, Rassi C, et al. Extending seasonal malaria chemoprevention to five cycles: a pilot study of feasibility and acceptability in Mangodara district, Burkina Faso. BMC Public Health. 2022;22:442. 10.1186/s12889-022-12741-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coldiron ME, Von Seidlein L, Grais RF. Seasonal malaria chemoprevention: successes and missed opportunities. Malar J. 2017;16:481. 10.1186/s12936-017-2132-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisanzio D, Keita MS, Camara A, Guilavogui T, Diallo T, Barry H, et al. Malaria trends in districts that were targeted and not-targeted for seasonal malaria chemoprevention in children under 5 years of age in Guinea, 2014–2021. BMJ Glob Health. 2024;9: e013898. 10.1136/bmjgh-2023-013898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Health. Annual Bulletin of the National Guinea Malaria Profile 2022. Available: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2023/01/Guinea-Malaria-Profile-1.
- 11.Beavogui AH, Delamou A, Camara BS, Camara D, Kourouma K, Camara R, et al. Prevalence of malaria and factors associated with infection in children aged 6 months to 9 years in Guinea: results from a national cross-sectional study. Parasite Epidemiol Control. 2020;11: e00162. 10.1016/j.parepi.2020.e00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayre D, Camara A, Barry Y, Deen TB, Camara D, Dioubaté M, et al. Combined epidemiologic and entomologic survey to detect urban malaria transmission, Guinea, 2018. Emerg Infect Dis. 2021;27:599–602. 10.3201/eid2702.191701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lama DEN. Direction Nationale de la Statistique (DNS) [Guinée]. 2017. Annuaire statistique sanitaire.
- 14.Bernal JL, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol. 2018;47:2082–93. 10.1093/ije/dyy135 [DOI] [PubMed] [Google Scholar]
- 15.Singh MP, Rajvanshi H, Bharti PK, Anvikar AR, Lal AA. Time series analysis of malaria cases to assess the impact of various interventions over the last three decades and forecasting malaria in India towards the 2030 elimination goals. Malar J. 2024;23:50. 10.1186/s12936-024-04872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delamou A, Ayadi AME, Sidibe S, Delvaux T, Camara BS, Sandouno SD, et al. Effect of Ebola virus disease on maternal and child health services in Guinea: a retrospective observational cohort study. Lancet Glob Health. 2017;5:e448–57. 10.1016/S2214-109X(17)30078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagarde M. How to do (or not to do) … Assessing the impact of a policy change with routine longitudinal data. Health Policy Plan. 2012;27:76–83. 10.1093/heapol/czr004 [DOI] [PubMed] [Google Scholar]
- 18.Cairns M, Ceesay SJ, Sagara I, Zongo I, Kessely H, Gamougam K, et al. Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: Case–control studies in 5 countries. PLoS Med. 2021;18: e1003727. 10.1371/journal.pmed.1003727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oumar AA. Impact of malaria prevention chemotherapy on morbidity and mortality in children 3–59 months of age in the Health District of Diré Mali. Health Sci Dis. 2021;22:39–42. [Google Scholar]
- 20.Richardson S, Moukenet A, Diar MSI, De Cola MA, Rassi C, Counihan H, et al. Modeled impact of seasonal malaria chemoprevention on district-level suspected and confirmed malaria cases in Chad based on routine clinical data (2013–2018). Am J Trop Med Hyg. 2021;105:1712–21. 10.4269/ajtmh.21-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambe JP, Balogun ST, Waziri MB, Nglass IN, Saddiq A. Impacts of seasonal malaria chemoprevention on malaria burden among under five-year-old children in Borno State. Nigeria J Trop Med. 2020;2020:9372457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Cola MA, Sawadogo B, Richardson S, Ibinaiye T, Traoré A, Compaoré CS, et al. Impact of seasonal malaria chemoprevention on prevalence of malaria infection in malaria indicator surveys in Burkina Faso and Nigeria. BMJ Glob Health. 2022;7: e008021. 10.1136/bmjgh-2021-008021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirakoya-Samadoulougou F, De Brouwere V, Fokam AF, Ouédraogo M, Yé Y. Assessing the effect of seasonal malaria chemoprevention on malaria burden among children under 5 years in Burkina Faso. Malar J. 2022;21:143. 10.1186/s12936-022-04172-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba E, Hamade P, Kivumbi H, Marasciulo M, Maxwell K, Moroso D, et al. Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study. Lancet. 2020;396:1829–40. 10.1016/S0140-6736(20)32227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diawara F, Steinhardt LC, Mahamar A, Traore T, Kone DT, Diawara H, et al. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita, Mali. Malar J. 2017;16:325. 10.1186/s12936-017-1974-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Druetz T, Corneau-Tremblay N, Millogo T, Kouanda S, Ly A, Bicaba A, et al. Impact evaluation of seasonal malaria chemoprevention under routine program implementation: a auasi-experimental study in Burkina Faso. Am J Trop Med Hyg. 2018;98:524–33. 10.4269/ajtmh.17-0599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cairns ME, Sagara I, Zongo I, Kuepfer I, Thera I, Nikiema F, et al. Evaluation of seasonal malaria chemoprevention in two areas of intense seasonal malaria transmission: Secondary analysis of a household-randomised, placebo-controlled trial in Houndé District, Burkina Faso and Bougouni District, Mali. PLoS Med. 2020;17: e1003214. 10.1371/journal.pmed.1003214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan J, Suau Sans M, Okot F, Rom Ayuiel A, Magoola J, Rassi C, et al. A quasi-experimental study to estimate effectiveness of seasonal malaria chemoprevention in Aweil South County in Northern Bahr El Ghazal. South Sudan Malar J. 2024;23:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available from the corresponding author upon reasonable request.