Abstract
Background
Atrial fibrillation (AF) is a frequent cardiovascular (CV) comorbidity in cancer.
Objectives
The purpose of this study was to examine clinical characteristics and contemporary management of patients with AF and cancer with a specific focus on antithrombotic treatments.
Methods
This was a prospective, multicenter, observational study of patients with a recent cancer diagnosis and electrocardiographically confirmed AF (the BLITZ-AF Cancer Registry). CHA2DS2VASc scores were calculated for study participants.
Results
Overall, 1,514 individuals were enrolled from June 2019 to September 2021 (mean age 74 ± 9 years, 47.5% of participants >75 years of age; 63.5% males). CV diseases were common: 20.9% had heart failure, 18.1% had coronary artery disease, 38.5% had valvular heart disease, and 9.8% had peripheral artery disease. Previous thromboembolic and hemorrhagic events occurred in 13.9% and 10.4% of subjects, respectively. The most common cancer types were lung (14.9%), colorectal (14.1%), prostate (8.8%), and non-Hodgkin lymphoma (8.1%). In total, 41.5% of the patients had a CHA2DS2VASc score ≥4. Before admission or prior to cardiologist consultation, 16.6% of subjects were not taking any antithrombotic therapy and 22.7% were receiving antiplatelet agents and/or low-molecular-weight heparin. At discharge or after cardiologic assessment, these percentages dropped to 7.7% and 16.6%, respectively. This trend was paralleled by an increase in the use of direct-acting oral anticoagulant, while the proportion of vitamin K antagonist declined.
Conclusions
This study demonstrates that there is underuse of appropriate antithrombotic therapy for AF in cancer patients highlighting the need to integrate early CV assessment in the management of these patients. (Non-interventional Study on Patients With Atrial Fibrillation and Cancer [BLITZ-AF Cancer]; NCT03909386)
Key words: antithrombotic, atrial fibrillation, cancer, comorbidities, oral anticoagulants
Central Illustration
Atrial fibrillation (AF) is a frequent cardiovascular (CV) comorbidity in cancer patients. According to epidemiological studies, the incidence of AF may be up to 5-fold higher in individuals with cancer than in those without.1 This is likely due to the combination of tumor-related direct or indirect cardiac injury, toxicity of oncological treatments, and systemic inflammation.2 Moreover, prognosis is worsened by the co-occurrence of AF and cancer. In the ROCKET AF (Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), a history of cancer was associated with a higher risk of bleeding and non-CV death.3 In the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction 48) trial, which uniquely included 1,153 subjects with new-onset or recurrent cancer after randomization, the diagnosis of malignancy during follow-up was associated with an increased rate of major bleeding and death.4 A greater risk of major bleeding and mortality was also documented in retrospective investigations comparing AF patients with vs without cancer.5
The enhanced susceptibility to bleeding events and the possibility of clinically relevant interactions with antineoplastic drugs make thromboprophylaxis with vitamin K antagonists (VKAs) particularly challenging in patients with concomitant cancer.6 Direct-acting oral anticoagulants (DOACs) represent an appealing alternative for the prevention of AF-associated thromboembolism. Secondary analyses of randomized controlled trials and real-world evidence suggest that the efficacy and safety profile favoring DOAC over VKA is maintained in subjects with both AF and cancer.7,8
The use of DOAC is more frequent when patients are seen by cardiologists.9 This finding may be ascribed to the tendency to prescribe more often anticoagulants and less often off-label low-molecular-weight heparin (LMWH) by cardiologists as compared with oncologists,10,11 as well as to Class I recommendation to start DOACs rather than VKA in AF.12 Nonetheless, the patterns of antithrombotic therapies and the associated clinical (bleeding or thrombotic) events before and after a cardiological evaluation of individuals with AF and cancer have not been characterized yet in large, prospective cohorts. Likewise, updated information about rhythm vs rate control strategies in oncological patients with AF is lacking.
The primary objective of the BLITZ-AF Cancer Registry was to examine the clinical characteristics and the contemporary management of AF in patients with cancer (diagnosed prior or after the AF diagnosis) in a real-world setting, with a specific focus on the use of antithrombotic and rate or rhythm control treatments.
Methods
BLITZ-AF Cancer was a prospective, noninterventional study conducted at 112 cardiology units in Italy, Belgium, the Netherlands, Spain, Portugal, and Ireland (Supplemental Table 1), aimed at providing real-world evidence on the epidemiology and management of AF in patients with cancer, with a focus on the use of different antithrombotic and anticoagulant treatment strategies, from those recommended by current guidelines, such as VKAs and DOACs, to those widely used even in the absence of documented efficacy in the prevention of thromboembolism in AF, such as antiplatelets and LMWHs. The Heart Care Foundation in Florence, Italy, was the promoter of the study and the ANMCO Research Centre of Heart Care Foundation served as the coordinating center. The protocol was submitted to the Catania 2 Institutional Review Board, coordinating Institutional Review Board for the project, as well as to the Ethics Committees of all participating centers. The Single Opinion (“Parere Unico”) was issued on February 12, 2019 (n. 55/2019/CECT2). The study is registered at ClinicalTrial.gov (NCT03909386).
Study participants
Patients attending an outpatient visit or discharged from the cardiology ward were eligible if they met the following criteria: age ≥18 years; documented cancer other than basal-cell or squamous-cell carcinoma of the skin diagnosed within 3 years; electrocardiographically confirmed AF within 1 year; and no concomitant interventional study. The choice to include patients with a documented diagnosis of cancer within 3 years was motivated by the intention to select a population with “active” cancer using a simple temporal criterion. The same concept applies to “electrocardiographically confirmed AF in the last 12 months,” where the objective was to select patients with an “active” arrhythmic problem, excluding subjects with distant and no longer clinically relevant AF. Paroxysmal AF was defined as self-terminating AF, usually within 48 hours but up to 7 days. Persistent AF was an AF episode lasting longer than 7 days or requiring termination by cardioversion, with drugs or by direct current cardioversion. Long-standing persistent AF was defined as AF that was present for ≥1 year. Permanent AF was when the presence of AF was accepted by the patient and physician. Patients who met the above criteria were asked to sign a consent to the participation to the study and the anonymous handling of their data for research purposes.
Data collection
After consenting, all patients underwent a baseline evaluation. Data on baseline characteristics were collected at study entry, including demographic data, risk factors and comorbidities, diagnosis and current status of AF and cancer, previous interventions for AF, medical history including thromboembolic (according to TOAST [Trial of ORG 10172 in Acute Stroke Treatment] criteria) and hemorrhagic (according to International Society on Thrombosis and Haemostasis (ISTH) definition) events, and biochemical laboratory parameters. Ischemic stroke included large-artery atherosclerosis, cardioembolic, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology. Hemorrhagic events included major bleeding (fatal bleeding, nonfatal bleeding, and intracranial hemorrhage) and clinically relevant nonmajor bleeding (ISTH definition). Information on the use of antithrombotic agents, type of therapy, dosages, and concomitant medications were recorded at study entry (before admission or cardiologist consultation) and at discharge or after cardiological evaluation. The CHA2DS2VASc, HAS-BLED, and European Heart Rhythm Association (EHRA) symptom scores were calculated for study participants.
Data were collected using a web-based electronic case report form with the central database located at the ANMCO Research Centre. The definitions of the variables were made explicit in the electronic case report form. Using a validation plan built into the data entry software, the data were checked for missing or contradictory entries and values outside the normal range.
Statistical analysis
Categorical variables are reported as number and percentages, while continuous variables are reported as mean ± SD or median (IQR). A multivariable logistic regression analysis was performed in order to identify the independent predictors of DOAC prescription at discharge or after consultation in patients with non-valvular AF (ie, no mitral stenosis or prosthetic mechanical valve), considering the following variables of clinical interest: sex, age (<65 years [as reference group-RG]; 65 to 74 years; ≥75 years), body mass index (<30 [RG], ≥30 kg/m2; unknown), chronic cardiac disease (chronic heart failure (HF)/coronary artery disease/valvular disease/other cardiac disease), diabetes, hypertension, peripheral vascular disease (no [RG]; yes; unknown), renal dysfunction (defined as chronic kidney dysfunction and/or creatinine >2.5 mg/dL), history of ischemic thromboembolic complications, hemorrhagic events, upper-gastrointestinal/pancreas/colorectal malignancy vs other cancer types, metastasis, long-standing persistent/permanent AF vs other type of AF, hemoglobin (<12 g/dL; ≥12 g/dL [RG]; unknown), and planned cancer treatment with open surgery/chemotherapy/immunotherapy/targeted therapy. When more than 2 categories were present, dummy variables were introduced to define a reference group. Furthermore, a logistic regression on DOACs prescription at discharge or after consultation was performed in patients with non-valvular AF, considering only the CHA2DS2VASc (continuous) as a covariate. A P value <0.05 was considered statistically significant. All the analyses were performed with SAS system software, version 9.4 (SAS Institute Inc).
Results
The BLITZ-AF Cancer investigators enrolled a total of 1,514 patients from June 26, 2019, to September 30, 2021 (Supplemental Table 1). Patient baseline characteristics are summarized in Table 1. The mean age was 74 ± 9 years and almost half of the participants were aged more than 75 years; male patients accounted for 63.5% of the total population. CV diseases were common: 20.9% of patients had HF, 18.1% coronary artery disease, 38.5% any valvular heart disease, with aortic stenosis being the most frequent valve disease, and 9.8% peripheral artery disease. Previous thromboembolic and hemorrhagic events had occurred in 13.9% and 10.4% of subjects, respectively. Concerning the 158 patients with previous hemorrhagic event, 10 had an hemorrhagic stroke, 55 a major bleeding, and 95 a minor bleeding (2 patients had more than 1 type of hemorrhagic event). Diabetes (21.8%), hypertension (71.9%), and hypercholesterolemia (40.4%) were the most represented CV risk factors. Chronic kidney disease (14.3%) and chronic obstructive pulmonary disease (14.1%) were the most prevalent non-CV comorbidities. The median CHA2DS2VASc score was 3 (IQR: 2-4) and 628 (41.5%) patients had a CHA2DS2VASc score ≥4, median HAS-BLED was 1 (IQR: 1-2) and 170 (11.2%) patients had HAS-BLED score ≥3.
Table 1.
Baseline Characteristics and Type of Atrial Fibrillation of the Patients Enrolled in BLITZ-AF Cancer Registry (N = 1,514)
| Age, y | 74 ± 9 |
| <65 | 192 (12.7) |
| 65-75 | 602 (39.8) |
| >75 | 720 (47.5) |
| Female | 553 (36.5) |
| Body mass indexa >25 kg/m2 | 857 (58.2) |
| Active smoking | 169 (11.2) |
| Diabetes | 330 (21.8) |
| Hypertension | 1,088 (71.9) |
| Hypercholesterolemia | 612 (40.4) |
| Chronic heart failure | 317 (20.9) |
| Coronary artery disease | 274 (18.1) |
| Valvular heart disease | 583 (38.5) |
| Aortic stenosis | 78 (13.4) |
| Mitral stenosis | 21 (3.6) |
| Prosthetic mechanic valve | 17 (2.9) |
| Peripheral vascular disease | 149 (9.8) |
| Previous stroke, TIA, systemic embolism, or venous thromboembolism | 210 (13.9) |
| Previous hemorrhagic eventb | 158 (10.4) |
| Hemorrhagic stroke | 10 (6.3) |
| Major bleeding | 55 (34.8) |
| Minor bleeding | 95 (60.1) |
| Chronic kidney disease | 216 (14.3) |
| Chronic obstructive pulmonary disease | 214 (14.1) |
| Liver disease | 75 (5.0) |
| Hypothyroidism | 143 (9.5) |
| Hyperthyroidism | 59 (3.9) |
| Systolic blood pressure (mm Hg) | 129 ± 18 |
| Heart rate (beats/min) | 79 ± 19 |
| Left ventricular ejection fractionc (%)a | 56.2 ± 9.9 |
| Platelets (103/mm3)d | 224 ± 99 |
| Hemoglobine (g/dL) | 12.2 ± 2.0 |
| Type of atrial fibrillation | |
| First-detected | 323 (21.3) |
| Paroxysmal | 460 (30.4) |
| Persistent | 192 (12.7) |
| Long-standing persistent | 33 (2.2) |
| Permanent | 506 (33.4) |
Values are mean ± SD or n (%).
TIA = transient ischemic attack.
Available for 1,473 patients.
2 patients had more than 1 type of hemorrhagic event.
Available for 1,310 patients.
Available for 1,011 patients.
Available for 1,453 patients.
Types of AF and symptoms
AF was first detected in 323 (21.3%) subjects, paroxysmal in 460 (30.4%), persistent in 192 (12.7%), long-standing persistent in 33 (2.2%), and permanent in 506 (33.4%). Thus, <50% of the enrolled patients had long-lasting AF. Symptoms attributable to AF were ranked using the EHRA score and were present in 590 (39.0%) patients (74.2% EHRA score II, 21.7% EHRA score III, and 4.1% EHRA score IV).
Rate control was the most often adopted strategy for AF treatment (57.3%), primarily with beta-blockers. Rhythm control was the primary option in 14.4% and was pursued to a similar extent with drugs or electrical cardioversion (Table 2). In the first-detected and paroxysmal AF subgroups, a strategy focused on rhythm control (sometimes associated with rate control) was more prevalent, while in persistent AF subgroups rate control was, as expected, the more frequent choice (Supplemental Table 2).
Table 2.
Treatments for Atrial Fibrillation in the BLITZ-AF Cancer Registry (N = 1,514)
| AF treatment strategy | |
| Rate control | 867 (57.3) |
| Rhythm control | 218 (14.4) |
| Rate and rhythm control | 334 (22.0) |
| Still under evaluation | 95 (6.3) |
| Modalities of rhythm control | |
| Pharmacological cardioversion | 234 (15.5) |
| Electrical cardioversion | 227 (15.0) |
| Catheter ablation | 62 (4.1) |
| Surgical therapy | 13 (0.9) |
| Before Admission/Consultation | After Admission/Consultation | |
|---|---|---|
| Antiarrhythmic therapy | 258 (17.0) | 309 (20.4) |
| Digoxin | 157 (10.4) | 182 (12.0) |
| Beta-blocker | 929 (61.4) | 1,033 (68.2) |
| Verapamil or diltiazem | 49 (3.2) | 53 (3.5) |
| Pacemaker | 92 (6.1) | |
| ICD | 35 (2.3) | |
| CRT | 10 (0.7) |
Values are n (%).
CRT = cardiac resynchronization therapy; ICD = implantable cardioverter-defibrillator.
Cancers and therapies
The most frequent cancer types were lung (14.9%), colorectal (14.1%), prostate (8.8%), and non-Hodgkin lymphoma (8.1%) (full list in Supplemental Table 3). Patients with hematological cancers, (non-Hodgkin lymphoma, leukemias, multiple myeloma, and Hodgkin lymphoma, n = 297, 19.6%) have significantly lower platelets (mean 178 ± 102) than those with other types of cancer (235 ± 94) (P < 0.0001). Among the 252 patients not taking any antithrombotic/anticoagulant therapy on admission, the percentage of those with hematological cancer was somewhat higher (21.4% vs 19.3% in patients untreated vs treated), but the difference was not significant. A total of 463 (30.6%) patients had documented metastases. Cancer treatments are listed in Table 3.
Table 3.
Treatments of Cancer in the BLITZ-AF Cancer Registry (N = 1,514)
| Previous | Planned | |
|---|---|---|
| Surgery | 751 (49.6) | 122 (8.1) |
| Radiation therapy | 353 (23.3) | 100 (6.6) |
| Chemotherapy | 749 (49.5) | 613 (40.5) |
| Immunotherapy | 130 (8.6) | 102 (6.7) |
| Targeted therapy | 144 (9.5) | 123 (8.1) |
| Hormone therapy | 195 (12.9) | 178 (11.8) |
| Stem cell transplant | 17 (1.1) | 13 (0.9) |
Values are n (%).
Almost 90% of patients included in BLITZ-AF Cancer had been treated with (49.5%) or were planned to receive (40.5%) chemotherapy, underwent surgery (49.6%), or were scheduled for surgery (8.1%). Radiotherapy had been used in 23.3% of patients and was planned in another 6.6%. Taking into account the age of the population and the type of cancers, the use of innovative therapies such as immunotherapy (previous 8.6%, planned 6.7%), targeted therapy (9.5% and 8.1%, respectively), and stem cell transplantation (1.1% and 0.9%) are not surprising. Hormone therapy was used in a portion of the cohort (12.9 performed or ongoing, and 11.8% planned).
Antithrombotic and anticoagulant treatments
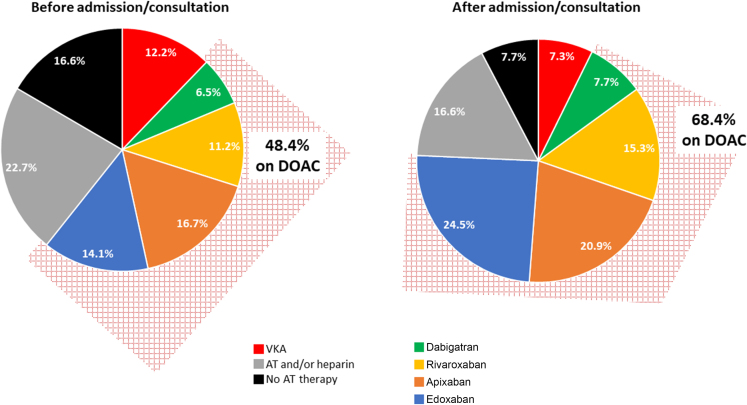
Before admission or prior to cardiologist consultation, 252 (16.6%) patients were not taking any form of antithrombotic/anticoagulant therapy, 344 (22.7%) were taking antiplatelet agents and/or LMWH, and 185 (12.2%) were on VKA. At discharge or after cardiologic assessment, the rates decreased and 117 (7.7%) patients were not taking any form of antithrombotic/anticoagulant therapy, 251 (16.6%) were taking antiplatelet agents and/or LMWH, and 110 (7.3%) were on VKA. As depicted in Figure 1, this trend was paralleled by a significant increase in the prescription of DOAC. Among the 252 patients not taking any antithrombotic/anticoagulant therapy before admission or cardiologists consultation, 114 had first-detected AF (as per protocol every patient who presents AF for the first time is considered a patient with first diagnosed AF, irrespective of the duration of the arrhythmia or the presence and severity of AF related symptoms), the date of onset of the arrhythmia was known in 88 cases, 81% of whom had an arrhythmia that occurred within the past 3 months. The 252 patients not taking any antithrombotic/anticoagulant therapy before admission or cardiologists consultation have lower HAS-BLED and CHA2DS2VASc with respect to other patients (HAS-BLED ≥3 4.4% vs 12.6% and CHA2DS2VASc ≥4 24.2% vs 44.9%) thereby identifying a subgroup at lower risk for both thromboembolic and hemorrhagic events (Table 4).
Figure 1.
Patterns of Antithrombotic Therapy in the BLITZ-AF Cancer Cohort, Before and After Admission or Evaluation at the Study Centers
The importance of cardiologist’s involvement. AT = antithrombotic; DOAC = direct-acting oral anticoagulant; VKA = vitamin K antagonist.
Table 4.
Hemorrhagic and Thromboembolic Risk Profile and Hematological Laboratory Values by Antithrombotic/Anticoagulant Therapy Before Admission/Consultation
| No AT/AC Therapy at Study Entry (n = 252) |
AT/AC Therapy at Study Entry (n = 1,262) |
|
|---|---|---|
| HAS-BLED ≥3 | 11 (4.4) | 159 (12.6) |
| CHA2DS2VASc ≥4 | 61 (24.2) | 567 (44.9) |
| Platelets (103/mm3)a | 220 ± 117 | 225 ± 95 |
| Hemoglobinb (g/dL) | 12.0 ± 2.2 | 12.2 ± 2.0 |
| Prior major bleeding or hemorrhagic stroke | 11 (4.4) | 54 (4.3) |
| Stage IV cancer (metastasis) | 66 (26.2) | 397 (31.5) |
Values are n (%) or mean ± SD.
AC = anticoagulant; AT = antithrombotic.
Available for 1,011 patients.
Available for 1,453 patients.
Given the potentially relevant drug-drug interactions (DDIs) between antithrombotic agents and other drugs, and specifically cancer treatments, we also evaluated the oncological treatments in patients taking antithrombotic/anticoagulant therapy and in the 252 not treated patients and we found no significant differences between the oncological therapies prescribed to the 2 groups of patients (Table 5).
Table 5.
Oncological Drugs by Antithrombotic Therapy Before Admission/Consultation
| No AT/AC Therapy at Study Entry (n = 252) | AT/AC Therapy at Study Entry (n = 1,262) | |
|---|---|---|
| Anthracycline and analogues | 23 (9.1) | 117 (9.3) |
| Nonanthracycline chemotherapy | 117 (46.4) | 632 (50.1) |
| Other antitumor antibiotics | 2 (0.8) | 20 (1.6) |
| Topoisomerase inhibitors | 10 (4.0) | 34 (2.7) |
| Alkylating agents | 54 (21.4) | 283 (22.4) |
| Antimetabolites | 44 (17.5) | 208 (16.5) |
| Microtubule targeting agents (vinca alkaloids) | 6 (2.4) | 64 (5.1) |
| Taxane derivative | 26 (10.3) | 95 (7.5) |
| Monoclonal antibodies | 25 (9.9) | 157 (12.4) |
| Multi-targeted tyrosine kinase inhibitors (TKIs) | 21 (8.3) | 107 (8.5) |
| Biological response modifiers and differentiation agents | 1 (0.4) | 7 (0.6) |
| Proteasome inhibitors | 1 (0.4) | 27 (2.1) |
| Histone deacetylase inhibitors (HDAC-I) | - | - |
| Immune checkpoint inhibitors (ICIs) [CTLA4/PD-1/PD-L1] | 19 (7.5) | 65 (5.2) |
Values are n (%).
AC = anticoagulant; AT = antithrombotic.
Previous bleeding may also be one of the reasons why OAC therapy is not prescribed. However, the timing of prior bleeding was not known, only if patients had a history of bleedings (major bleedings or hemorrhagic strokes or minor bleedings, as per ISTH definition). Only 63 of 596 (10.6%) patients not on OAC (either VKAs or DOACs) at study entry had a prior bleeding (of whom 31 patients with a history of a major bleeding or hemorrhagic stroke, 5.2%). At discharge/after cardiologist consultation, 54 out of 368 (14.7%) patients not prescribed OAC had a prior bleeding (of whom 29 patients with a history of a major bleeding or hemorrhagic stroke, 7.9%).
Among the 1,514 participants in BLITZ-AF Cancer Registry, 21 had mitral stenosis, 17 prosthetic mechanic valves, and 49 no information in this regard. Thus, 1,427 subjects could be categorized as non-valvular AF, of whom 997 (69.9%) were on DOAC at discharge/after cardiologist consultation. The distribution of antithrombotic/anticoagulant therapies in the 1,427 patients with cancer and non-valvular AF is shown in Supplemental Figure 1: DOAC prescription also increased in this subgroup following cardiologist evaluation, with a parallel reduction in the rate of use of VKA, antiplatelets, LMWH, and in the number of patients with no antithrombotic treatment. Multivariable logistic regression analysis showed that DOAC use in non-valvular AF was independently associated with female sex (OR: 1.58; 95% CI: 1.22-2.05), age (OR: 2.00; 95% CI: 1.39-2.88 and OR: 2.63; 95% CI: 1.84-3.76, respectively, for 65-74 years and ≥75 years vs <65 years), hypertension (OR: 1.43; 95% CI: 1.10-1.87), long-standing persistent or permanent AF (OR: 1.36; 95% CI: 1.05-1.78), hemoglobin <12 g/dL (OR: 0.57; 95% CI: 0.45-0.73), and planned cancer treatment (OR: 0.72; 95% CI: 0.57-0.92).
The prescription of DOAC increased with increasing CHA2DS2VASc score (Table 6), consistently with the association of some CHA2DS2VASc components with DOAC therapy (OR: 1.31; 95% CI: 1.20-1.42; P < 0.0001). In a very small subset of patients, percutaneous occlusion of the left atrial appendage occlusion had been performed (n = 26, 1.7%) or was planned (n = 6, 0.4%) at the time of the baseline visit.
Table 6.
Direct Oral Anticoagulants Prescription at Discharge or After Consultation in 1,427 Patients With Nonvalvular Atrial Fibrillation: Stratified by CHA2DS2VASc Score
| CHA2DS2VASc = 0 (n = 33) | CHA2DS2VASc = 1 (n = 130) | CHA2DS2VASc ≥2 (n = 1,264) | |
|---|---|---|---|
| DOAC | 7 (21.2) | 70 (53.9) | 920 (72.8) |
Values are n (%).
DOAC = direct-acting oral anticoagulant.
Discussion
The purpose of BLITZ-AF Cancer Registry was to obtain a real-world snapshot of the clinical/demographic characteristics and patterns of antithrombotic treatments in a large population of patients with cancer and AF. In this setting, data are scarce, mainly derived from small retrospective studies and secondary analyses of pivotal studies of DOAC in AF.4,13
Anticoagulation can be challenging in patients with cancer for various reasons, including DDIs and increased bleeding risk due to hematologic abnormalities such as anemia and thrombocytopenia. The most important finding of this registry is the high percentage of cancer patients who were not treated or treated inappropriately (receiving antithrombotic therapy not recommended for thromboprophylaxis in AF, that is, antiplatelet agents and/or LMWH), especially at study entry. On the other hand, after cardiologist evaluation, the percentage of patients treated with DOAC significantly increased, while the one of untreated patients decreased, as well as did the percentage of patients receiving antithrombotic therapy not recommended for thromboprophylaxis in AF, as is the case for LMWHs (Central Illustration).
Central Illustration.
Characteristics and Antithrombotic Management of Patients With Cancer and Atrial Fibrillation
∗Inappropriately treated is defined as receiving antithrombotic therapy not recommended for thromboprophylaxis in AF (ie, antiplatelet agents and/or low-molecular-weight-heparin). AF = atrial fibrillation; DOAC = direct-acting oral anticoagulant.
Baseline patient characteristics
The average age of the population enrolled in BLITZ-AF Cancer is 74 ± 9 years, fully comparable with that of the population included a few years ago in the BLITZ-AF study, which had enrolled over 4,000 patients from emergency wards of whom only 4.7% of patients had cancer.14
Looking at the other clinical features, as well as at CV risk factors and associated pathologies, the 2 populations appear at least partially overlapping (Supplemental Table 4), with the major differences concerning the double prevalence of HF in BLITZ-AF vs BLITZ-AF Cancer (43.2% vs 20.9%), well explained by the context in which the data were collected in the first study (emergency room of 154 Italian cardiology wards).
In a large network meta-analyses using individual patient-level data from the pivotal randomized trials of DOAC vs warfarin in patients with AF,15 the median age was 72 years, while 37.3% were female. Again the rates of diabetes (30.8%), hypertension (87.7%), coronary artery disease (29.9%), and HF (46.4%) reflected the same pattern as in BLITZ-AF, with small differences regarding diabetes and hypertension, slightly less represented in the latter study (23.7% and 77.2%, respectively).
AF characteristics
In the present registry, slightly <50% of the enrolled patients had a form of long-lasting AF. In the BLITZ-AF Study, permanent AF (30.9%), persistent (23.8%), and long-lasting persistent (4.3%), accounted for 59% of the total, followed by first detected (22.3%), paroxysmal (15.7%), and unknown (3%).14 After cancer diagnosis, the incidence of new-onset AF is higher compared with individuals without cancer due, at least in part to the presence of shared risk factors, complications of cancer itself and cancer treatments but also to the fact that patients with a recent cancer diagnosis are subjected to more frequent clinical examinations, even in in-patient settings, which may facilitate the diagnosis of arrhythmia.16 Median CHA2DS2-VASc score in our study was 3 (IQR: 2-4) and is similar to that observed in other AF trials without malignancy.17 Our data confirm that patients with malignancies are generally less symptomatic compared to those without malignancies: in the BLITZ-AF study, the arrhythmia was asymptomatic (EHRA score 1) in only 38.4% of patients as opposed to 61% in the present registry. This may reflect the greater proportion of paroxysmal arrhythmia in the cancer cohort or indeed a relative ‘masking’ of arrhythmia-associated symptoms by cancer symptoms.
AF treatments: anticoagulants and antithrombotics
It is well known that only a proportion of eligible patients with cancer and AF ranging from 30% to 70% receive anticoagulation.18,19 This registry extends these observations highlighting how a non-negligible proportion of these patients, is either not prescribed any treatment (16.6%) or is treated (22.7%) with antithrombotic or antiplatelet drugs, such as aspirin and LMWHs, which are not recommended for the prophylaxis of thromboembolic complications of AF. These numbers appear substantially higher compared with the BLITZ-AF study, in which the percentage of patients who did not receive any antithrombotic treatment was 6.8%. These findings suggest that antithrombotic management in cancer patients may be more challenging with treatment decision likely driven more by the perceived risk of bleeding rather than risk of thrombotic complications, although these are also higher in cancer patients. Other factors may also however be relevant including site of metastases, extent of disease and prognostic survival, or frailty status of the older patient.
The analysis of the 252 patients naive from any antithrombotic/anticoagulant treatment at study entry showed a hematologic and clinical profile (as expressed by hemoglobin and platelet values, history of previous bleeding/hemorrhagic stroke, tumor stage, and ongoing oncologic therapies) substantially superimposable, with the only significant differences represented by a lower thromboembolic and hemorrhagic risk profile (CHA2DS2VASc and HAS-BLED score) than that of treated patients. However, after cardiologic evaluation at study entry, the percentage of untreated patients still decreased, thus identifying an area of “therapeutic inertia” in the oncology patient.
Cancer treatments may involve significant interactions with anticoagulants. An in-depth discussion of DDIs of anticoagulant/antithrombotic drugs with oncology drugs, although very relevant in these patients, is beyond the scope of this article. The mechanism involved, which should always be kept in mind with regard to novel oral anticoagulants, is usually the induction or inhibition of CYP3A4 and/or P-glycoprotein, with associated changes in the concentration and efficacy/safety of novel oral anticoagulants.
However, in our population, no significant differences emerged with respect to the use of all classes of oncology drugs considered, between the 252 untreated patients and the others.
Previous bleeding may be another valid reason for not administering anticoagulant treatment: in our study, however, this occurrence is relatively uncommon and affects no more than 10% to 15% of patients, with even lower rates for major and intracranial bleeding.
The proactive role of cardiologists in improving AF treatment, already documented in other studies,3,4,20 is also confirmed by our registry. In fact, following study entry evaluation, the percentage of patients treated with oral anticoagulants rises because of the fact that the number of patients who are prescribed a DOAC increases, at the expense of antiplatelet drugs, LMWHs, as well as VKAs. This framework indicates that cardiologists pursue the implementation of DOACs in these patients, even if the residual use of other antithrombotic therapies, such as antiplatelet agents or LMWHs or the lack of antithrombotic prophylaxis remains substantial.
Analysis of the use of individual drugs within the DOACs category indicated that edoxaban was most frequently used in patients with AF and cancer.
This may be attributable to the published evidence in the ENGAGE AF study,21 which not only enrolled the largest number of patients with non-valvular AF but was the first trial that included a large subgroup of patients with AF and cancer. The ENGAGE AF subanalysis4 specifically reviewed data from 1,153 patients with newly diagnosed or relapsed cancer after randomization and confirmed the efficacy and better safety profile of edoxaban compared to warfarin, regardless of the presence of cancer.
To date, this represents the largest body of data with a DOAC in this clinical setting. Another additional element that may have conditioned the use of edoxaban in this patient setting stems from the fact that edoxaban represents the first DOAC with evidence-based benefit in neoplastic venous thromboembolism in the Hokusai VTE-Cancer study.22 Finally, a major issue in cancer patients concerns drug interactions, especially considering the very rapid pace of introduction of new drugs in oncology. All DOACs, interacting with CYP3A4 and P-glycoprotein, could interfere with the metabolism of many anticancer drugs, but the low interaction of edoxaban with CYP3A4 (<4%), unlike rivaroxaban and apixaban, could further explain why edoxaban was the most prescribed DOAC. Finally, as regards the prescription of DOACs, we also found some significant differences between the countries represented in the registry, in all likelihood due to different decisions or rules of each national regulatory authority.
Rate vs rhythm control strategies
Rate control was applied in almost 60% of cases, while a pure rhythm control strategy was chosen in 14.4% of the patients and a mixed strategy (rate and rhythm control) in 22%. The predominance of rate control contrasts with the observation that <50% of enrolled patients had a long-lasting arrhythmia. A possible explanation could be related to the high proportion of asymptomatic patients in our registry and the fact that oncological treatments are considered the number one (or the only) priority.
In patients initiated into a rhythm control strategy, electrical cardioversion and pharmacological cardioversion are used in a balanced way (15% each), while a minority of patients underwent catheter ablation (4.1%) or surgical therapy (0.9%).
The drugs most frequently used for rate control are beta-blockers (61%), while digitalis is still used in 10 to 12% of patients, with a marginal role for calcium channel blockers. Antiarrhythmics, bearing in mind the option in favor of pharmacological cardioversion made in 15.5% of the patients, are used in 17% of cases, which rises to 20.4% after cardiological evaluation. Amiodarone was the most widely used antiarrhythmic (56%), followed by flecainide (28%), sotalol (9%), and propafenone (about 5%). Less than 10% of the enrolled patients had a device (either pacemaker, implantable cardioverter-defibrillator or cardiac resynchronization therapy) on board.
Compared to nononcological patients, our registry seems to document a greater propensity for less aggressive treatments, which may be expected in this context.
Cancer types
The cancer types included in the BLITZ-AF Cancer cohort reflects the general cancer prevalence distribution, with lung, colorectal, and breast cancer accounting for more than 40% of cases, followed by hematological malignancies in 15%. By comparison, in the largest cohort of cancer patients included in a pivotal study comparing edoxaban to warfarin in nonvalvular atrial fibrillation,13 gastrointestinal malignancies were present in 20.5% of patients, followed by prostate in 13.7%, lung/pleura in 11%, bladder in 7.5%, breast 6.5%, hematological 5% with other localizations to follow with lower percentages.
Study limitations
An inherent limitation of registries is the modality of patients recruitment which in the current investigation occurred in outpatient clinics or cardiology departments. Thus, our data concern the subgroup of cancer patients with AF for whom the treating oncologist deemed it necessary to seek advice from a cardiologist. Most probably other subgroups of patients with cancer and AF are not represented in this register, such as those with very advanced disease, those with an episode of AF far in the past or patients with limited access to multidisciplinary disease management.
Despite consecutive enrollment was recommended in the protocol and strongly encouraged during the investigator meetings before the start of the study, no ad hoc validation was performed to verify this issue. Given that the enrollment rate has been slightly lower than expected in some sites, this may well be due to a limited selection bias.
Conclusions
The BLITZ-AF Cancer provides extensive information on a large cohort of individuals with AF and cancer in a real-life setting.
The data show that much remains to be done to optimize anticoagulant therapy in these patients, since a very high percentage are either not treated or receive drugs for which the evidence is not available. The role of the cardiologist, who must always be involved in the initial assessment and follow-up of these patients, appears fundamental in this context, as the rate of prescription of the most appropriate anticoagulant therapy increases significantly after the cardiology evaluation at study entry. Given the likely frailty and age of this patient cohort multidisciplinary working with gerontology may also be of benefit in such treatment decisions. Furthermore, our work emphasizes similarities and differences between the general population of patients with AF and those in whom arrhythmia and cancer coexist. The analysis of the 1-year follow-up data will also be able to answer many clinically relevant questions that are among the secondary objectives of the study.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE 1: BLITZ-AF Cancer registry shows that much remains to be done to optimize anticoagulant therapy in cancer patients with AF as a high percentage of patients are not treated or are treated with drugs for which the evidence is not available. Cardiologists should be involved in the care of these patients to help optimize therapies as prescriptions for appropriate anticoagulant therapy increase after the cardiology evaluation.
COMPETENCY IN MEDICAL KNOWLEDGE 2: Among patients with cancer and AF, the management of anticoagulant therapy still presents many critical issues. The active and constant involvement of the cardiologist throughout their course of treatment appears to be a fundamental component of the multidisciplinary management that should always be ensured for all cancer patients.
TRANSLATIONAL OUTLOOK: Further studies with an adequate follow-up period are needed to evaluate the best anticoagulation strategy for patients with cancer and AF. In addition, studies examining the impact of cardio-oncological assessments on clinical outcomes are needed.
Funding support and author disclosures
The study was realized by Heart Care Foundation with own research funds, partially supported by a not conditional grant by Daiichi Sankyo Italia. Dr Ameri received speaker and/or advisor fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Daiichi Sankyo, Janssen, and MSD, all outside the scope of this work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a complete list of the BLITZ-AF Cancer participating centers and investigators, supplemental tables, and a supplemental figure as well as a the BLITZ-AF Cancer Baseline Case Report Form, please see the online version of this paper.
Supplementary data
References
- 1.Jakobsen C.B., Lamberts M., Carlson N., et al. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19:1105. doi: 10.1186/s12885-019-6314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menichelli D., Vicario T., Ameri P., et al. Cancer and atrial fibrillation: epidemiology, mechanisms, and anticoagulation treatment. Prog Cardiovasc Dis. 2021;66:28–36. doi: 10.1016/j.pcad.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen S.T., Hellkamp A.S., Becker R.C., et al. Efficacy and safety of rivaroxaban vs warfarin in patients with non-valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes. 2019;5:145–152. doi: 10.1093/ehjqcco/qcy040. [DOI] [PubMed] [Google Scholar]
- 4.Fanola C.L., Ruff C.T., Murphy S.A., et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF - TIMI 48 trial. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastori D., Marang A., Bisson A., et al. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: a nationwide cohort study. Cancer. 2021;127:2122–2129. doi: 10.1002/cncr.33470. [DOI] [PubMed] [Google Scholar]
- 6.Mosarla R.C., Vaduganathan M., Qamar A., Moslehi J., Piazza G., Giugliano R.P. Anticoagulation strategies in patients with cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;73:1336–1349. doi: 10.1016/j.jacc.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallari I., Verolino G., Romano S., Patti G. Efficacy and safety of nonvitamin K oral anticoagulants in patients with atrial fibrillation and cancer: a study-level meta-analysis. Thromb Haemost. 2020;120:314–321. doi: 10.1055/s-0039-3400300. [DOI] [PubMed] [Google Scholar]
- 8.Shah S., Norby F.L., Datta Y.H., et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018;2:200–209. doi: 10.1182/bloodadvances.2017010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neal W.T., Claxton J.S., Sandesara P.B., et al. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J Am Coll Cardiol. 2018;72:1913–1922. doi: 10.1016/j.jacc.2018.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malavasi V.L., Fantecchi E., Gianolio L., et al. Atrial fibrillation in patients with active malignancy and use of anticoagulants: under-prescription but no adverse impact on all-cause mortality. Eur J Intern Med. 2019;59:27–33. doi: 10.1016/j.ejim.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Toma M., Rrapaj E., Spallarossa P., Guerra F., Ameri P. Patterns of anticoagulation for atrial fibrillation in cancer patients referred to cardio-oncological evaluation. Eur J Intern Med. 2020;82:128–129. doi: 10.1016/j.ejim.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 13.Fradley M.G., Ellenberg K., Alomar M., et al. Pattern of anticoagulation use in patients with cancer with atrial fibrillation and/or atrial flutter. JACC CardioOncol. 2020;2:747–754. doi: 10.1016/j.jaccao.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulizia M.M., Cemin R., Colivicchi F., et al. Management of atrial fibrillation in the emergency room and in the cardiology ward: the BLITZ-AF study. Europace. 2019;21:230–238. doi: 10.1093/europace/euy166. [DOI] [PubMed] [Google Scholar]
- 15.Carnicelli A.P., Hong H., Connolly S.J., et al. A collaboration between multiple institutions to better investigate non-vitamin K antagonist oral anticoagulant use in atrial fibrillation) investigators. Direct oral anticoagulants vs warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145:242–255. doi: 10.1161/CIRCULATIONAHA.121.056355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Neal W.T., Lakoski S.G., Qureshi W., et al. Relation between cancer and atrial fibrillation (from the REasons for geographic and racial differences in stroke study) Am J Cardiol. 2015;115:1090–1094. doi: 10.1016/j.amjcard.2015.01.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel M.R., Mahaffey K.W., Garg J., et al. Rivaroxaban vs warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 18.Ardeshirrouhanifard S., An H., Goyal R.K., et al. Use of oral anticoagulants among individuals with cancer and atrial fibrillation in the United States, 2010-2016. Pharmacotherapy. 2022;42:375–386. doi: 10.1002/phar.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ording A.G., Søgaard M., Nielsen P.B., et al. Oral anti-coagulant treatment patterns in atrial fibrillation patients diagnosed with cancer: a Danish nationwide cohort study. Br J Haematol. 2022;197:223–231. doi: 10.1111/bjh.18060. [DOI] [PubMed] [Google Scholar]
- 20.Melloni C., Dunning A., Granger C.B., et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and a history of cancer: insights from the ARISTOTLE trial. Am J Med. 2017;130:1440–1448.e1. doi: 10.1016/j.amjmed.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano R.P., Ruff C.T., Braunwald E., et al. Edoxaban vs warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 22.Raskob G.E., van Es N., Verhamme P., et al. Hokusai VTE cancer investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.