Abstract
Skeletal muscle has long been recognized as an inhospitable site for disseminated tumour cells (DTCs). Yet its antimetastatic nature has eluded a thorough mechanistic examination. Here, we show that DTCs traffic to and persist within skeletal muscle in mice and in humans, which raises the question of how this tissue suppresses colonization. Results from mouse and organotypic culture models along with metabolomic profiling suggested that skeletal muscle imposes a sustained oxidative stress on DTCs that impairs their proliferation. Functional studies demonstrated that disrupting reduction–oxidation homeostasis via chemogenetic induction of reactive oxygen species slowed proliferation in a more fertile organ: the lung. Conversely, enhancement of the antioxidant potential of tumour cells through ectopic expression of catalase in the tumour or host mitochondria allowed robust colonization of skeletal muscle. These findings reveal a profound metabolic bottleneck imposed on DTCs and sustained by skeletal muscle. A thorough understanding of this biology could reveal previously undocumented DTC vulnerabilities that can be exploited to prevent metastasis in other more susceptible tissues.
To successfully metastasize, a tumour cell must traffic to and survive within a distant tissue before colonizing it. This proliferative phase of metastasis, and the accompanying destruction of the architecture and function of the host organ, accounts for the high incidence of cancer-related mortality. Paget’s pioneering work helped establish that the nature of this final phase is organotropic, which was based on his hypothesis that tumour “seeds” thrive in some but not all human “soils”1. Decades of work dissecting this phenomenon revealed that DTCs flourish within foreign environments through numerous mechanisms, including metabolic adaptation2–7, stromal co-option8–12, engagement with extracellular matrix (ECM)13–21 and immune evasion22–24. Failure to acclimate often results in DTC death or cell cycle exit25,26. Therefore, the ultimate success of a DTC may be dictated by how extensively it can adapt to the microenvironment of its host organ. Many studies have enumerated mechanisms by which primary tumours or various insults facilitate DTC adaptation to common sites of metastasis, such as the lung9–11,14,15,27. In contrast, why DTCs never adapt to certain organs has received far less attention. Investigation of such sites could reveal unique metastasis-suppressive mechanisms.
Of all tissues, one could argue that skeletal muscle (SkM) represents the most metastasis suppressive. While tumours frequently invade SkM28, blood-borne metastases are exceptionally rare. A meta-analysis of autopsy samples of 41 different primary cancers from 3,827 individuals identified SkM metastases in only 16 patients (0.4%)29. Considering that the mass of SkM in a typical human far exceeds that of other tissues, a compelling question is why tumour cells rarely colonize SkM.
Three other research groups have asked this question, proposing tumour-suppressive mechanisms related to SkM mechanics, lineage specification and soluble effectors30–34. We hypothesized that a careful examination of the interplay between DTCs and the SkM microenvironment might reveal additional, unique mechanisms of metastasis suppression and expose DTC vulnerabilities that can be exploited at other more susceptible sites35. Here, we show that despite trafficking to and persisting within the SkM at a relatively high rate, DTCs fail to colonize this tissue. An array of studies that contrasted SkM with lung, an organ far more prone to metastasis, revealed a metabolic barrier for DTCs in the SkM niche. Namely, sustained environmental hydrogen peroxide (H2O2) exposure and insufficient antioxidant defences result in the induction of endogenous reactive oxygen species (ROS) and a highly oxidized state, particularly within DTC mitochondria. These findings implicate oxidative stress as a barrier that DTCs must overcome to colonize distant organs and show the ability of SkM to sustain this stress is a major component of its antimetastatic nature.
Results
Dissemination to SkM is not a rare event.
To determine whether breast cancer cells spontaneously disseminate to SkM, we interrogated SkM biospecimens from two patients with metastatic breast cancer (MBC) (Fig. 1a) diagnosed with oestrogen-receptor-positive (ER+) and progesterone-receptor-positive (PR+) disease. We utilized these markers along with pan-cytokeratin (pan-CK), an epithelial cell marker, in an attempt to identify breast tumour cells in the quadriceps, the tibialis anterior (TA) and the gastrocnemius. This antibody cocktail positively stained tumour cells within breast cells (Fig. 1b and Extended Data Fig. 1a), but did not stain resident cells in SkM from donors with no documented history of cancer (Extended Data Fig. 1b). In one of the donors with MBC, we found multiple individual ER+PR+pan-CK+ breast cancer cells located both inside and outside the quadriceps microvasculature (Fig. 1b and Extended Data Fig. 1c–f), which suggests that spontaneous dissemination to SkM may occur with unexpected frequency given the limited number of patients and tissue sampled.
Fig. 1 |. Breast cancer cells traffic to and persist within SkM.
a, Locations surveyed for the identification of human breast cancer cells in SkM from patients with MBC. b, Images of a multiplex immunohistochemistry panel of a breast tumour and SkM with disseminated breast cancer cells. ER and PR in purple, pan-CK in yellow. Haematoxylin and eosin (H&E)-stained muscle in second panel from the left. Scale bars, 20 μm (tumour cells in SkM), 50 μm (primary tumour) or 500 μm (SkM H&E). c, Schematic of the orthotopic (mammary fat pad, MFP) mouse study used to determine whether tumour cells spontaneously traffic to SkM. d, Schematic of the experiment used to determine the sensitivity of detection of the AluYb8 qPCR method. e, AluYb8 qPCR limit of detection, reflected by cycle of quantification (Cq) and log of the starting quantity (SQ). n = 3 replicates, repeated in triplicate. One-way analysis of (ANOVA), followed by an uncorrected Fisher’s least significant difference (LSD), was used to analyse the OP-9-only negative control against all others, where ****P < 0.0001 for all comparisons, except OP-9 only versus no template control (NTC). f, AluYb8 amplification (fold-change) in tumours 3 weeks after tumour resection. n = 5 inoculated and 3 uninoculated mice. Two-way ANOVA was performed, followed by uncorrected Fisher’s LSD, where P < 0.0001 for lung and quadriceps when comparing tumour-bearing tissue against uninoculated controls. P = 0.007 for tumour-bearing TA versus uninoculated and P = 0.002 for tumour-bearing gastrocnemius versus uninoculated. n = 5 inoculated and 3 uninoculated mice. g, Representative images for Ce3D-cleared lung and TA of MDA-MB-231-bearing mice. Insets are GFP+ tumours cells in lung and TA. Scale bars, 100 μm (insets) or 1 mm (whole tissue). h, Percentage of GFP+ tumour area normalized by total tissue area for lung, TA, gastrocnemius and quadriceps. One-tailed unpaired t-tests, with Welch’s correction, were performed for comparison of GFP area in tumour-bearing tissue against uninoculated, where P = 0.0003 for lung, P = 0.051 for TA, P = 0.039 for gastrocnemius and P = 0.084 for quadriceps. n = 5 inoculated and 3 uninoculated mice. For e, f and h, the centre line represents the mean, and error bars the s.e.m.
To achieve a more quantitative assessment of dissemination to SkM, we used an animal model of spontaneous metastasis utilizing MDA-MB-231 MBC cells expressing firefly luciferase enhanced green fluorescent protein (ffluc-eGFP-MDA-MB-231) implanted orthotopically into female NSG mice (Fig. 1c). Three weeks after resection of the primary tumour, bioluminescence imaging (BLI) revealed that all mice had substantial metastatic burden in their lungs. We compared DTC burden in lung and SkM at this time point using two parallel and complementary assays: (1) Alu quantitative PCR (qPCR) to measure the quantity of primate-specific (that is, non-murine) AluYb8 DNA36–38 in lung and in three SkM types (TA, quadriceps and gastrocnemius), and (2) immunofluorescence detection of GFP+ MDA-MB-231 cells in paired, Ce3D-cleared39 tissues.
Amplification of AluYb8 repeats from MDA-MB-231 cells diluted serially in mouse OP-9 cells demonstrated statistically reliable detection of one human cell in 106 mouse cells (Fig. 1d,e). When applied to the spontaneous metastasis model, AluYb8 qPCR revealed substantial metastatic burden in lung (7.5-fold increase in human AluYb8 DNA compared to uninoculated mice), which was consistent with the observed BLI signal (Fig. 1c,f). We also observed an increase in AluYb8 signals from three SkM sites, with 3.5-fold, 2.6-fold and 2.3-fold increases in the quadriceps, the gastrocnemius and the TA, respectively (Fig. 1f). Analyses of GFP in cleared tissues led to similar conclusions; GFP+ tumour cells occupied a greater percentage of lung tissue (8.6%) than any of the three muscles assessed (1.2% of the TA, 0.16% of the quadriceps and 0.08% of the gastrocnemius). Of note, GFP+ tumour cells were identified in all three SkM sites (Fig. 1g,h).
These data substantiated our observations in human samples that spontaneous dissemination to SkM is readily observed. To determine whether the differences observed were a result of enhanced trafficking to lung versus TA, quadriceps or gastrocnemius, we used an experimental model of metastasis in which the injection a prescribed number of cells into the left ventricle ensures equal input across mice (Extended Data Fig. 2a). Quantification of AluYb8 DNA across brain, lung, liver, bone marrow, TA, quadriceps and gastrocnemius 3 days after intracardiac injection revealed that dissemination to muscle is not rate-limiting. Indeed, the AluYb8 signal in TA was equivalent to that of lung at this early time point (Extended Data Fig. 2b; confirmed visually in a subset of tissues in Extended Data Fig. 2c). Analysis of the same set of tissues 7 weeks after intracardiac injection revealed the following findings: (1) despite equivalent trafficking to the TA and lung, DTCs emerge in lung but fall into the TA; (2) DTCs persist for at least 7 weeks in multiple muscle sites at or above the frequency found in some bone marrow, liver and brain specimens, all of which are more common sites of overt metastasis (Extended Data Fig. 2d); and (3) persistence of DTCs in SkM was common, given the high penetrance observed in this model (Extended Data Fig. 2d). These findings motivated us to perform a detailed exploration of how SkM suppresses DTC outgrowth.
Myotubes suppress breast cancer cell outgrowth in culture.
In contrast to tumour cells located in other tissues, DTCs identified within SkM commonly resided on or near the basal lamina of a myofibre and stained negative for Ki67 (Extended Data Fig. 2e,f). To test whether the niche surrounding SkM myofibres suppresses DTC outgrowth, we differentiated human SkM myoblasts (SkMc) into myotubes, which are multinucleated structures that approximate the myofibres of human and mouse tissue (Fig. 2a,b). Seeding breast tumour cells onto lung stroma (primary human lung fibroblasts (LFs)) or SkMc-derived myotubes allowed us to contrast breast tumour cell outgrowth on a permissive niche (lung) with one that we suspected was suppressive (Fig. 2a,b). Over a 10-day period, the SkMc niche significantly and substantially suppressed growth of five MBC cell lines (MDA-MB-231, Hs578T, BT549, HCC70 and MDA-MB-468) and two mammary cancer cell lines (4T1 and EO771) (Fig. 2c,d).
Fig. 2 |. Myotubes suppress breast and mammary cancer cells in culture, but not through a secreted or deposited factor.
a, Schematic of the experiment used to determine whether organotypic culture models of SkM suppress breast and mammary cancer cell outgrowth. b, Representative images of human lung (LF) and SkM (SkMc) organotypic niches. Scale bar, 10 mm. c, Outgrowth (YFP area fraction) of a panel of breast and mammary cancer cell lines on SkMcs and LFs. n = 8–10 replicates, repeated in triplicate. Multiple two-sided unpaired t-tests were performed, followed by Holm–Sidak’s multiple comparison test, where outgrowth on SkMcs versus LFs was ****P < 0.0001 for all comparisons. d, Representative images of breast and mammary cancer outgrowth on the SkMc niche. Scale bar, 10 mm. e, MDA-MB-231 outgrowth on LF and SkMc niches when treated daily with CM from LF or SkMc monoculture. n = 10–15 replicates, performed in triplicate. One-way ANOVA was performed, followed by Dunnett’s multiple comparisons test, to find P > 0.05. NS, not significant. f, MDA-MB-231 outgrowth on LFs and SkMcs when treated with EVs collected from SkMc monoculture. n = 7 replicates, performed in duplicate. Compared against untreated control, P > 0.1 for all conditions when one-way ANOVA, followed by Dunnett’s multiple comparisons test, was performed. g, MDA-MB-231 outgrowth on decellularized LFs or SkMcs, compared with outgrowth on intact LFs and SkMcs. n = 5–8 replicates, performed in triplicate. ****P < 0.0001 for the following when two-way ANOVA, followed by Sidak’s multiple comparisons test, was performed: intact LFs versus intact SkMcs, intact LFs versus decellularized LFs, intact SkMcs versus decellularized SkMcs. ***P = 0.0004 for intact LFs versus decellularized SkMcs, **P = 0.005 for intact SkMcs versus decellularized LFs, **P = 0.0088 for decellularized LFs versus decellularized SkMcs, ***P = 0.0005 for decellularized LFs versus decellularized SkMcs, and ****P < 0.0001 for intact LFs versus intact SkMcs when unpaired two-tailed t-tests were performed. h, MDA-MB-231 outgrowth when co-cultured on a LF–SkMc mixture, LF-only or SkMc-only. n = 5–8 replicates, performed in triplicate. ****P < 0.0001 when all conditions compared against LF-only using one-way ANOVA, followed by Dunnett’s multiple comparisons test. For c, e–h, the centre line represents the mean, and error bars the s.e.m.
Prior work has defined secretory molecules8, vesicular cargo10,16,18 and/or ECM components19,21,27 that regulate DTC dormancy and metastasis. To examine two of these potentially suppressive fractions, SkMc-derived conditioned medium (CM) and SkMc extracellular vesicles (EVs) were applied to LF and SkMc niches seeded with MDA-MB-231 tumour cells tagged with yellow fluorescent protein (MDA-MB-231-YFP). MDA-MB-231 outgrowth on LFs and SkMc was unaltered by daily treatment with SkMc-derived CM (Fig. 2e) or by SkMc EVs (Fig. 2f). MDA-MB-231-YFP cells seeded on decellularized LF or SkMc cultures proliferated 1.5-fold more on decellularized SkMc cultures (in which ECM is the primary constituent left behind40) than decellularized LF, which suggests that SkM ECM is not tumour suppressive (Fig. 2g). In fact, MDA-MB-231 cells outgrew four times more on decellularized SkMcs than they did on intact SkMc niches, which suggests that live myotubes are required for growth suppression. To test this hypothesis, we performed a SkMc–LF mixing experiment. Here, tumour outgrowth correlated inversely with the percentage of SkMc myotubes present (Fig. 2h). This result confirmed that live myotubes suppress tumour cell outgrowth.
The failure of SkMc-derived CM, EVs and ECM to recapitulate the growth-suppressive effect of live myotubes implicated that myotubes produce transient or reactive components that suppress breast tumour cell outgrowth, and/or outcompete breast tumour cells for key nutrients. Both possibilities raised the suspicion that SkM metabolism may underlie its metastasis-suppressive nature.
Glutathione metabolism is implicated in metastasis suppression.
Based on a growing body of evidence that metastases evolve towards the metabolism of their host tissue, we designed an experiment to reverse engineer SkM-specific metabolic barriers to DTC colonization4,41–43. We serially passaged a 4T1 subline derived from a rare SkM metastasis through Balb/c mice to achieve a 4T1 derivative that effectively colonized SkM (4T1-SkM; Fig. 3a). After intracardiac injection of 10,000 SkM-tropic 4T1-SkM cells into Balb/c mice, necropsy was performed and metastatic lesions in lung and SkM were excised. We performed metabolomics on the metastatic lesions and contrasted the results with healthy tissues from uninoculated mice. Cultured 4T1-parental and 4T1-SkM cell lines collected from culture were used as controls.
Fig. 3 |. GSH metabolism is enriched in SkM metastases.
a, Schematic of the derivation of a SkM-metastatic 4T1 subline (4T1-SkM). b, Schematic of the collected tissue samples and comparisons made for metabolomics analysis. c, KEGG MSEA of (SkM metastasis versus healthy SkM)/(lung metastasis versus healthy lung), referred to as comparison 3. Over representation analysis used the hypergeometric test; one-tailed P values were provided after adjusting for multiple testing. d, Heatmap displaying the row minimum (min) and maximum (max) for the raw values of the healthy SkM and SkM metastasis (Met) samples. Metabolites in the heatmap represent those that were at least 2-fold increased or 50% decreased in comparison 3, P < 0.1. Black circles denote the GSH metabolism metabolite set from KEGG. e, Heatmap displaying the row min–max for the raw values of the healthy lung and lung metastasis samples. Metabolites in the heatmap represent those that were at least 2-fold increased or 50% decreased in comparison 3, P < 0.1. Black circles denote the GSH metabolism metabolite set from KEGG. f, Schematic of the chemical reaction for GSH-mediated H2O2 detoxification. g, Dot plot of the ratio of reduced to oxidized glutathione (GSH:GSSG) for healthy SkM and lung. n = 6 healthy SkM, 3 healthy lung. Unpaired two-sided t-test was performed, where ****P < 0.0001 for healthy lung versus healthy SkM. h, Dot plot of GSH:GSSG ratio for SkM metastasis and lung metastasis. n = 4 SkM metastasis and 3 lung metastasis. Metastasis samples were collected from seven mice. Unpaired two-sided t-test was performed, where *P = 0.038 for lung metastasis versus muscle metastasis. i, Dot plot of the GSH:GSSG ratio in SkM compared with the GSH:GSSG in lung for both healthy and metastasis samples. Unpaired two-sized t-test was used, where ****P = 0.003. n = 3–5 per condition. For e–h, the centre line represents the mean, and error bars the s.e.m.
4T1-parental and 4T1-SkM cell lines were metabolically distinct from each other (Extended Data Fig. 3a), whereby 4T1-SkM cells shifted towards healthy SkM tissue. This shift was accentuated in vivo, as a principal component analysis placed SkM metastases in proximity of healthy muscle tissue (Extended Data Fig. 3a). These data indicate that tumour cells that colonize SkM adapt to become more muscle-like.
To understand the metabolic adaptations that are required specifically for the colonization of muscle, we normalized : (1) metabolites from SkM metastases to those from healthy SkM; (2) metabolites derived from lung metastases to those of healthy lung; then (3) these values by each other (i.e., (1) normalized by (2)). Metabolite set enrichment analysis (MSEA)44 was performed on the most divergent metabolites arising from this final comparison (Fig. 3b). The most enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways45 were purine metabolism and glutathione (GSH) metabolism (Fig. 3c). The level of enrichment measured for both sets was specific to this comparison; they were not observed when contrasting healthy SkM and lung tissue, for example (Extended Data Fig. 3c,d). Purine metabolism may reflect the biosynthetic needs of proliferative metastases, whereas GSH metabolism is invariably linked to ROS detoxification. Maintenance of cellular reduction–oxidation (redox) homeostasis requires a dynamic balance of ROS generation with the reducing capacity of the antioxidant system46 to prevent oxidative stress, proliferative stasis or even cell death47. Therefore, GSH metabolism enrichment might reflect a particular evolution by SkM metastases to overcome a harsh, oxidative environment. This hypothesis was particularly attractive because the volatile nature of ROS may explain why SkMc-derived CM could not suppress breast tumour cell outgrowth (Fig. 2e).
MSEA revealed that SkM metastases were enriched for several GSH intermediates (S-glutathionyl-<di>L-cysteine and 5-<di>L-glutamyl-<di>l-glutamine) compared with all other samples (Fig. 3d,e). We also observed trends towards increased glutamine and glutamate (a GSH precursor) in SkM metastases compared to lung metastases and healthy lung tissue (Supplementary Tables 1 and 2). Cysteine (another GSH building block) was increased in the SkM metastases compared with healthy SkM (Supplementary Tables 1 and 2). We observed a similarly strong increase in GSH metabolites such as glutamine and glutamate in the SkM metastases compared with the 4T1-SkM cells in tissue culture, which suggested that utilization of these substrates occurs specifically and abundantly within the SkM niche (Extended Data Fig. 3b).
To gain insight into oxidative stress, we examined the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG)48 (Fig. 3f). In a resting cell, the molar GSH:GSSG ratio exceeds 100:1, whereas in various models of oxidative stress, this ratio can decrease by one to two orders of magnitude49,50. Healthy SkM and SkM metastases exhibited a greater redox buffering capacity than lung or lung metastases (Fig. 3g). This trend did not hold true in culture (Extended Data Fig. 3e), which perhaps indicates that the metabolic programmes that benefit 4T1-SkM cells in SkM only become apparent and/or advantageous in vivo (Extended Data Fig. 3e and Supplementary Tables 3 and 4).
Given their common tissue of origin and the likelihood that due to haematogenous dissemination51, tumour cells that seed muscle and lung start at the same oxidized state, the substantially increased GSH:GSSG ratio of SkM metastases versus lung metastases (Fig. 3i) could be interpreted in two mutually exclusive ways. The first is that tumour cells capable of colonizing SkM experience less ROS and therefore have less oxidation of GSH to GSSG. The second is that proliferating within SkM requires tolerating increased ROS, and thus only DTCs that have and can maintain a high GSH:GSSG ratio colonize SkM. A corollary is that the vast majority of DTCs that reside within muscle cannot make this adaptation, fail to neutralize the higher ROS burden of the tissue49 and are effectively frozen in a single cell or micrometastatic state due to an unmanageable level of oxidative stress. Moving forward, we sought to determine which of these interpretations was more accurate.
Skeletal muscle DTCs exhibit increased oxidative stress.
To determine whether breast tumour cells in the muscle microenvironment exhibit symptoms of a GSH redox imbalance, particularly in the context of GSH metabolism, we used a glutaredoxin redox-sensitive GFP2 (Grx1-roGFP2) biosensor52,53 that allows dynamic and quantitative live imaging of GSH redox potential and, by association, the oxidation state of the cell (Fig. 4a). When plated in co-culture on differentiated SkMcs, MDA-MB-231-Grx1-roGFP2 (MDA231-roGFP2) cells became highly oxidized (Fig. 4b). Of note, the oxidation state of the MDA231-roGFP2 cells on SkMcs increased rapidly over the first several days and remained elevated throughout the course of study. Conversely, MDA231-roGFP2 cells grown in the LF niche experienced a modest increase in oxidation within the first 3 days then returned to baseline (Fig. 4b). This pattern of oxidation correlated closely with measured growth dynamics (Fig. 4c).
Fig. 4 |. The SkMc niche causes unchecked oxidative stress in DTCs.
a, Schematic of the Grx1-roGFP2 redox biosensor used to measure GSH-specific oxidative stress. b, Left: MDA231-roGFP2 oxidation state on LFs and SkMcs. n = 8 replicates, performed in triplicate. Two-way ANOVA, followed by Sidak’s test: *P = 0.036, 4 days; ****P < 0.0001, 6, 8 and 10 days. P = 0.07, 2 days. Right: representative image of MDA231-roGFP2 on SkMcs (oxidized) and LFs (reduced). Scale bar, 100 μm. c, MDA231-roGFP2 outgrowth on LFs and SkMcs. n = 10 replicates, performed in triplicate. Two-way ANOVA, followed by Sidak’s test: *P = 0.02, 0 day; ***P < 0.0001, 6, 8 and 10 days. P > 0.05, 2 and 4 days. d, MDA-MB-231 mean ROS intensity (arbitrary units (AU)) on LFs and SkMcs. n = 852 cells (LFs), 39 cells (SkMcs); repeated in triplicate. Unpaired two-tailed t-test, ****P < 0.0001. e, Representative MDA-MB-231 ROS on SkMcs and LFs. Scale bars, 50 μm (SkMc) or 100 μm (LF). f, ROS mean intensity for LFs, SkMcs and myotubes. n = 24 negative control, 23 LF, 24 SkMc wells. One-way ANOVA, followed by uncorrected Fisher’s LSD: ****P < 0.0001, negative control versus myotubes, LF versus myotubes, SkMc versus myotubes. All other *P > 0.05. g, Extracellular H2O2 in LFs and SkMcs. n = 8 replicates, repeated in triplicate. Two-way ANOVA, followed by Tukey’s test: ****P < 0.0001, LF versus SkMc (3 and 7 days); *P = 0.011, LF versus SkMc (10 days). h, Extracellular H2O2 in LF-only, SkMc-only or LF–SkMc mixture. n = 5 replicates, repeated in duplicate. Two-way ANOVA, followed by Sidak’s test: mean compared against LF-only: *P = 0.049, 1:20; *P = 0.04, 1:50; **P = 0.0002, 1:100; ***P = 0.0001, SkMc-only. All other P > 0.05. i, Timeline of the NOD-SCID mouse study to determine whether DTCs are oxidized in vivo. j, MDA231-roGFP2 oxidation in SkM and lung. n = 13 cells (muscle), 35 cells per lesions (lung). One-way ANOVA, followed by Dunnett’s test: compared against single cells- lung, P = 0.053, doublets; ***P = 0.0001; 3–10 and 11+ cells clusters. k, Visualization of metastases/DTCs in lung (reduced) and muscle (oxidized). Scale bar, 50 μm. n = 13 cells (muscle), 35 cells per lesions (lung). For c, d, f–h and j, the centre line represents mean, and error bars the s.e.m.
To support these findings, we used a cell-permeable fluorescent dye to more broadly quantify ROS abundance within MDA-MB-231 cells and the niches in which they reside. We consistently measured higher levels of ROS in tumour cells residing within the SkMc niche than those within the LF niche (4.5-fold increase on SkMc; Fig. 4d,e). Furthermore, SkMcs themselves exhibited increased ROS levels, particularly within myotubes (Fig. 4e,f), which had 6.7-fold the ROS levels measured in LFs and 3.1-fold those measured in unfused myocytes (Fig. 4e,f).
Since H2O2 is arguably the longest-lived ROS and is reduced by GSH, we hypothesized that increased H2O2 in the SkMc niche could account for the perturbations in redox balance observed. In culture, a maximum concentration of 0.42 μM H2O2 was measured in the LF niche on day 7, whereas the maximum H2O2 documented in SkMc cultures was nearly triple that (1.2 μM) and occurred early on. This was consistent with the early break in tumour cell oxidation state observed between LF- and SkMc-cultures (Fig. 4c) and sustained throughout the 10-day time course (Fig. 4b,g). Admixing LFs and SkMcs at increasing SkMc:LF ratios demonstrated clearly that H2O2 levels tracked with the quantity of myotubes in each well (Fig. 4h). These data demonstrate that ROS accumulate in SkMc niches in culture, disrupt redox balance of tumour cells within these niches and correlate with reduced tumour cell outgrowth. To what extent would this hold true in vivo?
Using the same Grx1-roGFP2 biosensor in MDA-MB-231 cells delivered by intracardiac injection revealed that single DTCs within SkM were highly oxidized (Fig. 4j,k). Micro- and macro-metastases were not observed within SkM (Fig. 4j). Of further note, single DTCs were oxidized to the same extent in lung as they were in SkM (Fig. 4j,k). However, oxidation resolved over the course of metastatic progression in this organ (Fig. 4j,k). Lung micrometasteses and macrometastases (clusters of more than 50 cells) were significantly more reduced than single cells (Fig. 4j,k). These data lent further credence to the notion that single DTCs might start at similar oxidation states regardless of tissue, and must balance their redox state to colonize a given site51. This motivated us to perform functional studies to determine whether sustaining oxidative stress would prevent lung metastatic progression, and whether enhancing antioxidant reserves would enable tumour cells to achieve redox balance and colonize SkM.
Increased oxidative stress suppresses tumour cell outgrowth on lung.
To determine whether increased ROS attenuated outgrowth on permissive lung-like niches, we spiked sublethal doses of H2O2 into organotypic niches containing MDA231-roGFP2 tumour cells. Here, the H2O2 dose required to achieve growth suppression (125 μM; Fig. 5b) was two orders of magnitude greater than the H2O2 concentration measured in SkMc niches (Fig. 4g). Given the volatility of H2O2, we asked whether lung colonization could be suppressed if DTCs were consistently exposed to ROS concentrations in line with those measured in the SkM niche.
Fig. 5 |. Sustained oxidative stress prevents DTC outgrowth in lung and muscle.
a, MDA231-roGFP2 outgrowth on LFs and SkMcs with 75 μM, 125 μM H2O2. n = 6 replicates, repeated in triplicate. Two-way ANOVA, with Dunnett’s test: compared to untreated LF, *P = 0.029, untreated SkMc; *P = 0.032, 125 μM/LFs (8 days). ***P = 0.0001, untreated SkMc; *P = 0.033, 75 μM/LFs; ***P = 0.0001, 125 μM/LFs (10 days). b, MDA231-roGFP2 oxidation state on LFs and SkMcs with 75 μM or 125 μM H2O2. n = 5 replicates, repeated in duplicate. Two-way ANOVA, with uncorrected Fisher’s LSD: compared against untreated LF, ****P < 0.0001, untreated SkMc (1, 5 and 10 days). *P = 0.011, 125 μM/LF (1 day). **P = 0.0096, 75 μM/LFs; ****P < 0.0001, 125 μM/LFs (10 days). c, Schematic of H2O2 generator DAAO biochemistry. d, Schematic of the experiment used to test whether increased tumoural H2O2 stunts outgrowth on niches. e, MDA231-DAAO or MDA231-WT cell outgrowth on LFs with l-alanine (l-ala) or d-alanine (d-ala) treatment. n = 4–5 replicates, repeated in triplicate. One-way ANOVA, with Tukey’s test: day 10, compared to untreated DAAO+, all d-alanine ****P < 0.0001. ****P < 0.0001, 10 mM d-alanine versus l-alanine, 10 mM d-alanine DAAO versus WT. All others P > 0.05. f, Representative images of MDA231-DAAO cell outgrowth on LFs. Scale bar, 10 mm. g, MDA231-DAAO or MDA231-WT cell outgrowth on SkMcs following l-alanine or d-alanine treatment. n = 4–5 replicates, repeated in triplicate. One-way ANOVA, with Tukey’s test: day 5, compared to untreated DAAO+, all d-alanine ****P < 0.0001 (SkMc). ***P = 0.0004, 10 mM d-alanine DAAO versus WT; *P = 0.012, 10 mM d-alanine versus l-ala. Day 10, compared to untreated DAAO+, all d-alanine ****P < 0.0001 (SkMc). *P = 0.01, 10 mM d-alanine in DAAO versus WT. **P = 0.0006, 10 mM d-alanine versus l-alanine. ****P < 0.0001, 20 mM d-alanine DAAO versus WT. **P = 0.0007, 20 mM d-alanine versus l-alanine. ****P < 0.0001, 100 mM d-alanine DAAO versus WT, 100 mM d-alanine versus l-alanine. h, Representative images of MDA231-DAAO cell outgrowth on SkMcs. Scale bar, 10 mm. i, Schematic of the experiment used to test whether increased H2O2 in LF stunts tumour outgrowth. j, MDA231-WT cell outgrowth on LFs, LF-DAAO or SkMcs following d-alanine treatment. n = 3 replicates, repeated in triplicate. Two-way ANOVA, with uncorrected Fisher’s LSD: day 2.5, **P = 0.0013, untreated LF-DAAO versus LF; P = 0.06, untreated LF-DAAO versus SkMc; **P = 0.007, 30 mM LF-DAAO versus LF; ****P < 0.0001, 50 mM LF-DAAO versus LF. Day 5, ****P < 0.0001, untreated LF-DAAO versus LF, untreated LF-DAAO versus SkMc, 30 mM LF-DAAO versus LF, 50 mM LF-DAAO versus LF. For a, b, e, g and j, the centre line represents the mean, and error bars the s.e.m.
To reconstruct these conditions, we utilized a chemogenetic system (D-amino acid oxidase (DAAO)54) that provides sustained, on-demand generation of H2O2 in a cell type of interest. Based on the DAAO from Rhodotorula gracilis, this vector produces H2O2 when DAAO converts d-amino acids (for example, d-alanine) to their corresponding imino acids (Fig. 5c). We first optimized DAAO amounts in MDA-MB-231 (MDA231-DAAO) cells on tissue culture plastic (Extended Data Fig. 4a) to establish the dynamics and scale of extracellular H2O2 accumulation that could be achieved. Serum-free culture medium containing increased doses of d-alanine or l-alanine was added to MDA231-DAAO or wild-type MDA-MB-231 (MDA231-WT) cells and sampled over 48 h. By the 24-h time point, MDA231-DAAO cells treated with 100 mM or 500 mM d-alanine accumulated significantly more H2O2 than all other conditions (Extended Data Fig. 4b). Daily administration of 100 mM d-alanine significantly reduced the proliferation of MDA231-DAAO cells without immediate toxicity (Extended Data Fig. 4c). Thus, the DAAO system was able to stimulate a tuneable and sustainable H2O2 bolus, but had to be delivered in tumour cell monoculture at concentrations of 100 mM or less.
To determine whether chemogenetic induction of H2O2 affected the outgrowth on LF and SkMc niches, we administered serum-free medium containing 0 mM, 10 mM, 20 mM or 100 mM d-alanine or l-alanine immediately after seeding MDA231-DAAO or MDA231-WT cells (Fig. 5d). In these more nutrient-limited conditions, dosing alanine at 100 mM compromised survival and outgrowth independent of the DAAO vector and thus was not suitable for comparison (Fig. 5e,f). However, MDA231-DAAO cell outgrowth on the LF niche was suppressed in the presence of 10 mM d-alanine (Fig. 5e,f). Importantly, outgrowth of controls were not affected at these alanine concentrations (Fig. 5e,f).
The induction of tumoural H2O2 also affected outgrowth on SkMc niches. Any MDA231-DAAO outgrowth on SkMcs was diminished back to baseline when treated with 10 mM or 20 mM d-alanine (Fig. 5g,h). This was consistent with increased levels of H2O2 ultimately resulting in cell death in monocultures (Extended Data Fig. 2b,c) and in co-cultures (Fig. 5e). Between the endogenous ROS produced by the microenvironment and those stimulated by the DAAO vector, there appeared to be sufficient H2O2 present by day 5 to induce a switch from dampened proliferation to diminished survival (Fig. 5e).
These data reveal that inducing and sustaining oxidative stress in tumour cells suppresses metastatic outgrowth in lung-like niches, and that this effect is nearly saturated in SkMc-comprised, H2O2-rich microenvironments. This prompted us to ask whether lung colonization could be suppressed if DTCs were exposed to a niche that produced ROS at concentrations in line with those measured in SkMc niches. To answer this question, we introduced the DAAO system into LFs. Akin to the results observed with MDA-MB-231 cells, LFs exhibited a dose-dependent response to d-alanine that allowed us to narrow in on a dose (30 mM) that did not compromise the viability of LFs. This dose also stimulated H2O2 concentrations on par with those measured in SkMc cultures (Extended Data Fig. 4d,e). Seeding MDA-MB-231 cells on these niches revealed that this level of H2O2 induction was sufficient to suppress MDA-MB-231 cell growth to the degree observed on SkMc niches (Fig. 5i,j). Going beyond this resulted in tumour cell death (Fig. 5j). These data point to ROS generation as a key, tumour-suppressive feature of SkMc niches.
Enhancing antioxidant capacity enables muscle colonization.
The data presented in Fig. 5 demonstrate that approximating SkMc niche ROS levels achieves comparable levels of tumour suppression; however, the consequence of restoring the redox balance by enhancing the antioxidant capacity of tumour cells delivered to SkM remained unknown. To address this question, we engineered MDA-MB-231 and EO771 cells to ectopically express catalase (CAT), a peroxisomal enzyme that rapidly converts H2O2 to H2O and O2, in their mitochondria (mCAT) or cytosol (cCAT; Extended Data Fig. 5a). Catalase acts in parallel to GSH to protect cells from ROS-inflicted oxidative damage55,56. Unlike GSH, catalase accomplishes detoxification without the need for cofactors or co-substrates, so it was a straightforward and potentially potent genetic manipulation.
To determine whether ectopic expression of catalase neutralized ROS in MDA-MB-231 cells in co-culture, we stained for ROS in control, mCAT and cCAT MDA-MB-231 (MDA231-Ctl, MDA231-mCAT and MDA231-cCAT, respectively) cells on SkMc and lung niches. Ectopic catalase expression significantly reduced ROS in MDA-MB-231 cells, irrespective of the niche (Fig. 6a). A difference in ROS neutralization between compartments was observed on SkMcs, whereby mCAT expression led to a 22% reduction in ROS intensity, whereas cytoplasmic expression resulted in a 52% reduction in ROS intensity.
Fig. 6 |. Enhanced tumoural antioxidant capacity enables SkM colonization in culture and in vivo.
a, ROS mean intensity of MDA231-Ctl, MDA231-mCAT and MDA231-cCAT cells in co-culture with LFs or SkMcs. For SkM, n = 39 (Ctl), 137 (mCAT) and 199 (cCAT). For LFs, n = 851 (Ctl), 200 (mCAT) and 233 (cCAT). Repeated in triplicate. One-way ANOVA, with Tukey’s test, where all comparisons ****P < 0.0001 except LF/mCAT versus LF/cCAT, P = 0.53. b, Dot plot of MDA231-Ctl, MDA231-mCAT and MDA231-cCAT cell outgrowth on SkMcs. n = 10 replicates, repeated in triplicate. One-way ANOVA, followed by Dunnett’s test: MDA231-Ctl versus mCAT, **P = 0.009; MDA231-Ctl versus cCAT, ****P = 0.0001. c, Representative images of MDA231-Ctl, MDA231-mCAT and MDA231-cCAT cell outgrowth on SkMcs. Scale bar, 20 mm. d, EO771-Ctl, EO771-mCAT and EO771-cCAT cell outgrowth on SkMcs. n = 10 replicates, repeated in triplicate. One-way ANOVA, with Dunnett’s test where ***P = 0.0001, MDA231-Ctl versus mCAT; **P = 0.001, MDA231-Ctl versus cCAT. e, Representative EO771-Ctl, -mCAT or -cCAT outgrowth on SkMc. Scale bar, 20 mm. f, Schematic of the NOD-SCID mouse study to examine whether ectopic catalase promoted muscle colonization in vivo. g, BLI total flux for MDA231-Ctl, MDA231-mCAT and MDA231-cCAT cells. n = 10 mice. Two-way ANOVA, followed by Dunnett’s test: week 11, ****P < 0.0001, Ctl versus mCAT. All others P > 0.05. h, Images of MDA231 total flux of TA ex vivo (week 11 for mCAT, week 16 for Ctl and cCAT). i, Immunofluorescence quantification (number of lesions per lesion size) of MDA231 at endpoint. n = 4 tissues. Chi-square test for trend performed, Ctl versus CAT (catalase cohorts grouped) *P = 0.016. j–l, Representative immunofluorescence images of MDA231-Ctl (j), MDA231-mCAT (j) and MDA231-cCAT (l) DTCs/lesions in SkM. Scale bar, 100 μm. m, Schematic of the C57BL/6 mouse study to examine whether ectopic catalase promoted colonization of SkM. n, BLI total flux for EO771-Ctl and EO771-mCAT cells. n = 14 mice. Two-way ANOVA, followed by uncorrected Fisher’s LSD: **P = 0.0032, week 3; P = 0.09, week 4. All other P > 0.8. o, Representative BLI total flux and immunofluorescence images for EO771-Ctl and EO771-mCAT cells. Scale bar, 100 μm. For a, b and d, the centre line represents the mean, and error bars the s.e.m. For i, the centre line represents the median, and whiskers the smallest and largest values.
Ectopic catalase expression promoted MDA-MB-231 cell outgrowth on SkMcs in a manner commensurate to the level of ROS reduction achieved by each vector (3.6-fold increase for mCAT, 4.7-fold increase for cCAT; Fig. 6a–c). Enhancement of antioxidant capacity also promoted EO771 cell outgrowth on SkMcs; mCAT and cCAT vectors induced a 4.3-fold and 3.4-fold increase, respectively, in EO771 cell outgrowth on SkMcs compared to control (Fig. 6d,e). Of note, this phenotype was not consistent across niches. Neither MDA231-mCAT nor MDA231-cCAT outgrew more than the MDA231-Ctl on LFs (Extended Data Fig. 5b). Ectopic catalase expression blunted the outgrowth of EO771 cells on LFs compared with EO771-Ctl cells (Extended Data Fig. 5b). These data reveal that levelling redox balance promotes outgrowth in culture on SkMc niches but not on LF niches.
To determine whether these findings applied in vivo, we inoculated luciferase-expressing MDA231-Ctl, MDA231-mCAT or MDA231-cCAT cells into the TA of NOD-SCID hosts and tracked outgrowth by BLI (Fig. 6f). A total of 40% of mice in the MDA231-mCAT cohort developed a detectable BLI signal in their TA before the study endpoint (Fig. 6g). In contrast, neither control nor cCAT cohorts developed a single muscle tumour (Fig. 6h). Immunofluorescence quantification of DTCs within the TA revealed that both the MDA231-mCAT and MDA231-cCAT tumour cells had an enhanced ability to transition out of the single-cell state (Fig. 6i–l). Whereas 86% of the MDA231-Ctl cells remaining in the TA after 16 weeks across 4 mice were single cells, TAs inoculated with MDA231-mCAT and MDA231-cCAT housed tumour cell clusters ranging from doublets to clusters of ten or greater (Fig. 6i), including the only bona fide SkM tumour observed in this model (Fig. 6k).
To determine whether these general findings held true in immune-competent settings, we inoculated the TA of C57BL/6 mice with syngeneic EO771-mCherry-luciferase cells expressing control or mCAT (EO771-Ctl and EO771-mCAT, respectively) (Fig. 6m). Tumour colonization was tracked by BLI over a 6-week period. Only 1 out of 14 mice (7%) in the EO771-Ctl cohort developed a BLI signal. In contrast, there was a strong increase in EO771-mCAT tumour burden in SkM after only 3 weeks of TA inoculation, with 5 out of 14 mice (36%) developing full-blown SkM lesions (Fig. 6n,o). These data show that restoring the tumoural redox balance overcomes proliferative barriers imposed by the SkM microenvironment, substantially enough in some cases to facilitate full-blown colonization of the tissue.
Breast tumour cells do not benefit from enhanced redox capacity in lung.
One might expect that increasing tumoural catalase leads to increased metastatic potential irrespective of organ site. Outgrowth of ectopic catalase variants on LFs suggested otherwise (Extended Data Fig. 5b). To test the impact of ectopic catalase expression on lung colonization in vivo, ffluc-eGFP-MDA231-Ctl, ffluc-eGFP-MDA231-mCAT and ffluc-eGFP-MDA231-cCAT cells were injected intravenously into mice and tracked by BLI (Extended Data Fig. 5c). In contrast to TA inoculation, lung BLI from intact mice (Extended Data Fig. 5d) and from dissected lung (Extended Data Fig. 5e) revealed that mCAT and cCAT expression significantly diminished MDA-MB-231 cell colonization compared to control. The number of lung metastases visualized by eGFP fluorescence on the surface of the lung supported this conclusion (average of 51 Ctl versus 11.6 mCAT versus 1.6 cCAT lesions; Extended Data Fig. 5f). The failure of ectopic catalase expression to promote lung colonization was perpetuated in immune-competent settings (Extended Data Fig. 5g–j). These results suggest that redox interactions between a DTC and its microenvironment are not equal across tissues, and that disrupting this balance too far in one direction or another substantially influences colonization.
Neutralizing mitochondrial ROS in the host stimulates SkM but not lung colonization.
We took advantage of the MCAT transgenic mouse model to test whether ectopic expression of mitochondrial catalase in the muscle niche would recapitulate the pro-growth phenotype observed when catalase was expressed intratumourally. The MCAT mouse expresses mitochondrial-targeted catalase to reduce H2O2 generation and is expressed in all organs surveyed, with the highest expression measured in cardiac muscle and SkM56. The TA of C57BL/6 WT or MCAT mice was inoculated with EO771-Ctl or EO771-mCAT cells (Fig. 7a), then colonization was tracked by BLI and, after necropsy, measured in SkM ex vivo (Fig. 7b). Only 1 out of 19 WT mice (5%; Fig. 7c) in the EO771-Ctl cohort developed a BLI signal, whereas 6 out of 19 WT mice (32%; Fig. 7d) in the EO771-mCAT cohort developed stable tumour burden over the course of the study. MCAT hosts promoted a similar degree of SkM colonization (3 out of 9 mice; Fig. 7e) after inoculation of EO771-Ctl cells, but despite initial dynamics suggesting otherwise, inoculation of EO771-mCAT cells into MCAT hosts did not exhibit in an additive effect (3 out of 9 mice; Fig. 7f).
Fig. 7 |. Reduction of tumoural or environmental mtRoS stimulates SkM colonization but hinders lung colonization.
a, Schematic of the C57BL/6 mouse study to examine whether ectopic catalase in the muscle environment promotes colonization. b, Representative BLI total flux images for EO771-Ctl and EO771-mCAT cells SkM in WT (left) and MCAT (right) mice. c–f, Quantification of BLI total flux for EO771-Ctl (c,e) and EO771-mCAT (d,f) in WT (c,d) or MCAT (e,f) mice. n = 19 mice per WT cohort, 9 mice per MCAT cohort. Two-way ANOVA, followed by uncorrected Fisher’s LSD: **P = 0.0065, EO771-Ctl versus EO771-mCAT in WT mice (week 3); P = 0.07, EO771-Ctl/WT mice versus EO771-mCAT/MCAT mice (week 4). ****P < 0.0001, EO771-Ctl/WT mice versus EO771-Ctl/MCAT mice (week 5). All other EO771-Ctl/WT mouse comparisons P > 0.15. g, Schematic of the C57BL/6 mouse study to examine whether ectopic catalase in the lung environment promotes colonization. h, BLI quantification for EO771-Ctl and EO771-mCAT cell injected into WT or MCAT mice. n = 15 WT, 10 MCAT mice per cohort. Two-way ANOVA, followed by Dunnett’s test: **P = 0.0003, EO771-Ctl in WT versus MCAT mice; ***P = 0.0001, EO771-Ctl versus EO771-mCAT in WT mice, EO771-Ctl/WT mice versus EO771-mCAT/MCAT mice (week 2). All others P > 0.05. i, Representative BLI images for EO771-Ctl and EO771-mCAT cells in WT and MCAT mice. j, BLI quantification of lungs ex vivo from WT and MCAT mice with EO771-Ctl and EO771-mCAT cells. n = 15 WT, 10 MCAT mice per cohort. One-way ANOVA, followed by Dunnett’s test: *P = 0.038, EO771-Ctl versus EO771-mCAT in WT mice; *P = 0.018, EO771-Ctl/WT mice versus EO771-mCAT/MCAT mice. P = 0.069, EO771-Ctl in WT versus MCAT mice. k, Quantification of EO771-Ctl and EO771-mCAT lesions in WT or MCAT mouse lungs. n = 15 WT, 10 MCAT mice per cohort. One-way ANOVA, followed by Dunnett’s test: ***P = 0.0001, EO771-Ctl versus EO771-mCAT in WT mice; **P = 0.005, EO771-Ctl/WT mice versus EO771-mCAT/MCAT mice. P = 0.996, EO771-Ctl in WT versus MCAT mice. l, Representative images of lung ex vivo (mCherry) from WT and MCAT mice with EO771-Ctl and EO771-mCAT. Scale bar, 1 mm. n = 15 WT, 10 MCAT mice per cohort. For c–f, h, j and k, the centre line represents the mean, and error bars the s.e.m.
These data suggest that the contribution of ROS from the environment is as important as the ROS generated (or transmitted) intratumourally in response to an oxidative environment. Resolving either stressor is sufficient for DTCs to progress within muscle. The failure of dual mCAT expression in the tumour and host to promote colonization beyond either individual manipulation might reflect increased anti-tumour T cell activity in the MCAT host57, particularly in light of the tumour ‘neo-antigens’ (GFP and luciferase) used in this study58. Or it might suggest that there is a fine-tuned range for DTCs in SkM at which the redox state must fall to stimulate outgrowth. The latter interpretation relates to observations made in lung, where tumoural mCAT expression hinders metastatic colonization rather than promoting it (Fig. 7g–l). Here, however, expression of mCAT in the host did not promote or inhibit metastasis when the total number of metastatic lesions was quantified (Fig. 7k).
These data confirm that an enhanced capacity to neutralize oxidative stress in the tumour or its surrounding tissue promotes colonization of muscle in a tissue-specific manner. They also implicate the mitochondria as a relevant compartment where ROS orchestrates metastasis-suppressive functions within SkM.
Tumour cells have a higher mitochondrial ROS burden in the muscle niche.
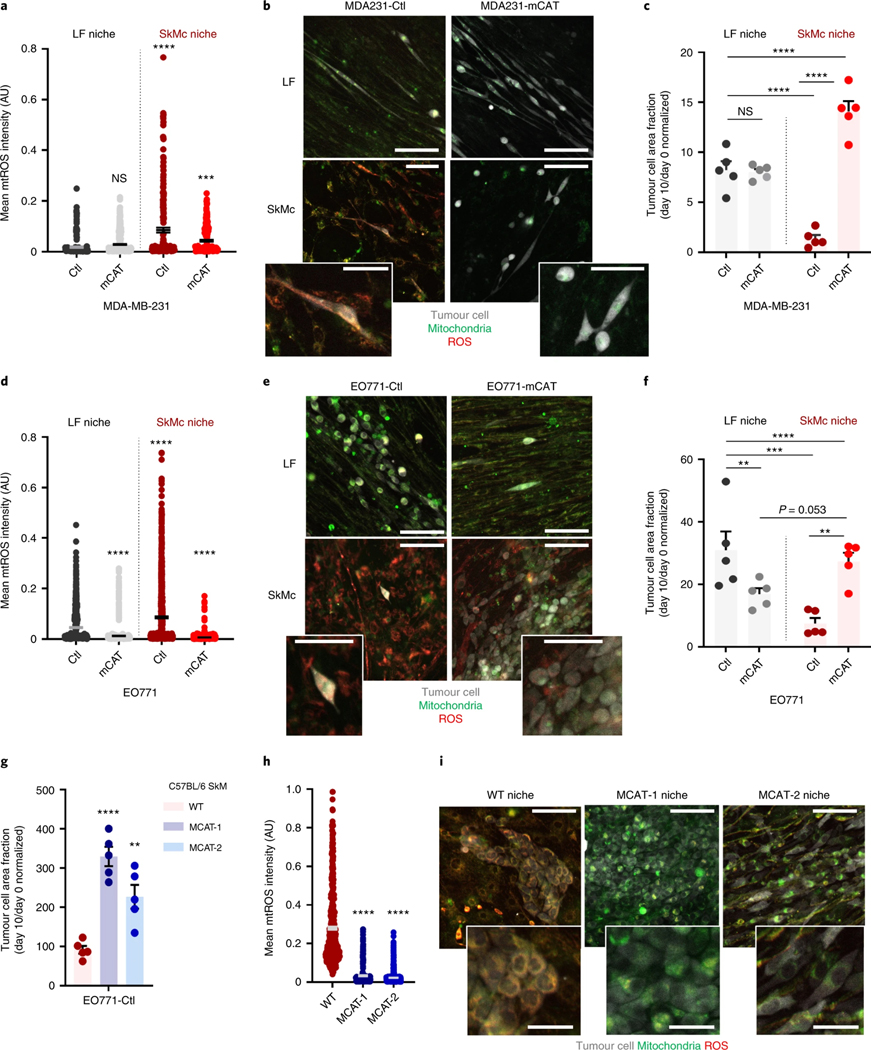
The ability of mCAT to stimulate SkM colonization pinpointed mitochondria as a relevant site of tumour-suppressive oxidative stress. To determine whether the amount of mitochondrial ROS (mtROS) differed between MDA-MD-231 cells on SkMc and LF niches, we measured mtROS via colocalization of a mitochondrial marker (MitoTracker) and a ROS dye (CellROX). The mtROS burden for MDA231-Ctl cells on SkMcs was 4.7-times higher than what was measured on LFs (Fig. 8a,b). Introduction of the mCAT vector into MDA-MB-231 cells significantly reduced the mtROS levels in SkMcs to 50.6% of what was measured with MDA231-Ctrl cells on LFs (Fig. 8a,b). This coincided with a level of outgrowth that eclipsed what was measured on LFs seeded with either MDA231-Ctl or MDA231-mCAT cells (Fig. 8c). Measuring mtROS in EO771 cells on LFs and SkMcs revealed similar levels of induction of mtROS on SkMcs, which was alleviated by mCAT and resulted in outgrowth that approached what occurred on LFs (Fig. 8d–f). These data highlight the inverse relationship between mtROS levels and outgrowth on the SkMc niche.
Fig. 8 |. mtRoS induced by the SkM niche resolves with mitochondrial catalase expression in tumour cells or in myocytes.
a, Mean mtROS intensity for MDA231-Ctl and MDA231-mCAT cells on LFs or SkMc. n = 208 MDA231-Ctl (LFs), 662 MDA231-mCAT (LFs), 202 MDA231-Ctl (SkMc), 229 MDA231-mCAT (SkMc); repeated in triplicate. One-way ANOVA, with Tukey’s test: ****P < 0.0001, MDA231-Ctl on LFs versus SkMc, MDA231-Ctl/LFs versus MDA231-mCAT/SkMc. P = 0.17, MDA231-Ctl versus MDA231-mCAT (LFs). b, Representative images of mtROS in MDA231-Ctl and MDA231-mCAT cells. Scale bar, 50 μm (inset) or 100 μm. c, MDA231 outgrowth on LFs or SkMc. n = 5 wells, repeated in triplicate. One-way ANOVA, with uncorrected Fisher’s LSD: ****P < 0.0001 for all except MDA231-Ctl versus MDA231-mCAT (LF, P = 0.84). d, Mean mtROS intensity for EO771-Ctl and EO771-mCAT cells on LFs or SkM. n = 1,129 EO771-Ctl (LFs), 980 EO771-mCAT (LFs), 1080 EO771-Ctl (SkMc), 1133 EO771-mCAT (SkMc); repeated in triplicate. One-way ANOVA, with Tukey’s test: ****P < 0.0001, MDA231-Ctl versus MDA231-mCAT (LFs), MDA231-Ctl on LFs versus SkMc, MDA231-Ctl/LFs versus MDA231-mCAT/SkMc, MDA231-Ctl versus MDA231-mCAT (SkMc). e, Representative images of mtROS in EO771-Ctl and EO771-mCAT cells. Scale bar, 50 μm (inset) or 100 μm. f, EO771 outgrowth on LFs or SkMc. n = 5 wells per cohort, repeated in triplicate. One-way ANOVA, with uncorrected Fisher’s LSD: P = 0.13, EO771-Ctl versus EO771-mCAT (LFs); **P = 0.0002, MDA231-Ctl on LFs versus SkMc; **P = 0.0011, EO771-Ctl versus EO771-mCAT (SkMc). P = 0.053, EO771-mCAT on LFs versus SkMc; P = 0.78, EO771-mCAT/LFs versus EO771-Ctl/SkMc; P = 0.48, EO771-Ctl/LFs versus EO771-mCAT/SkMc. g, EO771-Ctl outgrowth on WT, MCAT-1 or MCAT-2. n = 5 wells, repeated twice. One-way ANOVA, with Dunnett’s test: ****P < 0.0001, WT versus MCAT-1; **P = 0.0027, WT versus MCAT-2. h, Mean mtROS intensity for EO771-Ctl on WT, MCAT-1 and MCAT-2. n = 540 EO771-Ctl (WT), 346 EO771-Ctl (MCAT-1), 750 EO771-Ctl (MCAT-2); performed twice. One-way ANOVA, with Tukey’s test: ****P < 0.0001, WT versus MCAT-1, WT versus MCAT-2. P = 0.37, MCAT-1 versus MCAT-2. i, Representative images of mtROS in EO771-Ctl on WT, MCAT-1 or MCAT-2. Samples sizes same as h. Scale bars, 50 μm (inset) or 100 μm. For a, c, d and f–h, the centre line represents the mean, and error bars the s.e.m.
In light of our data revealing that reducing host mtROS promoted colonization of SkM in vivo, we probed whether culturing EO771 cells on syngeneic SkMcs established from MCAT mice resulted in reduced levels of tumoural mtROS. We established two unique myoblast cultures (MCAT-1 and MCAT-2) from the gastrocnemius of MCAT mice and a single myoblast culture from the gastrocnemius of the WT littermate. Outgrowth was enhanced significantly when EO771-Ctl cells were cultured on either the MCAT-1 or MCAT-2 niche as opposed to the WT myoblast niche (Fig. 8g). Tumoural mtROS was decreased by 88.2% and 91.8% on MCAT-1 and MCAT-2 SkMcs versus WT SkMcs (Fig. 8h,i), indicating that the SkM microenvironment imposes mitochondrial redox stress on co-cultured tumour cells. These data reveal that the mtROS burden in muscle DTCs is greater than that of lung DTCs, and suggest a direct role for mtROS in regulating DTC outgrowth. More specifically, the data point to increased mtROS levels in DTCs that reside in SkM - driven by both internal and environmental stressors - as DTC suppressive.
Discussion
In this manuscript, we applied a qPCR assay, paired with immunofluorescence microscopy of optically cleared tissue, to show that DTC trafficking and persistence within SkM occurs with unexpected frequency, a finding substantiated by examination of human specimens. A metabolomic interrogation of SkM tissue prompted us to probe the role of redox metabolism on metastatic colonization, and revealed that DTCs in SkM are under sustained oxidative stress in culture and in vivo. Functional studies demonstrated that DTCs endowed with enhanced antioxidant capacity via mitochondrial catalase expression could bypass this oxidative bottleneck. Complementary experiments that targeted catalase to the mitochondria of host tissue revealed that microenvironmental ROS significantly influences DTC redox state. In line with this, chemogenetic induction of H2O2 in both tumour and microenvironmental compartments blocked progression of MDA-MB-231 cells in otherwise permissive lung-like culture microenvironments. These findings reveal a metabolic bottleneck to metastasis in SkM, and suggest more broadly that there is a fine-tuned range where redox state must fall for DTCs to retain outgrowth potential. We surmise that disrupting this already-fragile redox balance is a unique DTC vulnerability that can be exploited for metastasis prevention approaches.
Whereas boosting antioxidant metabolism to quench ROS is a bedrock of strategies aimed at preventing tumour initiation59–61, recent findings suggest that antioxidants might actually abet metastasis. For example, patient-derived melanoma cells display increased ROS levels in blood and liver compared to cells that are subcutaneously implanted51. In these models, treatment with the GSH precursor N-acetylcysteine (NAC) increased the number of circulating cancer cells and metastatic burden in the lung and lymph node51. Similar observations have been made in models of colorectal cancer liver metastasis62. Recent work has implicated blood-borne free iron as the source of oxidative stress, which suggests that tumour cells that disseminate haematogenously may encounter oxidative stress by default63. Our study applies SkM as a filter and shows that DTCs within an antimetastatic site cannot achieve redox balance and never progress as a result, which also suggests that sustained oxidative stress might prevent colonization of other organs. Indeed, the fact that single DTCs in lung and SkM experience high oxidative stress suggests that this state might be exploited for a selective approach to target DTCs. Identification of unique dependencies required to survive this state may provide molecular targets that enable elimination of metastasis-initiating populations.
Our study also revealed that a strong increase in antioxidant capacity enables SkM colonization. But it does not address why this takes place so rarely. For instance, gain-of-function mutations and/or amplifications of enzymes that promote antioxidant capacity would be expected to yield SkM metastasis. Yet metastases at these sites are rarely observed. Is this because the level of antioxidant capacity required is never achieved? Or is it because the microenvironment must also be complicit? SkM homeostasis is consistently disrupted by exercise64, which triggers the emergence of quiescent satellite cells64, but this process is clearly insufficient to promote DTC outgrowth in patients who have recovered from cancer65. Mechanistic differences underlying the response of satellite cells and DTCs to disruption of tissue homeostasis may provide further insight into DTC-specific growth-suppressive stresses that can be therapeutically leveraged.
Our work provides evidence that trafficking to SkM and DTC survival within this environment is not the major bottleneck to colonizing this tissue. Instead, a hostile microenvironment that induces and sustains a profound redox imbalance—with mitochondria potentially playing a key role—is the core mechanism by which SkM suppresses DTC outgrowth. Critically, we provide evidence that if this phenomenon could be perpetuated in lung, it would also restrict metastasis there. This presents a challenge to uncover means by which oxidative stress can be prolonged and/or ultimately leveraged to compromise DTC survival, effectively converting permissive niches into antimetastatic sites.
online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41556–022-00881–4.
Methods
Human studies.
Specimen collection.
Human specimens were obtained and utilized in accordance with Peter MacCallum Cancer Centre Human Research Ethics Committee’s guidelines and ethical regulations (IR protocol: 10474). Patients provided informed consent to donate tissue post-mortem and enrolled in The BROCADE Rapid Autopsy Project. After rapid autopsy, SkM samples from the TA, quadriceps and gastrocnemius were placed in 10% neutral-buffered formalin and embedded as formalin-fixed paraffin-embedded (FFPE) blocks. For this study, SkM was collected post-mortem from two female donors (aged 54 years and 61 years) with MBC and known ER+PR+ disease at the time of diagnosis. Healthy SkM from a de-identified female donor was acquired as a FFPE block from Origene Technologies (PA154769F7), and pathology verification from haematoxylin and eosin (H&E) review showed that it comprised 75% SkM and 25% interstitial tissue. Sample pathology was within normal limits. Two slides of 4-μm sections were stained as controls for pan-CK, ER and PR antibody staining in SkM (see below for detail). An ER+PR+ breast tumour specimen from one de-identified female donor was obtained from the Cancer Epidemiology Research Cooperative at the Fred Hutchinson Cancer Research Centre (FHCRC) and used as a positive staining control. The pathology report confirmed that the tumour was ER+PR+.
Multiplex immunohistochemistry.
Pieces of TA, quadriceps and gastrocnemius muscle were embedded in a single paraffin block. Serial sections at a thickness of 4 μm were cut and mounted onto positively charged slides. A total of 50 slides per patient were sectioned. If cells of interest were identified, additional sections (4 slides preceding/4 slides proceeding) were stained with the multiple immunohistochemistry (mIHC) panel or with H&E to confirm histology. Fully automated mIHC was performed on a Discovery Ultra instrument (Ventana Medical Systems (VMSI)). Tissue sections were deparaffinized in Discovery wash buffer (VMSI, 950–510) followed by heat-induced epitope retrieval in Discovery CC1 solution (VMSI, 950–500). mIHC was performed sequentially, including wash steps with Discovery reaction buffer (VMSI, 950–510) to removed unbound antibodies. First, the sections were incubated with a primary antibody cocktail containing ER (Genetex, 6F11) at 1:50 and PR (Genetex, 16) at 1:50 in Ventana antibody diluent with casein (VMSI, 760–219). The biomarker cocktail was bound with Discovery anti-mouse HQ (VMSI, 760–4814), followed by Discovery anti-HQ HRP (VMSI, 760–4820) and visualized with a Discovery Purple kit (VMSI, 760–229). Previously bound HRP was quenched with Discovery inhibitor (VMSI, 760–4840) before the application of the next biomarker in the sequence. The sections were incubated with pan-CK (Abcam, AE1/AE3+5D3) at 1:100, labelled with Discovery anti-mouse NP multimer (VMSI, 760–4816), followed by Discovery anti-NP AP multimer (VMSI, 760–4827) and visualized with a Discovery Yellow kit (VMSI, 760–229). Sections were counterstained with haematoxylin II (VMSI, 790–2208) and Bluing reagent (VMSI, 760–2037). Slides were dehydrated in a series of ethanol and xylene baths and mounted in Cytoseal XYL (Fisher Scientific, 22–050-262).
Imaging and analysis.
The slides were scanned at a magnification of ×40 using an Aperio Versa 200 scanner (Leica Biosystems) and evaluated with Halo (Indica Labs). Serial registration of the sequential images was performed in Halo (Indica Labs). Cells that stained positively for the ER/PR cocktail and pan-CK were identified in the mIHC sections. Vascular tissue and SkM bundles were confirmed using the flanking H&E sections. The findings were verified by a pathologist (L.T.).
Animal studies.
All animal work was performed in accordance with the FHCRC-approved IACUC protocol 51075 and AAALAS guidelines and ethical regulations. NOD/SCID(NOD.CB17-Prkdcscid/J(NOD.SCID) (NOD-SCID, 394) and BALB/c (028) female mice were purchased from Charles River and used at 6–8 weeks of age. C57BL/6NJ (005304) and B6.Cg-Tg(CAG-OTC/CAT)4033Prab/J (mCAT, 016197) female mice were purchased from The Jackson Laboratory and used at 6–10 weeks of age. In this manuscript, we use MCAT to denote the B6.Cg-Tg(CAG-OTC/CAT)4033Prab/J mouse strain to distinguish from the tumour cell line expression of catalase (termed mCAT). Female NOD-SCID-Il2rg−/− (NSG) mice were obtained from the FHCRC CCEH core and used at 6 weeks of age. SkM specimens from female B6.Cg-Tg(CAG-OTC/CAT)4033Prab/J mice (aged 11 weeks) were also given as a gift from the Rabinovitch Lab at the University of Washington.
Intracardiac injection.
Anaesthetized mice were positioned in dorsal recumbency on a VisualSonics Vevo 2100 ultrasound imaging system to guide injection of 2 × 105 MDA-MB-231 cells or 1 × 104 4T1-SkM tumour cells in 100 μl PBS. Before injection, tumour cell–PBS suspensions were pipetted repeatedly with a p1000 micropipette to ensure a well-mixed solution, and drawn into a 1-ml syringe mounted with a 22-gauge needle. The needle was guided via ultrasound imaging into the left ventricle.
Intramuscular injection.
Anaesthetized mice were positioned in dorsal recumbency with the right hindlimb extended. EO771 mouse mammary tumour cells expressing GFP–luciferase (1,000 cells) were intramuscularly injected (30 μl) into the TA of 6–8-week-old female C57BL/6 mice. Similarly, MDA-MB-231 human breast cancer cells expressing GFP–luciferase (500 cells) were intramuscularly injected (30 μl) into the TA of 6–8-week-old female NOD-SCID mice. The tumour cell number was titrated such that the tumour cells persist within the TA without colonization of the tissue or local invasion.
Intravenous injection.
In preparation of the injection, mice were physically restrained and the animal’s tail was heated with a heat lamp or warm water to increase vessel dilation. A total of 5 × 105 MDA-MB-231 cells or 1 × 106 EO771 tumour cells (100 μl) were loaded into a 1-ml syringe with a 27-gauge needle and administered into the tail vein of 6–8-week-old female NOD-SCID or C57BL/6 mice, respectively.
Orthotopic injection.
A total of 1 × 106 ffluc-eGFP-MDA-MB-231 cells were injected into the inguinal mammary gland of 6–8-week-old female NSG mice in 30 μl of a 1:1 solution of LrECM (growth-factor reduced Cultrex; Trevigen) with PBS. Tumours were resected at a final volume of ~250 mm3 approximately 4 weeks later. BLI was performed on a IVIS spectrum (Perkin Elmer) 1 week after resection following an intraperitoneal delivery of 100 μl d-Luciferin (10 mg ml−1, BioVision) to confirm successful resection, and BLI was performed once a week to track metastasis.
BLI.
BLI of mice and organs of interest ex vivo was performed using an IVIS spectrum (Perkin Elmer) with Living Image software (4.7.3). Mice were intraperitoneally injected with 100 μl of PBS containing d-luciferin sodium salt (10 mg ml–1; Biovision, 7902) 5 min before imaging, followed by general anaesthesia (2% isoflurane). The exposure time of images was determined by the signal intensity (autoexposure), which ranged from 1 to 60 s. The bioluminescent signal was quantified with “region of interest” measurement tools in Living Image (Perkin Elmer) software. After ex vivo imaging of organs, samples were placed in 4% paraformaldehyde (PFA) fixative for downstream processing.
Tumour monitoring.
Tumour burden was monitored once a week after injection throughout the duration of the study via BLI (see above) or tumour volume measurements. Tumour volumes were measured every other day once mammary fat pad tumours became palpable. Per the standard operating procedure of the FHCRC, maximal tumour burden for a single external (palpable) tumour was 2 cm average diameter, and this size was not exceeded in our study. In the event that metastases arose and exceeded a BLI reading of 1 × 108 photons s−1 cm−3 sr−1 or significantly affected the health of the animal (for example, changes in weight, abnormal resting posture, failure to groom or loss of mobility), the mouse was euthanized and organs of interest collected.
AluYb8 qPCR.
Limit of detection assay.
Mouse OP-9 cells and human MDA-MB-231 cells were grown in culture until sufficient cell numbers were generated. MDA-MB-231 cells were mixed with OP-9 cells such that the following dilutions were made: 1:1, 1:10, 1:102, 1:103, 1:104, 1:105, 1:106 and 1:107. Genomic DNA was then extracted using a Qiagen DNeasy Blood and Tissue kit (69504). DNA was sheared into 500-bp fragments using a Covaris S220 ultrasonicator, and DNA concentrations were measured using a Nanodrop (ThermoFisher). PCR reactions were prepared using the sheared DNA samples, TaqMan Gene Expression Master Mix (4369016, ThermoFisher) and amplicon-specific primer/probe sets (human AluYb8 and mouse β-actin, Invitrogen). Fw-Yb8-ALU-S68: GTC AGG AGA TCG AGA CCA TCCT; Rv-Yb8-ALU-AS244: AGT GGC GCA ATC TCG GC; Probe-Yb8-ALU-167: 6-FAM-AGC TAC TCG GGA GGC TGA GGC AGG A-TAMRA. The mouse β-actin primer/probe pair used was Mm02619580_g1 FAM (ThermoFisher). Samples were run in triplicate on a Bio-Rad CFX96 real-time system using the following cycling conditions: (1) 50 °C for 2 min; (2) 95 °C for 10 min; (3) 95 °C for 15 s, followed by 63 °C for 1 min, repeated for 39 more cycles; (4) 63 °C for 3 min. AluYb8 Cq values were normalized by β-actin Cq values. Additional details are provided in Supplementary Table 5.
Mouse tissue.
After necropsy, organs of interest were immediately flash frozen (liquid N2). Tissue samples were homogenized at 4 °C using 2.8 mm ceramic beads (19–628-3, OMNI Bead Ruptor 24). Organs were processed using 5.65 m s−1 speed for 3 cycles of 30 s (5 s of dwell time). Genomic DNA was prepared as described above. PCR reactions were prepared using the sheared DNA samples, TaqMan Gene Expression Master Mix (4369016, ThermoFisher) and amplicon-specific primer/probe sets (human AluYb8 and mouse β-actin, Invitrogen). AluYb8 primers are described above. Mouse β-actin primer/probe sets for day 3 was Mm02619580_g1 VIC (ThermoFisher). For week 7, Mm02619580_g1 FAM (ThermoFisher) was used. Samples were run in triplicate using the same cycling conditions described above. Additional details are provided in Supplementary Table 5.
Ce3D clearing of lung and muscle with stained GFP+ DTCs.
Mice were euthanized and perfused with 25 ml ice-cold PBS through the left ventricle. The left lobe of the lung and the left hindlimb SkM (TA, gastrocnemius and quadriceps) were collected and fixed in 1% PFA/PBS overnight, rocking at 4 °C. Tissues were blocked in 1 ml of 1% normal mouse serum (MP Biomedicals), 1% BSA (Sigma), 0.3% Triton X-100 (Sigma) in PBS for 8 h at 37 °C in a shaking incubator. Primary antibodies were added to blocking solution, and tissues were incubated in primary antibody solution for 72 h while shaking at 37 °C. For lung, primary antibodies used were chicken anti-GFP (Abcam ab13970, 1:500), while SkM samples were stained with chicken anti-GFP and rabbit pan-laminin (Sigma L9393, 1:200). Tissues were washed for 24 h in 0.2% Triton X-100 and 0.5% 1-thioglycerol (Sigma) in PBS while shaking at 37 °C. Lung and muscle were submerged in 1 ml block solution containing goat anti-chicken 488 (1:250) and goat anti-rabbit 568 (1:250; for SkM only) and shaken for 48 h at 37 °C. 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI; 2 μg ml–1) was used to label cellular nuclei. Afterwards, the wash step was repeated for another 24 h.
Tissues were cleared using Ce3D clearing solution (40% N-methylacetamide (Sigma) v/v, 86% w/v Histodenz (Sigma), 0.1% Triton X-100 and 0.5% 1-thioglycerol in PBS). Tissues were covered and incubated in 1 ml Ce3D clearing solution while shaking at 37 °C for 5 days. Cleared tissues were stored in Ce3D clearing solution at 4 °C until imaged. Imaging to quantify DTCs was performed on a Zeiss LSM700 confocal microscope using a 0.3 NA ×10 air objective. Z-stacks of lungs and muscles were tile-scanned at ×10, and structures of interest were confirmed to be cellular in nature at ×20. Cleared lung and muscle from three uninoculated mice were used as control; these were used to subtract staining noise from our analyses.
Cell culture.
Primary human SkM myoblasts (SkMcs) were obtained from Lonza and propagated in SkGM or SkGM-2 growth medium (CC-3160, CC-3245). Primary human LFs were obtained from Lonza and propagated in high-glucose DMEM (Gibco,11965–118) supplemented with 10% FBS (Gibco 16140089) and 1% penicillin–streptomycin (P/S; Gibco 15140122). Mouse SkM myoblasts derived from C57BL/6 WT or MCAT mice were propagated in F10 (Gibco, 11550043) medium supplemented with 20% FBS, 1% P/S and 10 ng ml−1 bFGF (PeproTech, AF-100–18B) and subcultured on 10% Matrigel-coated plates (Corning, 356234). All primary human and mouse cells were used in experiments before passage 11. Differentiation medium (2% horse serum (Gibco 26050070) in high-glucose DMEM) was added to differentiate the SkMc and mouse myoblasts.
Human breast cancer cell lines (MDA-MB-231, Hs578T, BT549, HCC70 and MDA-MB-468), 293T cells and Phoenix Ampho cells were purchased from the American Type Culture Collection (ATCC). High-glucose DMEM supplemented with 10% FBS was used to culture the MDA-MB-231 and Hs578T lines. RPMI 1640 medium supplemented with 10% FBS was used to culture the BT549 and HCC70 lines. Leibovitz L-15 medium (Gibco, 11415064) supplemented with 10% FBS was used to culture the MDA-MB-468 cells. Mouse stromal cells (OP-9) were purchased form the ATCC. The mammary cancer cell line 4T1 was obtained from the Karmanos Cancer Institute. EO771 cells were a gift from H. K. Lyerly (Duke University). The mouse lines were cultured in high-glucose DMEM supplemented with 10% FBS. All cells were maintained at 37 °C, 5% CO2, with the exception of the MDA-MB-468 cells, which were grown in 0% CO2 conditions. All cell lines tested negative for mycoplasma.
Generation of C57BL/6 WT and MCAT myoblasts.
Female WT and MCAT C57BL/6 mice (aged 11 weeks) were obtained as a gift from P. Rabinovitch at the University of Washington. TA, quadriceps and gastrocnemius muscles were minced and enzymatically digested. To obtain pure myoblast populations, four rounds of pre-plating were performed every 2 days preceding the initial myoblast isolation. Pre-plating entailed creating a cellular suspension, plating the suspension on tissue-culture-treated dishes for 45 min, then replating the suspension on 10% Matrigel-coated tissue culture dishes. Myoblasts were cultured until sufficient cell numbers were generated and frozen back.
SkM and lung co-cultures.
SkMcs or LFs were seeded alone at a density of 8,333 cells per well (100 μl) in 96-well culture plates. Plates were then left undisturbed on a flat surface for 15 min to allow even cell seeding before incubation. Growth medium (Lonza SkGM-2 for SkMcs; 10% FBS in DMEM hi-glucose for LFs) was changed every other day. After 4 days, differentiation medium (2% horse serum in DMEM hi-glucose) was added to both SkMc and LF wells to initiate the maturation and fusion of the myocytes. To avoid experimental variability, the conditions of the media for SkMc and LF niches were identical from the differentiation step onwards. Differentiation media were refreshed every other day. After an additional 4 days, YFP- or RFP-expressing tumour cells were suspended in supplement-free DMEM/F12 (Gibco, 11330–032). Tumour cells were seeded at a density of 100 cells per well in a total volume of 100 μl after washing cultures twice with PBS. Cells were allowed to settle for 15 min at room temperature, then a drip of reduced growth factor basement membrane extract (BME) PathClear Cultrex (Biotechne 3433–005-01) in DMEM/F12 was slowly added to each well (final concentration of 20%). The BME Cultrex drip condensed for 15 min at room temperature before polymerizing fully at 37 °C. If required, cultures were imaged immediately after seeding on a Zeiss LSM700 confocal microscope using a 0.3 NA ×10 air objective. The objective was centred to each well before acquisition of 512 × 512 pixel, 6 × 6 tiles (zoom = 0.7) that captured the near-entirety of each well. Cultures were maintained with changes of medium every 72 h and imaged in 2–5 day intervals as required by the experiment.
Preparation of CM.
SkMc and LF cells were grown in a 96-well plate as established above. The number of wells seeded per experiment was doubled to account for the wells used for CM. To differentiate cells, exosome-depleted differentiation medium (2% horse serum, DMEM hi-glucose) was added. To remove exosomes, horse serum was centrifuged at 4 °C for 70 min at 100,000g. Supernatant was filtered by pouring contents into a vacuum-connected 0.22-μm filter on a sterile bottle. A total of 1 × 102 MDA-MB-231 cells were seeded on the experimental SkMc and LF wells (100 cells per μl, termed SkMc+MDA and LF+MDA, respectively). Medium was removed from all wells, collecting only the medium from the SkMc-only and LF-only wells (termed SkMc-CM and LF-CM, respectively). SkMc-CM was delivered to the SkMc+MDA and LF+MDA wells. As a control, LF-CM was added to SkMc+MDA and LF+MDA wells. Fresh differentiation medium was added to the SkMc-only and LF-only wells. Collection and distribution steps were repeated every day or every third day during the 10-day outgrowth period.
Preparation of EVs.
SkMc and LF cells were seeded in 96-well plates as established above, as well as an additional three 15-cm plates for EV collection. As described in the main SkM and LF co-culture section, cells were propagated and differentiated over the course of 8 days. Serum-free DME/F12 was added and allowed to incubate for 3 days before supernatant was collected for EVs using established methodologies10. EV-containing medium was transferred to a 50-ml conical tube and centrifuged at 4 °C for 10 min at 500g. Supernatant was collected and transferred to a new conical tube. Samples were then centrifuged at 4 °C for 20 min at 20,000g. Supernatant was transferred to 12-ml tubes compatible with the ultracentrifuge’s SW40-Ti rotor (Beckman Coulter). Samples were centrifuged at 4 °C for 70 min at 100,000g. Supernatant was completely removed and pellets were resuspended 1 ml 1× PBS. Samples were then centrifuged at 4 °C for 70 min at 100,000g. Supernatant was removed and a small volume (20–100 μl) of 1× PBS was used to resuspend the pellet. The protein concentration of EVs was quantified using a BioRad DC assay. Varying concentrations (5, 10 or 20 μg ml−1) of EVs were administered to the organotypic culture niches after MDA-MB-231 seeding (day 0). Collection and distribution steps were repeated every third day during the 10-day outgrowth period.
Decellularization.
SkMc and LF niches were seeded as described above. Triton X-100 (0.5%, 50 μl) was added to each well and incubated for 5 min at room temperature. Cell lysis was monitored using an inverted microscope and allowed to continue until no intact cells were visible. Wells were then gently washed with 1× PBS (200 μl). Double-distilled H2O was added to each well and incubated for 3–5 min at room temperature. Wells were gently washed in 1× PBS (200 μl) 3–5 times. A total of 1.5 × 103 tumour cells were seeded on top of the thin ECM layer. Tumour cells were grown in DMEM-hi glucose with 1% FBS. No BME Cultrex was added to the decellularized wells. Tumour cells were cultured for the 10-day period and imaged at day 0, 5 and 10.
SkMc–LF mixture.
SkMc and LF cells were plated in admixture, with the ratio of SkMcs to LFs increasing sequentially. LF to SkMc ratios included 1:1, 1:10, 1:20, 1:50 and 1:100. The final cell seeding density of 8,333 per 96 wells was maintained. Once both cell types were seeded, the procedure for differentiating the niches and seeding the tumour cells were the same as those described above.
H2O2 spike-in.
SkMc and LF niches were seeded as described above. A total of 1 × 102 MDA231-roGFP2 cells were plated on these niches, along with the BME Cultrex drip. After allowing the tumour cells to fully settle into the niche, the culture medium was removed and DME/F12 containing 75 μM or 125 μM H2O2 was added to each well. The H2O2 solution was incubated in the wells for 2 h and was then replaced with fresh serum-free DME/F12. This was repeated every day.
Alanine studies.
SkMc and LF niches were plated and cultured as described above. A total of 1 × 102 MDA231-WT or MDA231-DAAO cells were seeded atop these niches, along with the BME Cultrex drip. After allowing the tumour cells to fully settle into the niche, the culture medium was removed and wells were treated with serum-free DME/F12 medium containing 10 mM, 20 mM or 100 mM d-alanine (A7377–100G, Sigma) or l-alanine (A7627–100G, Sigma). This was repeated every day. Additionally, 5 × 103 MDA231-WT or MDA231-DAAO cells were seeded on plastic and allowed to settle overnight. The next day, wells were treated with serum-free DME/F12 medium containing 10 mM, 20 mM, 100 mM or 500 mM d-alanine or l-alanine. Medium was collected at 1 h, 4 h, 8 h, 24 h and 48 h time points and tested for H2O2 using an Amplex Red assay (A22188, Invitrogen). The tumour area fraction (mCherry+) was measured on a Zeiss CellDiscoverer7 microscope (×10 magnification).
LF-DAAO.
Generation and validation.
To generate the LF-DAAO cell line, LF cells were infected with high-titre pLX311-HyPer3-DAAO lentivirus and selected with blasticidin (5 μg ml−1) for a pure DAAO+ population. Vector expression was confirmed through dose-dependent extracellular H2O2 generation (Amplex Red) after alanine treatments. For this validation, SkMc, LF and LF-DAAO niches were seeded and cultured as described in the primary co-culture section. Wells were treated with serum-free DME/12 medium containing 10 mM, 20 mM, 30 mM, 40 mM, 50 mM, 70 mM or 100 mM d-alanine or l-alanine. Medium was collected at 4 h, 8 h, 24 h and 48 h time points and tested for H2O2 using an Amplex Red assay (A22188, Invitrogen).
Co-culture.
SkMc, LF and SkMc cells were seeded and cultured as described in the primary co-culture section. Tumour cells were seeded at a density of 100 cells per well in a total volume of 100 μl after washing cultures twice with PBS. Cells were allowed to settle for 15 min at room temperature, then a drip of BME was added. After allowing the tumour cells to fully settle into the niche, wells were treated with serum-free DME/F12 medium containing 30 mM or 50 mM d-alanine. This was repeated every day for the entirety of the 5-day experiment. The tumour area fraction (mCherry+) was measured at day 0, 2.5 and 5 on a Zeiss CellDiscoverer7 microscope (×10 magnification). The study endpoint was day 5 instead of day 10 because alanine treatment of the LF–DAAO cells caused niche rolling between day 5 and 10, thus data after this time point were unusable.
Quantification of tumour cell outgrowth in organotypic niches.
A macro was written using NIH ImageJ software to remove bias from data quantification. For the YFP or RFP channel, day 0 and 10 images were subjected to the following edits: contrast was enhanced such that 0.5% of pixels were saturated; the image was then sharpened, and a constant threshold was applied to all samples within a given experiment to eliminate variability. The total area fraction of the 6 × 6 tiled image occupied by YFP+ cells was then calculated. For each image, the measured area fraction at day 10 was normalized by the corresponding day 0 value to account for any variations in seeding density from well-to-well. Values obtained for each condition were averaged, and these averages were subsequently normalized to the control where indicated.
Immunofluorescence staining.
Serial tissue sections (thickness of 25 μm) of SkM, lungs and brains were generated with a Leica Cryostat CM3050 S (Leica Microsystems). Sections were thawed, rehydrated in PBS and incubated in 0.1 M glycine/PBS for 30 min. Tissues were then extensively rinsed with PBS and permeabilized/blocked in 0.3% Tritonx-100/5% BSA for 1 h at room temperature. Tissue sections were then incubated with the following antibodies: a rat monoclonal antibody targeting Cd49f (BD, 555734, clone GoH3; 1:250); an Armenian hamster monoclonal antibody targeting CD31 (Millipore, MAB1398z; 1:300); a rabbit polyclonal to pan-laminin (Sigma, L9393; 1:500); a mouse monoclonal antibody targeting laminin-2 alpha (Sigma, L0663, clone 4H8–2; 1:300); a chicken polyclonal antibody targeting GFP (Abcam, ab13970; 1:1,000); and a rabbit polyclonal antibody targeting RFP (Rockland, 600–401-379; 1:200). Proliferating cells were identified with an Alexa Fluor647-conjugated monoclonal antibody targeting Ki67 (BD, 561126, clone B56; 1:100). DAPI (2 μg ml−1) was used to label cellular nuclei. Secondary antibodies used were goat anti-chicken 488, goat anti-rat 568 or 647, goat anti-rabbit 568 or 647, goat anti-mouse 647 (Invitrogen), all used at 1:500. Tissues were imaged on a Zeiss LSM 710 confocal microscope using a 0.3 NA ×10 air objective, a 0.55 NA ×20 air objective or a 1.4 NA ×63 oil-immersion objective.
Organotypic cultures were stained after fixation (4% PFA for 15 min, 4 °C) with Alexa Fluor568 Phalloidin (Invitrogen A12380, 1:200) to detect F-actin or with the following antibodies: rabbit polyclonal to pan-laminin (Sigma, L9393; 1:500) and mouse monoclonal antibody to myosin (Sigma, M4276; 1:400). Secondary antibodies used were goat anti-rabbit 488, 568 or 647 and goat anti-mouse 568 or 647 (Invitrogen), all at 1:500. DAPI (2 μg ml−1) was used to label cellular nuclei. Stained organotypic cultures were imaged on a Zeiss LSM 710 confocal microscope using a 0.3 NA ×10 air objective.
Bone marrow whole-mounting and staining.
OCT-embedded femurs were shaved at 50-μm intervals on a Leica Cryostat CM3050 S (Leica Microsystems) to expose bone marrow on one face, reversed and sectioned to expose bone marrow on the opposite face. Sliced femurs were thawed from OCT, placed in a 1.5-ml Eppendorf tube, washed twice with PBS and blocked overnight (4 °C) in a solution containing sterile-filtered (0.2 μm) 20% goat serum plus 0.5% Triton X-100 in PBS under constant, gentle agitation by circumferential rotation. Following blocking/permeabilization, bones were incubated for ~72 h in 5% BSA containing a combination of chicken anti-GFP (Aves Labs, GFP-1010; 1:250) and rabbit anti-pan-laminin (Sigma, L9393; 1:500). Bones were washed the following day for a minimum of 6 times in PBS before washing overnight at 4 °C. Femurs were then counterstained in 5% BSA containing DAPI (2 μg ml−1) and the following secondary antibodies: goat anti-chicken 488 (Abcam, ab150169; 1:400) and goat anti-rabbit 647 (Invitrogen, 1:400). Femurs were washed again the following day in PBS and finally placed into a solution of PBS plus 0.02% sodium azide to preserve the bone before imaging.
For imaging, whole mounts were generated by gently placing femurs in aqueous mounting medium (Fluoromount-G, Southern Biotech) in a 3.5-cm plate containing a number 1.5 coverslip insert (MatTek, P35G-1.5–20-C), and covering the femur with a 18 × 18-mm coverslip to press the marrow flush against the imaging window. Tile scans were generated by placing this culture dish on a Zeiss LSM700 and scanning the entire bone using four laser lines and a 0.3 NA ×10 air objective to capture the entirety of the bone along with the entire imageable depth of the marrow (≤100 μm). Images of individual DTCs were acquired using a 0.55 NA ×20 air objective.
Lentiviral production.
A 293T packaging cell line was transfected using FuGene6 (3:1 v/w, E2692, Promega) with 6.5 μg DNA containing the lentiviral construct of interest (GFP, YFP, luciferase, mCherry, Grx1-roGFP2 or pLX311-HyPer-DAAO) as well as DNA encoding the packaging proteins VSVG and pPAX2 (6.5 μg each). At the time of transfection, 293T cells were cultured in Opti-MEM medium (51985034, Gibco). Medium was removed 24 h after transfection and refreshed with hi-glucose DMEM with 10% FBS. Virus was collected an additional 48–72 h after the medium change. Lentiviral supernatant was 0.45-μm filtered and pelleted at 500g for 10 min, followed by a 150× concentration step using Clontech’s Lenti-X (631232). Concentrated lentivirus was stored at −80 °C until use.
Mammalian cells were infected lentivirally by culturing in growth medium, 4 μg ml−1 polybrene and concentrated lentivirus. Medium was changed the next day and then selection with antibiotic was conducted 3 days later. When infecting with virus encoding a construct that lacked antibiotic resistance, pure populations of fluorescent cells were flow sorted instead by gating on the 10% brightest cells.
Grx1-roGFP2 redox imaging and analysis.
pEIGW Grx1-roGFP2 was a gift from T. Dick (Addgene plasmid 64990; http://n2t.net/addgene:64990; RRID: Addgene 64990). Grx1-roGFP2 imaging settings and validation were followed in accordance with a previously published protocol by Morgan et al.52. The Grx1-roGFP2 biosensor was excited using the 405 nm and 488 nm laser line and detected the emission through a 500–554 nm bandpass filter on a Zeiss LSM700 confocal microscope. Images of the region of interest were taken at ×10 magnification (0.3 NA ×10 air objective) and saved as .lsm files. Raw data were exported to ImageJ software as 16-bit .tif files and converted into 32-bit images for analysis. Background was subtracted and a threshold set per channel to avoid analysis-created artefacts. Ratiometric images were created by dividing the 405 nm (oxidized) channel by the 488 nm (reduced) channel, pixel by pixel. ImageJ LookUp Table ‘Fire’ was used to create false-coloured composite images. Numerical values were generated by plotting the Z-profile for each composite 405 nm/488 nm image. For Grx1-roGFP2 imaging of DTCs in mouse tissue, we followed methods by Fujikawa et al.66 for redox stabilization and tissue processing steps. Imaging settings were consistent with those described for cultured cells.
Amplex Red extracellular H2O2 assay.
The manufacturer’s protocol was followed for this assay (Invitrogen, A22188). CM (100 μl) was removed from the experimental 96-well plate and placed directly into a new 96-well plate. To maintain a 1:1 ratio, 100 μl of the Amplex Red reagent/HRP solution (100 μM Amplex Red reagent, 0.2 U ml–1 HRP, 1× reaction buffer) was then added to each well. The reactions were incubated for 30 min at room temperature. A serial dilution of H2O2 was included as a positive control and used to generate a standard curve. The 1× reaction buffer without H2O2 was included as negative control. A medium-only control was included to account for baseline noise. Fluorescence was measured on a SpectraMax microplate reader, using an excitation of 530–560 nm and emission detection at ~590 nm. H2O2 measurements were generated based on a standard curve and baseline subtraction of no-H2O2 medium-only control.
CellROX Deep Red 647 ROS assay.
The manufacturer’s protocol was followed for this assay (C10422, Molecular Probes by Fisher Scientific). A total of 100 μl of 10 μM CellROX reagent was added to experimental wells in a 96-well plate containing the cells of interest covered by the ~100 μl BME Cultrex/medium layer (final concentration of ~5 μM). The wells were incubated for 1 h at 37 °C to enable full penetration of the basement membrane layer and incorporation into the cells. After incubation, the medium was removed, and wells washed three times with 1× PBS. Wells were imaged immediately on a Zeiss LSM700 at ~644/665 nm.
MitoTracker Green mitochondrial dye.
The manufacturer’s protocol was followed for this assay (M7514, Invitrogen). A total of 100 μl of 200 nM MitoTracker reagent was added to experimental wells in a 96-well plate containing the cells of interest covered by the ~100 μl BME Cultrex/medium layer (final concentration of ~100 nM). The wells were incubated for 1 h at 37 °C to enable full penetration of the BME layer and incorporation into the cells. After incubation, the medium was removed, and wells washed three times with 1× PBS. Wells were imaged immediately on a Zeiss LSM700 confocal microscope (0.3 NA ×10 air objective). When used in conjunction with CellROX DeepRed 647, both reagents are prepared as described above and added to each well together.
Quantification of CellROX/MitoTracker intensity.
Raw data exported as 8-bit or 12-bit .tif files were analysed using CellProfiler, an open-source software, to quantify the following intensities: (1) ROS intensity in tumour cells; (2) ROS intensity in muscle cells; and (3) mitochondrial ROS intensity in tumour cells. (1) Tumour cells were identified as objects found within the immunofluorescence channel pertaining to the fluorescent marker of the tumour cell (mCherry/GFP) and refined based on shape, size and intensity of the objects. Tumoural ROS were identified by measuring the pixel intensity found in the ROS channel (CellROX647) within the confines of the objects identified as tumour cells. The ROS intensity values in the manuscript refer to the mean pixel intensity within each area of the cell in the corresponding ROS channel image. (2) Muscle cells were identified as objects found in the ROS channel (CellROX647) and refined based on shape, size and intensity. A mask was applied to exclude tumour cell populations in the muscle cell quantification. ROS intensity is reported as described above. Myotube intensity was found using ImageJ, a NIH open-source software. Myotubes were manually selected from the ROS channel image, and their average pixel intensities were recorded. A second CellProfiler pipeline again quantified tumour and muscle cell ROS intensity, this time excluding the myotubes by masking out the selected regions to obtain separate data for myocytes and myotubes. (3) To measure mitochondrial ROS, tumour cells were first identified as objects found within immunofluorescence channel pertaining to the fluorescent marker of the tumour cell and refined based on shape, size and intensity of the objects. Tumour cell mitochondria were defined as objects that existed in the 488 channel (MitoTracker Green 488) and overlapped with objects in the primary tumour cell mask. Mitochondrial ROS in tumour cells were identified by measuring the pixel intensity found in the ROS channel (CellROX647) within the confines of the objects identified as mitochondria. ROS intensity values in the manuscript refer to the mean pixel intensity. With the exception of outlining myotube areas, this analysis was automated and did not require subjective user input. CellProfiler pipelines will be provided upon request.
Retroviral production.
Expression of mCAT (pBMN-IRES-GFP-mCAT), cCAT (pBMN-IRES-GFP-cCAT) and empty vector control (pMBN-IRES-GFP, termed Ctl) in MDA-MB-231 and EO771 cells was achieved through retroviral transfection. pTS1-mCherry expression in EO771 cells was also achieved through retroviral transfection. First, Phoenix Ampho packaging cells were transfected using FuGene6 (3:1 v/w, E2692, Promega) with 12 μg DNA containing the retroviral expression vector of interest. At the time of transfection, Phoenix Ampho cells were cultured in Opti-MEM medium (51985034, Gibco). Medium was removed 24 h after transfection and refreshed with hi-glucose DMEM with 10% FBS. Virus was collected an additional 48–72 h after the medium change. Retrovirus supernatant was 0.45-μm filtered and pelleted at 500g for 10 min. The retrovirus was then 150× concentrated using Clontech Retro-X (631455). Concentrated retrovirus was stored at −80 °C until use.
Mammalian cells were infected by culturing in hi-glucose DMEM with 10% FBS, 4 μg ml−1 polybrene and concentrated retrovirus. Medium was changed the next day and after at least 7 days of culture, pure populations of fluorescent cells were flow sorted by gating on the 10% brightest cells.
Western blotting.
Tumour cells were lysed in 2% SDS/PBS containing cOmplete protease inhibitor cocktail (11697498001, Roche) and PhosSTOP phosphatase inhibitor cocktail (4906845001, Roche). Lysate (20–40 μg) was then separated on a Tris-glycine 4–20% gel. Human and murine catalase were probed with a rabbit monoclonal antibody (Abcam ab16731; 1:2,000). Loading of each lane was assayed by probing simultaneously for β-actin using a mouse polyclonal antibody (Sigma, A5441, clone AC-15; 1:7,500). Blots were developed on a Li-Cor Odyssey. Densitometry was performed using Odyssey software v.3.0.
Metabolomics.
Sample collection.
Lung and SkM (TA and bulk muscles from the upper arm) were collected upon necropsy and imaged for mCherry+ 4T1-SkM lesions (Zeiss AxioZoom). Once a mCherry+ lesion was identified, the region was excised, leaving as little tissue margin as possible. The sample was weighed and then flash frozen using liquid N2. Healthy lung and SkM samples were collected through the same process. 4T1-parental and 4T1-SkM cells were plated in 6-well plates at an initial density of 50,000 cells per well and collected 48 h later. Wells were washed twice with 1× PBS, cells were scraped into 1.5-ml microcentrifuge tubes and spun at 16,000g for 3 min. Samples were then flash frozen and stored at −80 °C until ready to process.
Preparation.
Before liquid chromatography–mass spectrometry analysis, tissue samples were weighed and resuspended in pre-chilled (−20 °C) methanol:acetonitrile:water (5:3:2, v/v) to a final concentration of 30 mg ml−1, cell samples were placed on ice and re-suspended with methanol:acetonitrile:water (5:3:2, v/v) at a concentration of 2 × 106 cells ml−1, and medium samples were extracted with the same solution at a dilution of 1:25 (v/v). Suspensions were vortexed continuously for 30 min at 4 °C. Insoluble material was removed by centrifugation at 18,000g for 10 min at 4 °C and supernatants were isolated for metabolomics analysis by ultra-high performance liquid chromatography–mass spectrometry. Analyses were performed per previously published methods67,68.
Analysis.
The following comparisons (>2-fold increase or 50% decrease, P < 0.1) were made: (1) SkM metastasis versus healthy SkM; (2) lung metastasis versus healthy lung; and (3) comparison (1) versus comparison (2) MetaboAnalyst5.0 was used for MSEA, implementing KEGG’s ‘metabolism-related metabolite set’ library. Over representation analysis (ORA) was implemented using the hypergeometric test to evaluate whether a particular metabolite set is represented more than expected by chance within the given compound list. One-tailed P values are provided after adjusting for multiple testing. Fold-enrichment was calculated by dividing the observed number of hits by the expected number of hits (Hits/Expect columns of the ORA). Heatmaps were generated using the Broad Institute’s Morpheus software: https://software.broadinstitute.org/morpheus.
pLX311-HyPer3-DAAO-NES cloning.
pAAV-HyPer-DAAO-NES was a gift from T. Michel (Addgene plasmid 119164; http://n2t.net/addgene:119164; RRID:Addgene 119164). The HyPer3-DAAO-NES sequence was cloned into the lentiviral construct pLX311-luciferase (Addgene 11773) using an In-Fusion HD Cloning Plus kit (638909, Takara Bio). The following cloning primers were used: Fw–gtgtcgtgaggctagcgccaccatggagatggcaagc; Rv–gataggcttaccgatatcttacagggtcagccgctccag.
Statistics and reproducibility.
Statistical analyses were conducted with GraphPad Prism 7 software. Data that were distributed normally were compared via unpaired, two-tailed t-test (if only two conditions were tested) or via one-way or two-way analysis of variance (ANOVA) (for experiments containing three or more conditions). One-way ANOVA was used when the experiment contained only one independent variable, whereas two-way ANOVA was used when the experiment contained two independent variables. Post-testing to correct for multiple comparisons was chosen based on whether a decision was made a priori to compare conditions to untreated (Dunnett’s) or to compare all treatment groups with each other (Tukey’s). If multiple comparisons were made but tested independently of each other, Sidak’s multiple comparisons test was used. Please refer to figure legends for individual n and P values, and the specific statistical tests used. Unless noted otherwise, data are reported such that the centre line represents the mean, and error bars represent the s.e.m. Data depicted in the figures represent one experiment with multiple technical replicates, unless otherwise noted. Experiments were repeated independently at least twice, with similar results obtained. MSEA results were ranked using open-source MetaboAnalyst5.0 software.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
Extended Data Fig. 1 |. Human SkM from a metastatic breast cancer patient harbours panCK+/eR+/PR+ breast cancer cells.

a) Representative multiplex IHC images from an ER+/PR+ primary breast tumour specimen, used as a positive staining control. ER/PR (breast cell marker) staining in purple, pan-cytokeratin (panCK, epithelial cell marker) staining in yellow. Scale bar: 1 mm (left), 100 μm (centre) and 10 μm (right). b) Representative multiplex IHC images from human SkM, used as a negative staining control. ER/PR staining in purple, panCK staining in yellow. Scale bar, 10 μm. c) Representative IHC image of the three human SkM sites sampled (tibialis anterior, quadriceps and gastrocnemius) from a patient with metastatic breast cancer (MBC). Scale bar, 1 cm. d) Multiplex IHC panels of three panCK+/ER+/PR+ cells located inside the quadriceps muscle. ER/PR staining in purple, pan-cytokeratin staining in yellow. Scale bar 100 μm, Inset 10 μm. e-f) Two micropictographs of serial sections (4 μm) of FFPE SkM tissue from an individual with metastatic breast cancer. The slides were stained with hematoxylin and eosin or sequentially stained with a multiplex IHC panel consisting of ER/PR (purple) and panCK (yellow). Two ER+/PR+/panCK+ breast cancer cells were identified in a vessel located within the quadriceps muscle. Scale bar, 10 μm.
Extended Data Fig. 2 |. Aluyb8 qPCR reveals that DTCs travel to and persist within multiple mouse SkMs following intracardiac injection of breast cancer cells.

a) Schematic of mouse study to examine frequency of DTCs across organ site at early and late timepoints. b) AluYb8 amplification (fold change) at Day 3 post ic injection. n = 11 inoculated and 3 uninoculated mice. Lung, brain and TA, P < 0.0001 when multiple unpaired two-tailed t-tests, followed by the Holm-Sidak method, were run for comparison of tumour-bearing tissues to uninoculated controls. c) Representative IF images of matched brain, lung, SkM and bone marrow (BoMa) with DTCs at the early timepoint. Scale bar: 100 μm for lung, brain and BoMa. 10 μm for muscle. d) AluYb8 amplification (fold change) at Week 7. n = 11 inoculated and 3 uninoculated mice. Lung, brain, liver, BoMa and TA, **** P < 0.0001 when multiple unpaired two-tailed t-tests, followed by Holm-Sidak method, were run for comparison of tumour-bearing tissues to uninoculated controls. e) Representative IF images of matched brain, lung, SkM and BoMa with DTCs at the late timepoint. Scale bar: 100 μm for lung, brain and BoMa; 10 μm for muscle. f) Representative IF images of DTCs or clusters of tumour cells in the brain, lung or SkM. Ki67+ marks proliferating cells. Scale bar: 100 μm for lung, brain and BoMa; 10 μm for muscle. n = 15 cells each. For c, e and g, centre line represents the mean, and error bars the standard error of the mean (s.e.m.).
Extended Data Fig. 3 |. Metabolic comparison of SkM-metastatic 4T1 cells pre- and post-injection reveal that 4T1-SkM adapt to SkM.
a) PLS-DA score plot comparing 4T1-parental and 4T1-SkM cells in tissue culture against 4T1-SkM SkM metastases, 4T1-SkM lung metastases, healthy SkM and healthy lung. b) Fold change enrichment of GSH-related metabolites (defined by KEGG’s GSH metabolism set) for 4T1-SkM v. 4T1-parental in culture, SkM metastases v. 4T1-SkM in culture, and lung metastases v. 4T1-SkM in culture. c) Metabolite Set Enrichment of the metabolites that were 2-fold enriched in healthy SkM versus healthy lung. Over Representation Analysis used the hypergeometric test; one-tailed P-values were provided after adjusting for multiple testing. d) Table displaying the mean values for the GSH-related metabolites in healthy SkM and healthy lung, followed by the fold-change difference between the two. e) Dot-plot of the ratio of reduced to oxidized glutathione (GSH:GSSG) for 4T1-SkM and 4T1-parental in culture, healthy SkM and healthy lung, and SkM- and lung- metastases. n = 3 replicates for 4T1-parental and 4T1-SkM culture samples. n = 4 muscle- and 3 lung- metastases, 6 healthy muscle and 3 healthy lung. An one-way ANOVA, followed by uncorrected Fisher’s LSD, was performed where *** P = 0.0004 for 4T1-parental v 4T1-SkM, **** P < 0.0001 for healthy SkM v. lung, * P = 0.037 for SkM metastases v. lung metastases. For e, centre line represents the mean, and error bars the standard error of the mean (s.e.m.).
Extended Data Fig. 4 |. H2o2 is generated upon D-alanine treatment in a dose-dependent fashion in DAAo-expressing tumour cells and lung fibroblasts.
a) Workflow to test if D-/L-alanine stimulated H2O2 in MDA231-WT and -DAAO. b) Extracellular H2O2 of MDA231-WT and -DAAO treated with D-/L-alanine. n = 3 replicates, performed twice. Two-way ANOVA was used, followed by Dunnett’s test. Compared against untreated MDA231-DAAO mean- 1 h: *P = 0.028,100 mM D-ala. 4 h: ***P = 0.0001, 100 mM D-ala; **P = 0.0017, 20 mM D-ala; **P = 0.0019, 100 mM D-ala MDA231-WT. 8 h: ***P = 0.0001, 100 mM L-ala, 20 mM L-ala. *P = 0.023, 100 mM D-ala MDA231-WT; * P = 0.018, 20 mM D-ala MDA231-WT. 24 h: ***P = 0.000, 100 mM D-ala; *P = 0.011, 100 mM L-ala. 48 h: ***P = 0.000, 100 mM D-ala. All other P > 0.05. c) MDA231-WT and -DAAO outgrowth with D-/L-alanine. n = 3 replicates, performed twice. Two-way ANOVA, followed by Dunnett’s test: compared against untreated MDA231-DAAO- 48 h: ***P = 0.0001, 100 mM D-ala; **P = 0.0011, 100 mM D-ala MDA231-WT; **P = 0.0036, 20 mM D-ala MDA231-WT; *P = 0.04, untreated MDA231-WT. 72 h: ***P = 0.0001, 100 mM D-ala, untreated MDA231-WT, 20 mM L-ala MDA231-WT. All others P > 0.05. d) Workflow to test if D-/L-alanine stimulates H2O2 in LF-DAAO. e) Extracellular H2O2 in LF-DAAO and LF treated with D-/L-alanine. n = 3 replicates per condition, performed twice. Two-way ANOVA, followed by uncorrected Fisher’s LSD: ****P < 0.0001, LF-DAAO 100 mM D-ala v. LF 100 mM D-ala, LF-DAAO 100 mM D-ala v. LF-DAAO 100 mM L-ala (4 h). 8 h: *P = 0.03, LF-DAAO 50 mM D-ala v. LF 50 mM D-ala; ****P < 0.0001, 70-, 100-mM D-ala LF-DAAO v. LF. 24 h: * P = 0.033, LF-DAAO 20 mM D-ala v. LF 20 mM D-ala; ** P = 0.004, LF-DAAO 30 mM D-ala v. LF 30 mM D-ala. **** P < 0.0001, 40-, 50-, 70-, 100-mM D-ala LF-DAAO v. LF. 48 h: *** P = 0.0006, LF-DAAO 30 mM D-ala v. LF 30 mM D-ala. **** P < 0.0001, 40-, 50-, 70-, 100-mM D-ala LF-DAAO v. LF. Other LF-DAAO v. LF comparisons with D-ala P > 0.05. For b-c and e, error bars represent the s.e.m.
Extended Data Fig. 5 |. Targeting catalase to the tumour cell mitochondria does not promote lung colonization.
a) Representative Western blots of MDA231 and EO771 -Ctl, -mCAT and -cCAT. b) Dot-plots of MDA231 and EO771 -Ctl, -mCAT or -cCAT outgrowth on LF. n = 10–15 replicates, performed in triplicate. One-way ANOVA, followed by Dunnett’s test, was performed. Mean of each condition compared against Ctl mean: P = 0.43, MDA231-Ctl v mCAT; P = 0.35, MDA231-Ctl v cCAT,; **** P < 0.0001, EO771-Ctl v mCAT, EO771-Ctl v cCAT. c) NOD-SCID study to examine if ectopic catalase promoted lung colonization. d) BLI measurements (total flux, photon/second) for MDA231-Ctl, -mCAT and -cCAT. n = 5 mice per cohort. Two-way ANOVA, followed by Tukey’s test, was performed. Week5- *P = 0.032, MDA231-Ctl v mCAT; *P = 0.027, MDA231-Ctl v cCAT. Week6- ****P < 0.0001, MDA231-Ctl v. mCAT, Ctl v. cCAT, e) BLI total flux for MDA231-Ctl, -mCAT and -cCAT in lung ex vivo. n = 5 mice per cohort. One-way ANOVA, followed by Tukey’s test, was performed: *P = 0.028, Ctl v mCAT; *P = 0.026, Ctl v cCAT. f) Quantification of MDA231-Ctl, -mCAT and -cCAT lesions in lung, with representative images of tumour burden. Arrows point to small GFP+ lesions. n = 5 mice per cohort. Scale bar, 1 mm. One-way ANOVA was performed, followed by Dunnett’s test, to determine **P = 0.0044, MDA231-Ctl v. mCAT; *** P = 0.0008, MDA231-Ctl v. cCAT. g) C57BL/6 study to examine if ectopic catalase promoted lung colonization. h) BLI total flux for EO771-Ctl and -mCAT. n = 5 mice per cohort. Two-way ANOVA, followed by Sidak’s test, was performed. Week2- *P = 0.039, EO771-Ctl v mCAT. i) BLI total flux for EO771-Ctl and -mCAT tumours in lung ex vivo. n = 5 mice per cohort. Unpaired two-tailed t-test was performed, where EO771-Ctl v mCAT, *P = 0.026. j) Quantification of EO771-Ctl and -mCAT lesions in lung, with representative images of tumour burden. Scale bar, 1 mm. Unpaired two-tailed t-test was performed where **P = 0.0036. For b, e-f and i-j, centre line represents mean, and error bars the s.e.m.
Supplementary Material
Acknowledgements
We are grateful to M. J. Bissell (Lawrence Berkeley National Laboratory) and to H. Blau (Stanford University) for inspiring this work. We remain indebted to the Breast Origin Cancer Tissue Donated after Death (BROCADE) programme, funded by the National Breast Cancer Foundation of Australia, for their hard work and especially to the women with MBC who selflessly donated to this study. We would like to thank P. Rabinovitch (University of Washington) and his group for the mCAT transgenics that enabled generation of the C57BL/6 myoblasts used within this manuscript. We thank W. J. Valente and J. Bielas (FHCRC) for providing the pBMN-IRES-GFP-mCAT and -cCAT vectors, and S. Beronja (FHCRC) for his critical feedback. This study was supported principally by the W. M. Keck Foundation (to C.M.G., K.C.H. and P.S.N.), and by start-up funds from the FHCRC (to C.M.G.). The Comparative Medicine and Proteomics Shared Resources of the FHCRC/University of Washington Cancer Consortium helped support this work and are funded by the NCI (P30 CA015704). BioRender.com was used to create mouse/human schematics. S.B.C. was supported by a Cellular and Molecular Biology Training Grant from the NIH (T32GM007270). J.D. was supported by a Postdoctoral Breakthrough award (W81XWH-18–1-0028) by the DoD Breast Cancer Research Program. P.S.N., L.D.T. and R.F.D. are also supported by P50CA097186.
Footnotes
Code availability
Code used in this study (for example, ImageJ macros for image analysis) are freely available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Additional information
Extended data is available for this paper at https://doi.org/10.1038/s41556–022-00881–4.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41556–022-00881–4.
Reprints and permissions information is available at www.nature.com/reprints.
Data availability
Raw data for Figs. 1e,f,h, 2c,e–h, 3d,e,g–i, 4b–d,f–h,j, 5a,b,e,g,j, 6a,b,d,g,i,n, 7c–f,h,j,k and 8a,c,d,f–h, and Extended Data Figs. 2b,d, 3b–e, 4b–c,e and 5b,d–f,h–j have been provided as individual source data files. Full metabolomics data pertaining to Fig. 3 and Extended Data Fig. 3 can be found in Supplementary Tables 1–4. Metabolomics data have been deposited in Metabolomics Workbench (study number ST002058) and can be accessed directly via its project: https://doi.org/10.21228/M89135. Additional details pertaining to the AluYb8 qPCR method and the studies described in Fig. 1 and Extended Data Fig. 2 are provided as Supplementary Table 5. Source data are provided with this paper.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889). [PubMed] [Google Scholar]
- 2.Elia I. et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elia I. et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 8, 15267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuy F. et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 22, 577–589 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Tasdogan A. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan MR et al. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 29, 1410–1421.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knott SRV et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghajar CM et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta GP et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765–770 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Peinado H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan RN et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiratsuka S, Watanabe A, Aburatani H. & Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Kim S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oskarsson T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wculek SK & Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Silva B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkan D. et al. Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudreau N, Sympson CJ, Werb Z. & Bissell MJ Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267, 891–893 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen OW, Ronnov-Jessen L, Howlett AR & Bissell MJ Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl Acad. Sci. USA 89, 9064–9068 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pommier A. et al. Unresolved endoplasmic reticulum stress engenders immune- resistant, latent pancreatic cancer metastases. Science 360, aao4908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantel K. et al. Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res. 51, 4712–4715 (1991). [PubMed] [Google Scholar]
- 24.Malladi S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coller HA Cell biology: the essence of quiescence. Science 334, 1074–1075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuilman T, Michaloglou C, Mooi WJ & Peeper DS The essence of senescence. Genes Dev. 24, 2463–2479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrengues J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willis RA The Spread of Tumours in the Human Body (Butterworth & Co., 1952). [Google Scholar]
- 29.Disibio G. & French SW Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 132, 931–939 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Weiss L. Biomechanical destruction of cancer cells in skeletal muscle: a rate-regulator for hematogenous metastasis. Clin. Exp. Metastasis 7, 483–491 (1989). [DOI] [PubMed] [Google Scholar]
- 31.Parlakian A. et al. Skeletal muscle phenotypically converts and selectively inhibits metastatic cells in mice. PLoS ONE 5, e9299 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djaldetti M, Sredni B, Zigelman R, Verber M. & Fishman P. Muscle cells produce a low molecular weight factor with anti-cancer activity. Clin. Exp. Metastasis 14, 189–196 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Fishman P, Bar-Yehuda S. & Vagman L. Adenosine and other low molecular weight factors released by muscle cells inhibit tumor cell growth. Cancer Res. 58, 3181–3187 (1998). [PubMed] [Google Scholar]
- 34.Bar-Yehuda S, Barer F, Volfsson L. & Fishman P. Resistance of muscle to tumor metastases: a role for A3 adenosine receptor agonists. Neoplasia 3, 125–131 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghajar CM Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer 15, 238–247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider T, Osl F, Friess T, Stockinger H. & Scheuer WV Quantification of human Alu sequences by real-time PCR—an improved method to measure therapeutic efficacy of anti-metastatic drugs in human xenotransplants. Clin. Exp. Metastasis 19, 571–582 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Preston Campbell J. et al. TRIzol and Alu qPCR-based quantification of metastatic seeding within the skeleton. Sci. Rep. 5, 12635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batzer MA & Deininger PL Alu repeats and human genomic diversity. Nat. Rev. Genet. 3, 370–379 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Li W, Germain RN & Gerner MY Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc. Natl. Acad. Sci. USA 114, E7321–E7330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco-Barraza J, Beacham DA, Amatangelo MD & Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. 2016, 10.9.1–10.9.34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muir A, Danai LV & vander Heiden MG Microenvironmental regulation of cancer cell metabolism: Implications for experimental design and translational studies. Dis. Model. Mech. 11, dmm035758 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan MR & vander Heiden MG Determinants of nutrient limitation in cancer. Crit. Rev. Biochem. Mol. Biol. 54, 193–207 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schild T, Low V, Blenis J. & Gomes AP Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer Cell 33, 347–354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong J, Wishart DS & Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinformatics 68, e86 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Kanehisa M. & Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bansal A. & Celeste Simon M. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 217, 2291–2298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powers SK, Li, Ji L, Kavazis AN & Jackson MJ Reactive oxygen species: impact on skeletal muscle. Compr. Physiol. 1, 941–969 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Townsend DM S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 7, 313–324 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zitka O. et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 4, 1247–1253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai YC, Ashraf SS, Rokutan K, Johnston RB & Thomas JA S-thiolation of individual human neutrophil proteins including actin by stimulation of the respiratory burst: evidence against a role for glutathione disulfide. Arch. Biochem. Biophys. 310, 273–281 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Piskounova E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan B, Sobotta MC & Dick TP Measuring EGSH and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 51, 1943–1951 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Gutscher M. et al. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Steinhorn B. et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 9, 4044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glorieux C. & Calderon PB Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 398, 1095–1108 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Schriner SE et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Ligtenberg MA et al. Coexpressed ÿcatalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. J. Immunol. 196, 759–766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grzelak CA et al. Elimination of fluorescent protein immunogenicity permits modeling of metastasis in immune-competent settings. Cancer Cell 40, 1–2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yun J. et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diehn M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 780–783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorrini C, Harris IS & Mak TW Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Nguyen A. et al. PKLR promotes colorectal cancer liver colonization through induction of glutathione synthesis. J. Clin. Invest 126, 681–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ubellacker JM et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snijders T. et al. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 6, 283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hojman P, Gehl J, Christensen JF & Pedersen BK Cell metabolism perspective molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 27, 10–21 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Fujikawa Y. et al. Mouse redox histology using genetically encoded probes. Sci. Signal. 9, rs1 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Nemkov T, Hansen KC & D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. 31, 663–673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nemkov T, Reisz JA, Gehrke S, Hansen KC & D’Alessandro A. in Methods in Molecular Biology Vol. 1978 (ed. D’Alessandro A) 13–26 (Humana Press, 2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data for Figs. 1e,f,h, 2c,e–h, 3d,e,g–i, 4b–d,f–h,j, 5a,b,e,g,j, 6a,b,d,g,i,n, 7c–f,h,j,k and 8a,c,d,f–h, and Extended Data Figs. 2b,d, 3b–e, 4b–c,e and 5b,d–f,h–j have been provided as individual source data files. Full metabolomics data pertaining to Fig. 3 and Extended Data Fig. 3 can be found in Supplementary Tables 1–4. Metabolomics data have been deposited in Metabolomics Workbench (study number ST002058) and can be accessed directly via its project: https://doi.org/10.21228/M89135. Additional details pertaining to the AluYb8 qPCR method and the studies described in Fig. 1 and Extended Data Fig. 2 are provided as Supplementary Table 5. Source data are provided with this paper.