Abstract
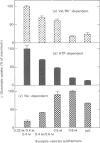
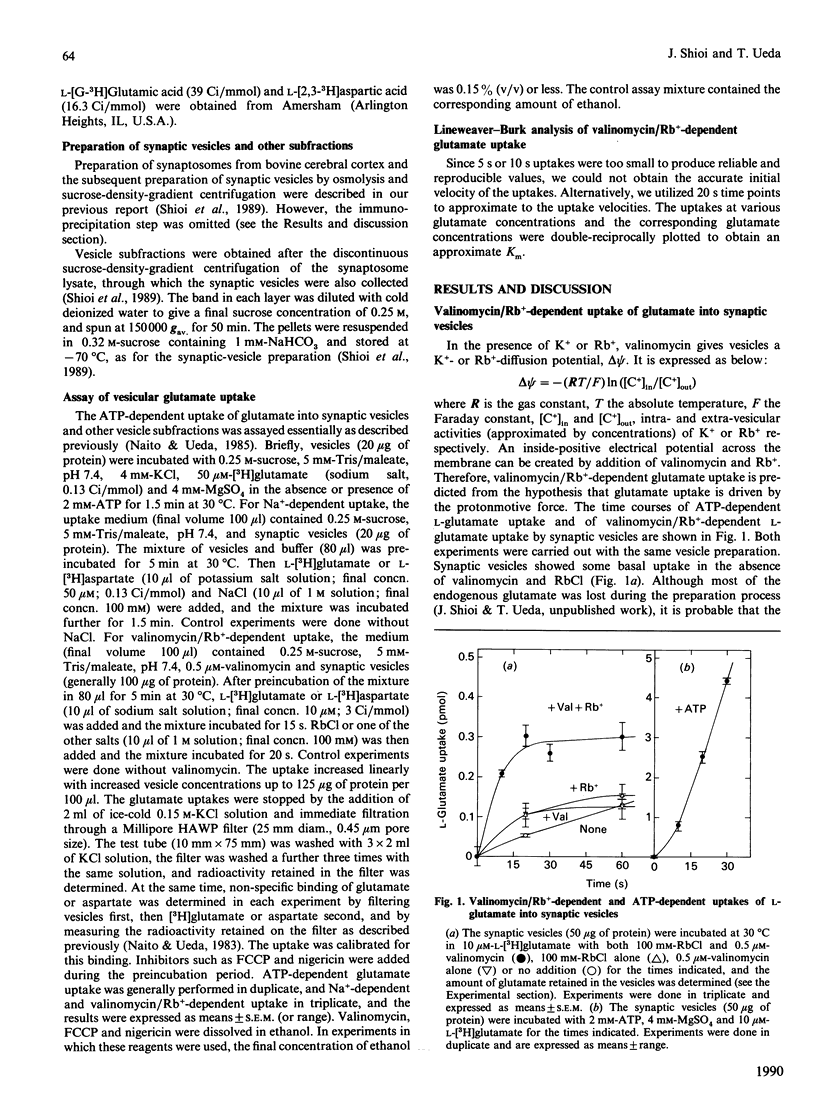
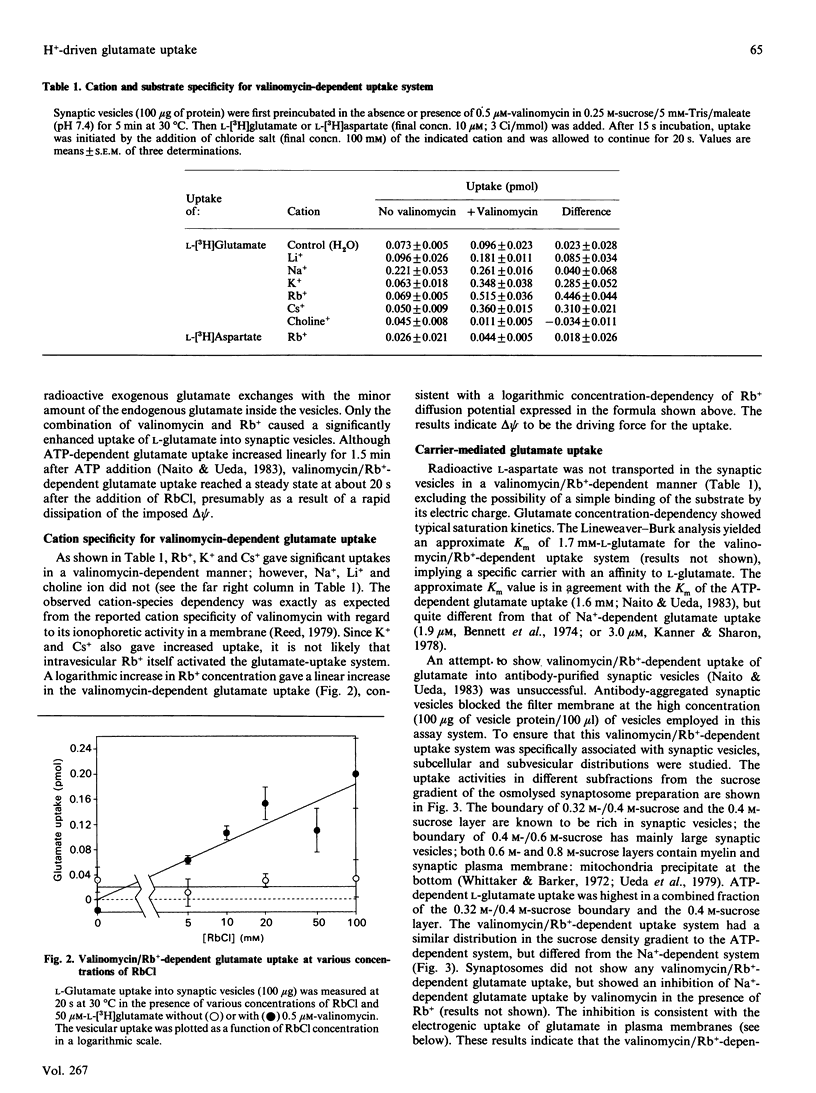
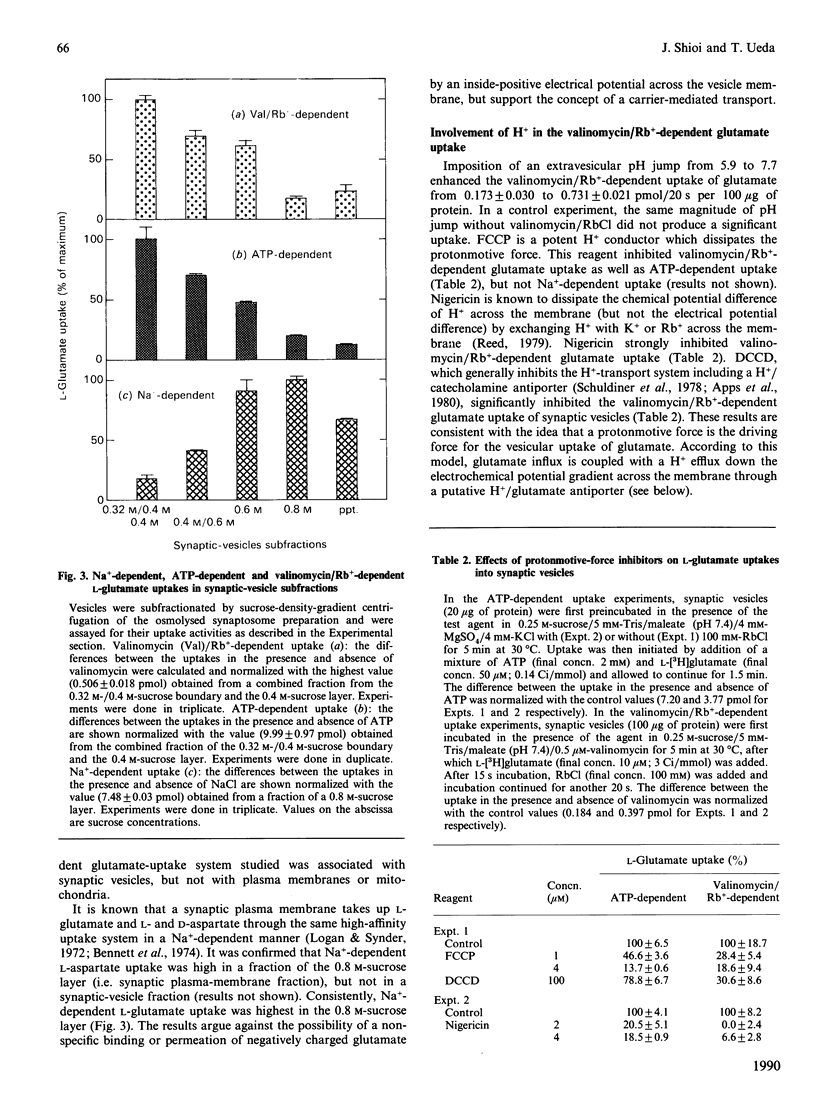
L-Glutamate is a major excitatory neurotransmitter in the central nervous system. MgATP-dependent glutamate uptake and H(+)-pumping ATPase activity were reported in highly purified synaptic vesicles [Naito & Ueda (1983) J. Biol. Chem. 258, 696-699; Shioi, Naito & Ueda (1989) Biochem. J. 258, 499-504], and it is hypothesized that an electrochemical H+ gradient across the vesicle membrane, the so-called protonmotive force, elicits the neurotransmitter uptake. An inside-positive diffusion potential across the vesicle membrane was established with valinomycin plus Rb+. This artificial electrical potential promoted the uptake of glutamate, but not aspartate, in the synaptic vesicles prepared from bovine cerebral cortex. The uptake was inhibited by the protonmotive-force dissipators carbonyl cyanide p-trifluoro-methoxyphenylhydrazone or nigericin, and was enhanced by concomitant imposition of a pH jump (alkalinization) in the external medium. Subcellular and subvesicular distributions showed the uptake system to be predominantly associated with small synaptic vesicles. The results support the hypothesis that glutamate uptake into synaptic vesicles is coupled with a H+ efflux down the electrochemical potential gradient, which is generated by H(+)-pumping ATPase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K., Pryde J. G., Sutton R., Phillips J. H. Inhibition of adenosine triphosphatase, 5-hydroxytryptamine transport and proton-translocation activities of resealed chromaffin-granule 'ghosts'. Biochem J. 1980 Aug 15;190(2):273–282. doi: 10.1042/bj1900273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Jr, Mulder A. H., Snyder S. H. Neurochemical correlates of synaptically active amino acids. Life Sci. 1974 Sep 15;15(6):1045–1056. doi: 10.1016/s0024-3205(74)80002-0. [DOI] [PubMed] [Google Scholar]
- Carlson M. D., Kish P. E., Ueda T. Characterization of the solubilized and reconstituted ATP-dependent vesicular glutamate uptake system. J Biol Chem. 1989 May 5;264(13):7369–7376. [PubMed] [Google Scholar]
- Cidon S., Sihra T. S. Characterization of a H+-ATPase in rat brain synaptic vesicles. Coupling to L-glutamate transport. J Biol Chem. 1989 May 15;264(14):8281–8288. [PubMed] [Google Scholar]
- Davies L. P., Johnston G. A. Uptake and release of D- and L-aspartate by rat brain slices. J Neurochem. 1976 May;26(5):1007–1014. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Fischer-Bovenkerk C., Kish P. E., Ueda T. ATP-dependent glutamate uptake into synaptic vesicles from cerebellar mutant mice. J Neurochem. 1988 Oct;51(4):1054–1059. doi: 10.1111/j.1471-4159.1988.tb03068.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Fykse E. M., Christensen H., Fonnum F. Comparison of the properties of gamma-aminobutyric acid and L-glutamate uptake into synaptic vesicles isolated from rat brain. J Neurochem. 1989 Mar;52(3):946–951. doi: 10.1111/j.1471-4159.1989.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978 Sep 19;17(19):3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- Kish P. E., Fischer-Bovenkerk C., Ueda T. Active transport of gamma-aminobutyric acid and glycine into synaptic vesicles. Proc Natl Acad Sci U S A. 1989 May;86(10):3877–3881. doi: 10.1073/pnas.86.10.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan W. J., Snyder S. H. High affinity uptake systems for glycine, glutamic and aspaspartic acids in synaptosomes of rat central nervous tissues. Brain Res. 1972 Jul 20;42(2):413–431. doi: 10.1016/0006-8993(72)90540-9. [DOI] [PubMed] [Google Scholar]
- Maycox P. R., Deckwerth T., Hell J. W., Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988 Oct 25;263(30):15423–15428. [PubMed] [Google Scholar]
- Naito S., Ueda T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J Biol Chem. 1983 Jan 25;258(2):696–699. [PubMed] [Google Scholar]
- Naito S., Ueda T. Affinity-purified anti-protein I antibody. Specific inhibitor of phosphorylation of protein I, a synaptic protein. J Biol Chem. 1981 Oct 25;256(20):10657–10663. [PubMed] [Google Scholar]
- Naito S., Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985 Jan;44(1):99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Sihra T. S., Sanchez-Prieto J. Calcium-dependent and -independent release of glutamate from synaptosomes monitored by continuous fluorometry. J Neurochem. 1987 Jul;49(1):50–57. doi: 10.1111/j.1471-4159.1987.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Sihra T. S. Synaptosomes possess an exocytotic pool of glutamate. Nature. 1986 Jun 19;321(6072):772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- Njus D., Kelley P. M., Harnadek G. J. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853(3-4):237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Ionophores. Methods Enzymol. 1979;55:435–454. doi: 10.1016/0076-6879(79)55058-7. [DOI] [PubMed] [Google Scholar]
- Riveros N., Fiedler J., Lagos N., Muñoz C., Orrego F. Glutamate in rat brain cortex synaptic vesicles: influence of the vesicle isolation procedure. Brain Res. 1986 Oct 29;386(1-2):405–408. doi: 10.1016/0006-8993(86)90181-2. [DOI] [PubMed] [Google Scholar]
- Robinson M. B., Coyle J. T. Glutamate and related acidic excitatory neurotransmitters: from basic science to clinical application. FASEB J. 1987 Dec;1(6):446–455. doi: 10.1096/fasebj.1.6.2890549. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto J., Sihra T. S., Nicholls D. G. Characterization of the exocytotic release of glutamate from guinea-pig cerebral cortical synaptosomes. J Neurochem. 1987 Jul;49(1):58–64. doi: 10.1111/j.1471-4159.1987.tb03394.x. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H., Kanner B. I. Role of a transmembrane pH gradient in epinephrine transport by chromaffin granule membrane vesicles. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3713–3716. doi: 10.1073/pnas.75.8.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J., Naito S., Ueda T. Glutamate uptake into synaptic vesicles of bovine cerebral cortex and electrochemical potential difference of proton across the membrane. Biochem J. 1989 Mar 1;258(2):499–504. doi: 10.1042/bj2580499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm-Mathisen J., Leknes A. K., Bore A. T., Vaaland J. L., Edminson P., Haug F. M., Ottersen O. P. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983 Feb 10;301(5900):517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. The transmitter role of glutamate in nervous systems. Jpn J Physiol. 1987;37(4):559–572. doi: 10.2170/jjphysiol.37.559. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]