Abstract
Brain functioning relies on orchestrated synaptic vesicle dynamics and controlled neurotransmitter release. Multiple biomolecular condensates coexist at the pre- and post-synapse and they are driven by condensation that combines binding, phase separation, and percolation. In pre-synapses, intrinsically disordered regions (IDRs) of synaptic proteins are drivers of condensation that enable clustering of synaptic vesicles (SVs). Although sequences of IDRs are poorly conserved across evolution, our computational analysis reveals the existence of non-random compositional biases and sequence patterns (molecular grammars) in IDRs of pre-synaptic proteins. For example, synapsin-1, which is essential for condensation of SVs, contains a conserved valence of arginine residues and blocks of polar and proline residues that are segregated from one another along the linear sequence. We show that these conserved features are crucial for driving synapsin-1 condensation in vitro and in cells. Our results highlight how conserved molecular grammars drive the condensation of key proteins at the pre-synapse.
Keywords: biomolecular condensates, intrinsically disordered protein regions, synapse, Biological Sciences, Biochemistry, Physical Sciences, Applied Physical Sciences
Functional neurotransmission relies on synaptic vesicle (SV) cycles at the synapse. SVs cluster at pre-synapses forming biomolecular condensates with synapsins, which are abundant synaptic proteins (1)(2). Synapsin-SV condensates enable reversible sequestration and maintenance of soluble synaptic proteins that are essential for distinct steps of SV cycles (3). Extant data suggest that pre-synapses are complex emulsions defined by multiple coexisting phases (4). Here, we report that pre-synaptic proteins feature intrinsically disordered regions (IDRs) with evolutionarily conserved, distinct and complementary molecular grammars. The grammars with synapsin-1 IDR influence condensation via homotypic interactions in vitro, and synapsin co-condensates in cells.
We analyzed IDRs of pre-synaptic proteins and uncovered non-random compositional biases and binary sequence patterns (5) – molecular grammars (6) – within synapsin-1 and other SV proteins. Cytosolic IDRs of integral SV proteins are enriched in negatively charged and aromatic residues whereas IDRs of soluble pre-synaptic proteins are enriched in proline and positively charged residues (Fig. 1A–C). Grammars of IDRs of pre-synaptic proteins are distinct from IDRs in proteins from other condensates (8).
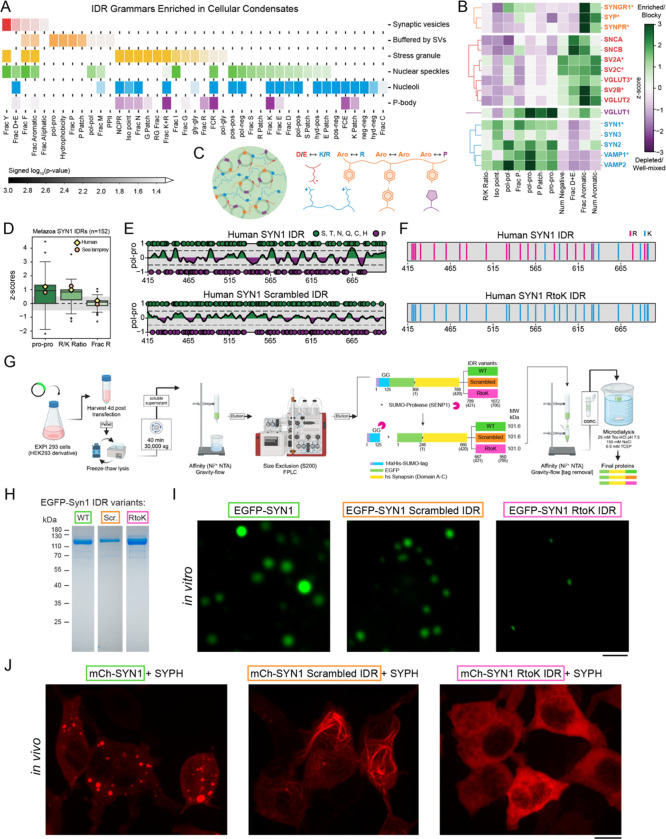
Figure 1: IDRs of proteins that make up synaptic condensates feature distinct molecular grammars.
A. IDR grammars of SV proteins are distinct from those of IDRs in other condensates.
B. IDR compositions and sequence patterns of pre-synaptic proteins fall into distinct clusters.
C. Schematic illustrating the chemical complementarity across IDRs within SV clusters.
D. Molecular grammars are conserved across 152 metazoans, including lamprey, which is the oldest vertebrate predecessor of humans.
E. Schematic showing how the synapsin-1 scrambled IDR in which polar and proline blocks are evenly distributed to reduce blockiness.
F. Schematic of the synapsin-1 IDR showing the substitution of Arg with Lys.
G. Purification protocol for synapsin-1 IDR (WT) and the two variants SCR and RtoK.
H. Coomassie gel indicating the final recombinant proteins used for the biophysical assays.
I. Microscopy images of EGFP-synapsin-1 (8 μM) with WT, SCR, and RtoK IDR reconstituted in 25 mM Tris-HCl (pH 7.5), 0.5 mM TCEP, 150 mM NaCl and 3% PEG 8,000. Scale bar, 5 μm.
J. Images of HEK cells expressing EGFP-synapsin-1 WT (left), EGFP-synapsin-1 scrambled IDR (middle), or EGFP-synapsin-1 RtoK IDR (right) with untagged synaptophysin. Mutants disrupted formation of normal synapsin condensates. IDR scramble formed filaments resembling cytoskeleton, and RtoK completely abolished condensation. Scale bar, 10 μm.
The synapsin-1 IDR features segregated blocks of polar/proline residues and a preference for Arg over Lys (Fig. 1D). We designed two variants of synapsin-1 to test how conserved grammars affect condensation. In the SCR variant, we scrambled the IDR sequence to disrupt proline/polar blocks (Fig. 1E). In the RtoK variant, Arg residues within the IDR were substituted with Lys (Fig. 1F). We cloned, expressed, and purified the WT and the two mutants each with an EGFP fluorophore (Fig. 1G,H). WT synapsin-1 readily formed condensates; synapsin-1 SCR formed smaller and more sparse condensates. Synapsin-1 RtoK did not form condensates under equivalent conditions (Fig. 1I) suggesting that substitution of Arg to Lys weakens homotypic interactions (9).
Next, we co-expressed each synapsin-1 construct in cells with synaptophysin, an integral SV protein implicated in the formation of small vesicles (10). The IDR of synaptophysin is enriched in aromatic residues (Fig. 1B). Co-expression of WT synapsin-1 recapitulated co-condensation with synaptophysin (11). However, co-expression of synapsin-1 SCR with synaptophysin condensates generated a gain-of-function phenotype for cytoskeleton binding without forming condensates. Co-expressed synapsin-1 RtoK remained fully soluble (Fig. 1J). Substitution of Arg with Lys in the synapsin-1 IDR abrogated co-condensation with synaptophysin in cells.
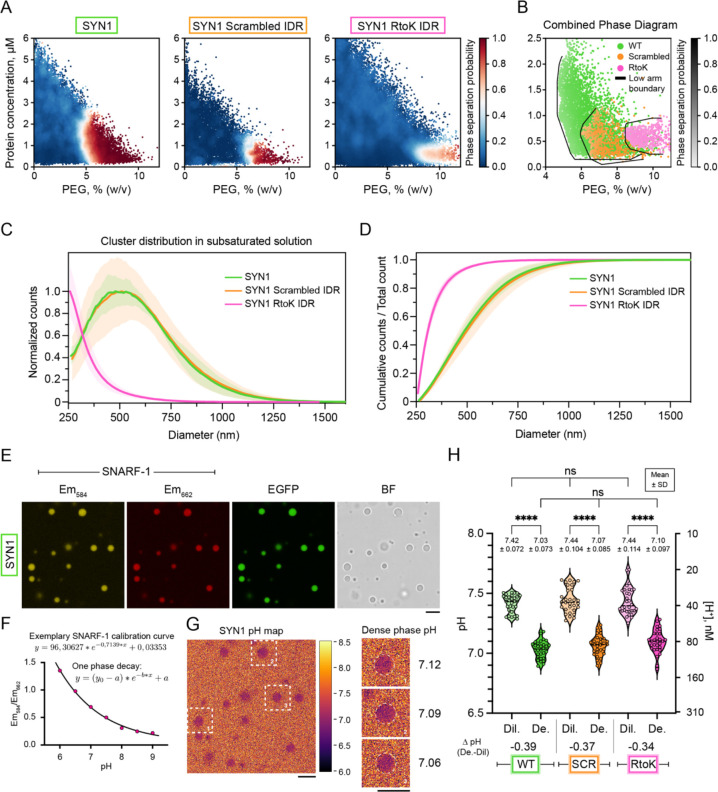
For rigorous quantification of driving forces, we mapped comparative phase boundaries for the synapsin-1 variants using a microfluidics-based “PhaseScan” approach (12). Synapsin-1 WT, synapsin-1 SCR and synapsin-1 RtoK showed distinct phase boundaries (Fig. 2A) with the synapsin-1 RtoK being the weakest driver of phase separation (Fig. 2B). We also deployed microfluidics resistive pulse sensing (MRPS) to quantify size distributions of clusters in subsaturated solutions (13). Substitution of Arg to Lys alters the size distribution showing a pronounced shift toward smaller species (Fig. 2C,D). Even though the driving forces for phase separation are weakened when the IDR is scrambled, clustering in subsaturated solutions is equivalent for synapsin-1 WT and synapsin-1 SCR. This highlights how driving forces for forming pre-percolation clusters and phase separation can be decoupled (13).
Figure 2. Molecular grammars of synapsin-1 IDR influence synapsin-1 condensation.
A. Low concentration arms of phase boundaries of EGFP-synapsin-1 WT (left, N = 29,187 points), EGFP-synapsin-1 scrambled IDR (middle, N = 13,203 data points), or EGFP-synapsin-1 RtoK IDR (right, N = 64,675 data points). Red and blue data points correspond to individual microdroplets classified as phase separated or homogeneous, respectively. The heatmap quantifies the phase separation probability.
B. Combined phase boundaries from A showing the low concentration arm.
C. Cluster size distributions in subsaturated solutions for synapsin-1 WT (green), SCR (orange), and RtoK (magenta) variants. Measurements were performed at equivalent conditions (8 μM Synapsin 1 variants with 3% PEG 8,000 in 25 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 mM TCEP) using a C-2000 cartridge in an nCS1 instrument. The plot shows counts that are normalized to the maximum value as a function of the particle diameter.
D. Cumulative counts for cluster size distributions shown in C. Error bars (shaded) represent standard deviation.
E. Representative micrographs of EGFP-synapsin-1 condensates (8 μM protein in 3% PEG 8,000) co-incubated with the ratiometric pH-sensitive reporter SNARF-1. SNARF-1 was excited at 561 nm and recorded at 582–586 (yellow) and 660–664 nm (red). Scale bar, 5 μm.
F. Carboxy SNARF-1 calibration curve for reconstitution buffer (25 mM Tris-HCl, 150 mM NaCl, 0.5 mM TCEP) at different pH (pH 6 – pH 9) a single plane. Data were fit to a one-phase-decay function.
G. Left: Example micrograph indicating a pH map for EGFP-synapsin-1 WT condensates calculated by applying the z-slice calibration curve. Heatmap represents pH. Right: Magnified regions of selected condensates (dashed line) with indicated average pH values of the dense phase. Scale bar, 5 μm.
H. pH analysis for the dense and dilute phases of condensates for synapsin-1 WT (green), SCR (orange), and RtoK (magenta) variants. Average pH values are shown in the graph with standard deviation. Violin plots with median and quartiles indicated by the dashed lines. (One-way ANOVA, ****: p<0,0001).
Motivated by recent work on nucleoli (8), we asked if phase separation generates a pH gradient because this could help with proper loading of neurotransmitters into SVs (14) (15). Using the ratiometric pH-sensitive dye SNARF-1 (Fig. 2E–2G), we found that synapsin-1 WT, SCR, and RtoK showed equivalent abilities to accumulate protons in dense phases (Fig. 2H). Therefore, while molecular grammars in synapsin-1 control driving forces for condensation, the interphase pH gradients remain unchanged, presumably because sequences have identical net charge (8).
Our work shows how insights from computational analysis can be tested via designed mutations. This approach bypasses the often-used approach of deleting IDRs to assess their contributions – an approach that rests on the erroneous notion that IDRs engage mainly in non-specific interactions. Instead, we show how systematic designs that modulate conserved grammars can be brought to bear to investigate how these regions contribute to phase behavior. In the context of the pre-synapse, the results presented here and investigations of the synergies of molecular grammars in synaptic boutons (4) are likely to enable new insights into our understanding of the neuronal transmission that is relevant in healthy brains and in diseases characterized by the loss of functional synapses.
Materials and Methods.
Details of the materials and methods are available in Supplementary Information.
Supplementary Material
Acknowledgments
We thank the Advanced Medical Bioimaging Core Facility at Charité University Clinic, Berlin for microscopy. This work was supported by DZNE, grants from the DFG (MI 2104 and SFB1286/B10 to DM); ERC Grants (MemlessInterface 101078172 to DM, DiProPhys 101001615 to TPJK); the NIH (NIA 2RF1 NS078165-12 to JRM, NINDS R01NS121114 to RVP, and F32GM146418-01A1 to MRK) and AFOSR (FA9550-20-1-0241 to RVP). CH is supported by the Innovative Minds Program of the German Dementia Association.
Footnotes
Competing Interest Statement: RVP is a member of the scientific advisory board and shareholder in Dewpoint Therapeutics Inc. TPJK is the co-founder of Fluid Analytics, Wren Therapeutics, Xampla, and Transition Bio. These affiliations did not contribute to, motivate, or influence the current study. All other authors have no competing interests to declare.
References
- 1.Milovanovic D., Wu Y., Bian X., De Camilli P., A liquid phase of synapsin and lipid vesicles. Science 361, 604 607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann C., et al. , Synapsin condensation controls synaptic vesicle sequestering and dynamics. Nature Commun 14, 6730 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denker A., Kröhnert K., Bückers J., Neher E., Rizzoli S. O., The reserve pool of synaptic vesicles acts as a buffer for proteins involved in synaptic vesicle recycling. Proc Natl Acad Sci USA 108, 17183 17188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sansevrino R., Hoffmann C., Milovanovic D., Condensate biology of synaptic vesicle clusters. Trends Neurosci. 46, 293–306 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Cohan M.C., et al. , Uncovering non-random binary patterns within sequences of intrinsically disordered proteins. J Mol Biol 434, 167373 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., et al. , A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–699.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taoufiq Z., et al. , Hidden proteome of synaptic vesicles in the mammalian brain. Proc Natl Acad Sci USA 117, 33586–33596 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King M. R., et al. , Macromolecular condensation organizes nucleolar sub-phases to set up a pH gradient. Cell 187, 1889–1906.e24 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Fisher R. S., Elbaum-Garfinkle S., Tunable multiphase dynamics of arginine and lysine liquid condensates. Nature Commun 11, 4628 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron P. L., Südhof T. C., Jahn R., De Camilli P., Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol 115, 151–164 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park D., et al. , Cooperative function of synaptophysin and synapsin in the generation of synaptic vesicle-like clusters in non-neuronal cells. Nature Commun. 12, 263 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arter W. E., et al. , Biomolecular condensate phase diagrams with a combinatorial microdroplet platform. Nature Commun 13, 7845 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kar M., et al. , Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc Natl Acad Sci USA 119, e2202222119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farsi Z., et al. , Single-vesicle imaging reveals different transport mechanisms between glutamatergic and GABAergic vesicles. Science 351, 981–984 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Kosmidis E., et al. , Regulation of the mammalian-brain V-ATPase through ultraslow mode-switching. Nature 611, 827–834 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.